©The Author(s) 2016.
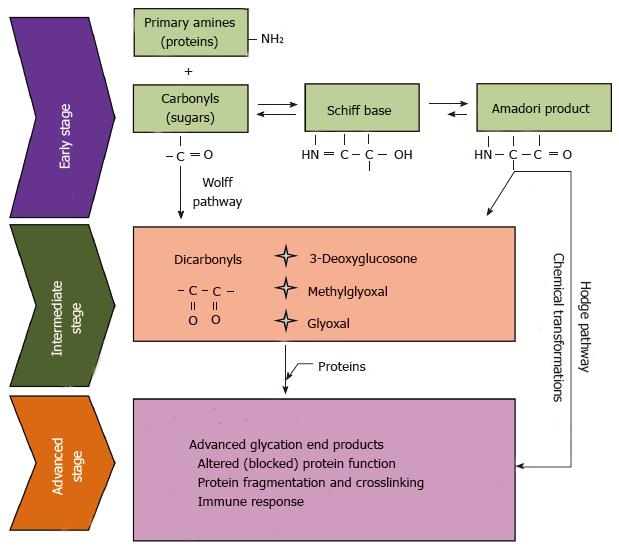
Figure 1 Maillard reaction.
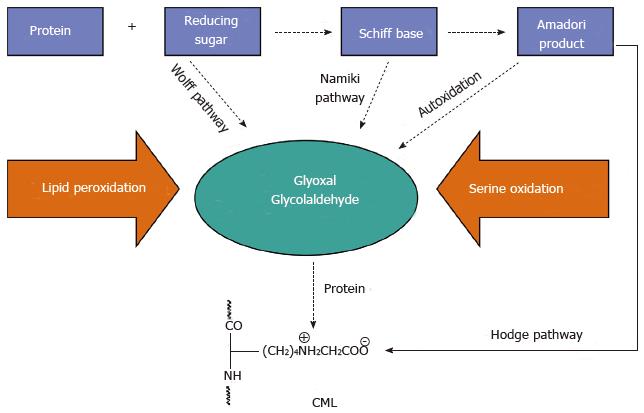
Figure 2 Pathways of Nε-(carboxymethyl)lysine formation.
CML: Nε-(carboxymethyl)lysine.
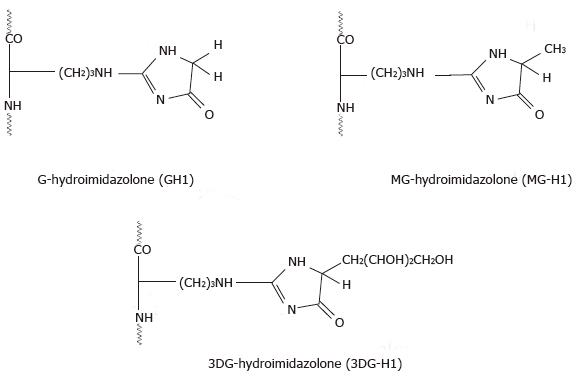
Figure 3 Hydroimidazolones[67].
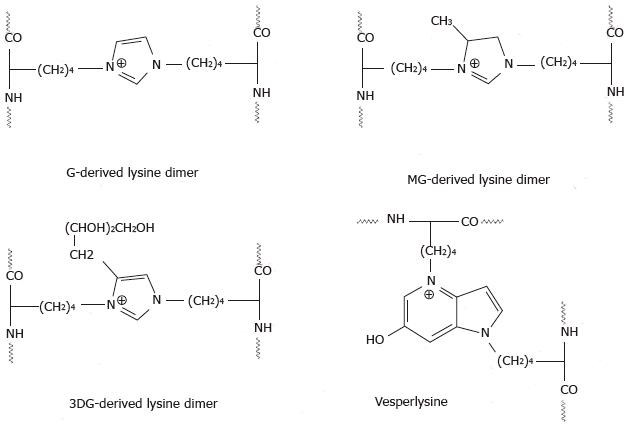
Figure 4 Lysine-lysine advanced glycation end products crosslinks[67].
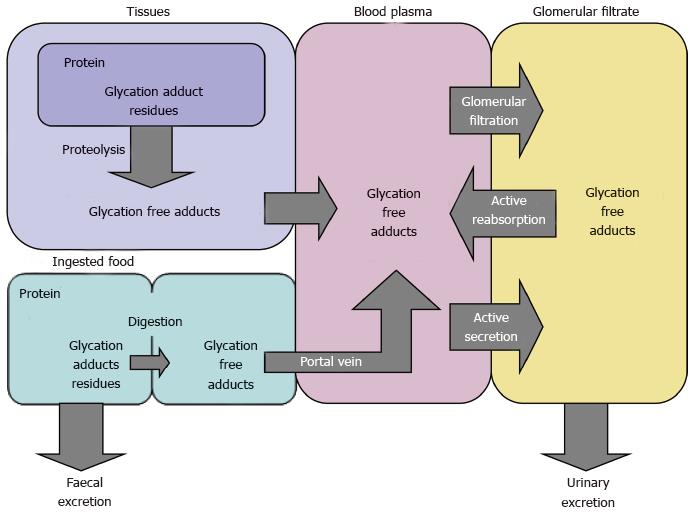
Figure 5 Biodistribution scheme illustrating flows of formation and removal of protein glycation free adducts[86].
