Published online Feb 8, 2017. doi: 10.5409/wjcp.v6.i1.52
Peer-review started: June 13, 2016
First decision: July 4, 2016
Revised: October 26, 2016
Accepted: November 16, 2016
Article in press: November 17, 2016
Published online: February 8, 2017
Processing time: 235 Days and 10.7 Hours
To evaluate the risk profile of sulfur hexafluoride in voiding urosonography (VUS) based on a large cohort of children.
Since 2011 sulfur hexafluoride (SH, SonoVue®, Bracco, Italy) is the only ultrasound contrast available in the European Union and its use in children has not been approved. Within a 4-year-period, 531 children with suspected or proven vesicoureteral reflux (f/m = 478/53; mean age 4.9 years; 1 mo-25.2 years) following parental informed consent underwent VUS with administration of 2.6 ± 1.2 mL SH in a two-center study. A standardized telephone survey on adverse events was conducted three days later.
No acute adverse reactions were observed. The survey revealed subacute, mostly self-limited adverse events in 4.1% (22/531). The majority of observed adverse events (17/22) was not suspected to be caused by an allergic reaction: Five were related to catheter placement, three to reactivated urinary tract infections, five were associated with perineal disinfection before voiding urosonography or perineal dermatitis and four with a common cold. In five patients (0.9%) hints to a potential allergic cause were noted: Perineal urticaria was reported in three interviews and isolated, mild fever in two. These were minor self-limited adverse events with a subacute onset and no hospital admittance was necessary. Ninety-six point two percent of the parents would prefer future VUS examinations with use of SH.
No severe adverse events were observed and indications of self-limited minor allergic reactions related to intravesical administration of SH were reported in less than 1%.
Core tip: This was a two-center study on 531 children with suspected or proven vesicoureteral reflux undergoing off-label voiding urosonography using sulfur hexafluoride (SH). We investigated the SH risk profile with intravesical administration. No acute allergic adverse event was observed with the off-label-use of SH for radiation free assessment of vesicoureteral reflux. Only a few subacute, minor-to-moderate adverse events were reported (4.1%). Hints of self-limited minor allergic reaction related to intravesical administration of sulfur hexafluoride were reported in less than 1%. This underlines the demand for an approval of SH in pediatric applications.
- Citation: Sauer A, Wirth C, Platzer I, Neubauer H, Veldhoen S, Dierks A, Kaiser R, Kunz A, Beer M, Bley T. Off-label-use of sulfur-hexafluoride in voiding urosonography for diagnosis of vesicoureteral reflux in children: A survey on adverse events. World J Clin Pediatr 2017; 6(1): 52-59
- URL: https://www.wjgnet.com/2219-2808/full/v6/i1/52.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v6.i1.52
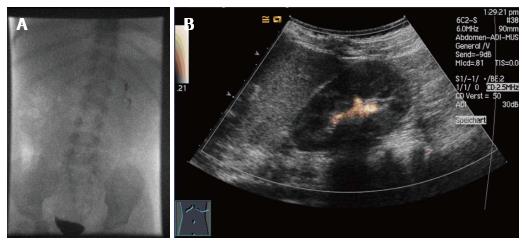
The role of vesicoureteral reflux (VUR) in the pathophysiology of renal damage is controversially discussed[1,2]. Voiding urosonography (VUS) is a radiation-free imaging modality and has become an established alternative to the most common radiological modality for detection of VUR, that is fluoroscopic voiding cystourethrography (VCUG)[3-7]. Recent studies have demonstrated its equal or higher sensitivity when compared to VCUG, especially in cases of high grade VUR[3,8,9]. Known negative effects of ionizing radiation underline the demand for VUS to replace VCUG for assessment of VUR in children (Figure 1).