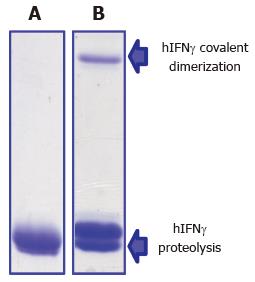
Figure 6 Covalent dimerization and proteolysis of Escherichia coli-derived hIFNγ (A) after storage for one month in solution (0.
4 mol/L NaCl, Tris-HCl pH 8.2) at 4 °C under nitrogen (B). Protein species were separated on 15% denaturing (sodium dodecyl sulfate) polyacrylamide gel.
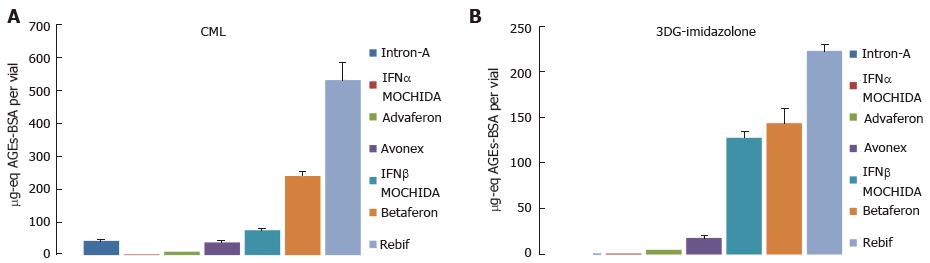
Figure 7 Nε-(carboxymethyl)lysine (A) and 3DG-imidazolone (B) in interferon pharmaceuticals.
Three vials of each drug were analyzed by competitive enzyme-linked immunosorbent assay in duplicates, and the pooled results were presented as mean ± SD. One μg-eq AGEs-BSA corresponds to the amount of CML or 3DG-imidazolone present in one microgram of the referent AGEs-BSA. The latter was prepared by incubating 10 mg/mL BSA with 0.5 mol/L glucose for 3 mo at 37 °C. AGEs: Advanced glycation endproducts; CML: Nε-(carboxymethyl)lysine; BSA: Bovine serum albumin.
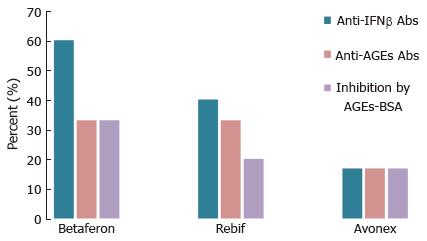
Figure 8 Percent sera of MS patients treated with Betaferon, Avoex and Rebif, containing anti-IFNβ, anti-advanced glycation endproducts Abs, and responding to Advanced glycation endproducts-bovine serum albumin inhibition of binding to IFNβ.
For detailed information on the experimental conditions see ref. No. 211. AGEs-BSA: Advanced glycation endproducts-bovine serum albumin.
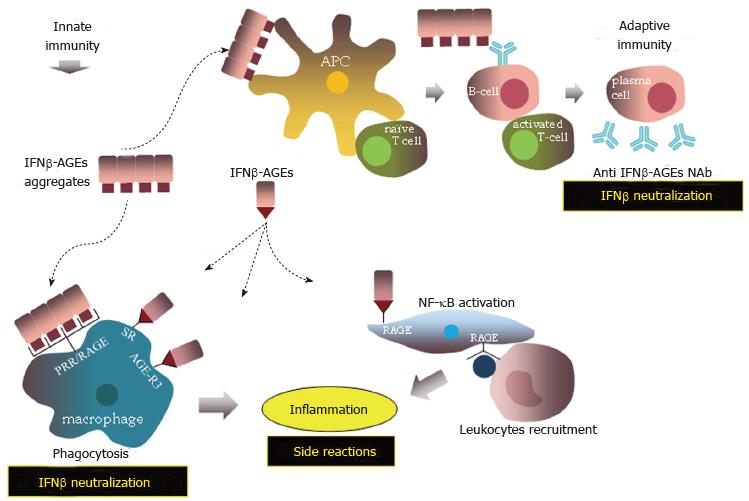
Figure 9 Proposed immune responses against advanced glycation end products modified IFNβ (IFNβ-advanced glycation endproducts).
AGEs: Advanced glycation endproducts.
- Citation: Tsekovska R, Sredovska-Bozhinov A, Niwa T, Ivanov I, Mironova R. Maillard reaction and immunogenicity of protein therapeutics. World J Immunol 2016; 6(1): 19-38
- URL: https://www.wjgnet.com/2219-2824/full/v6/i1/19.htm
- DOI: https://dx.doi.org/10.5411/wji.v6.i1.19