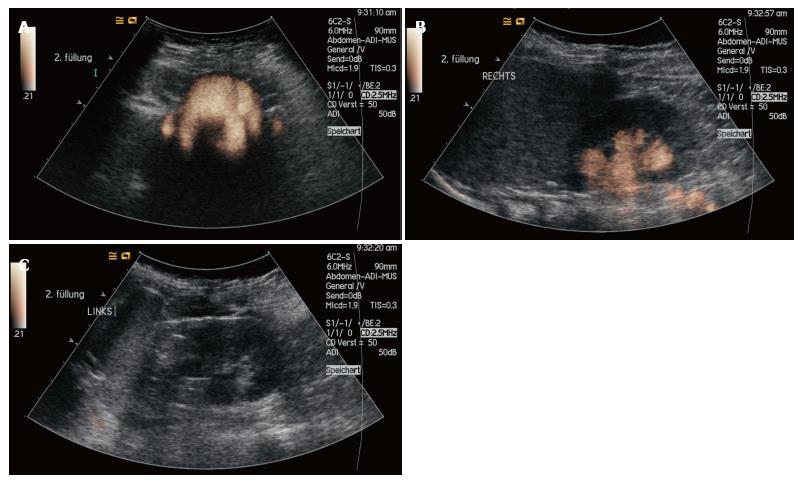
First generation urosonography contrast agents (US-CA) consisted of stabilized galactose-based air-filled microbubbles. Until its withdrawal in 2011, Levovist® (Bayer-Schering, Berlin, Germany) was approved for pediatric application and was commonly used for VUS, which is the most frequent pediatric application of US-CA[10]. Since that time, sulfur hexafluoride (SH, SonoVue® Bracco, Milan, Italy) remains as the only US-CA available in the European Union. To date, it has not been approved for the use in children. SH is a second-generation US-CA composed of a stabilized aqueous suspension of sulfur hexafluoride microbubbles with a phospholipid shell[11,12]. Due to the clinical demand it has gained widespread off-label acceptance in Europe. VUS implies the intravesical administration of SH and saline solution using a transurethral catheter. While scanning the bladder and kidneys during filling and voiding, the observer assesses whether microbubbles ascend to the ureters and/or the renal collecting system (Figure 2).
To date there are only few studies evaluating the safety profile of intravesical application of SH in children. These studies mainly address diagnostic efficacy of VUS using SH[13-15] and only additional limited data concerning adverse reactions were reported on the side. Severe side effects were not reported in any of these studies. Riccabona[16] underlined these results in a survey among European departments of radiology concerning adverse events associated with SH in intracavity use: No adverse reaction was recorded. Additionally, Papadopoulou et al[17] reported no adverse allergic event in VUS with SH application in a single Pediatric Medical Center.
To date, there is no prospective multicenter study focusing on SH related adverse events in VUS. The aim of this prospective two-center-study was to evaluate the risk profile of SH in VUS based on a large cohort of children with suspected or proven VUR.
Within a 4-year period, 531 children with suspected or proven VUR (f/m = 478/53; mean age 4.9 years; range 1 mo-25.2 years) underwent VUS in one of the two participating study hospitals (n = 487 in tertiary care hospital and n = 44 in secondary care hospital), which perform VUS in daily routine for several years. Indications for VUS were history of recurrent febrile urinary tract infections (UTI) or follow-up of previously detected VUR under conservative treatment or after surgical therapy, as recommended in the guidelines of the European Association of Urology/European Society for Pediatric Urology. In case of follow-up, a continuous oral antibiotic prophylaxis was performed in 370 children.
All legal guardians were informed about the off-label use of SH and their informed written consent was obtained prior to the examination. All study work was conducted in accordance with the Helsinki Declaration. Parents were asked to take part in a standardized telephone survey after VUS.
Ultrasound of the urinary tract was conducted in supine and prone position with a 5/6-MHz convex abdominal transducer (Acuson-Sequoia 512, Siemens Healthcare, Erlangen, Germany or Aplio 400, Toshiba Medical Systems, Neuss, Germany). Then the bladder was catheterized under aseptic conditions by using a 5/6 CH infant feeding tube (Unomedical, Birkerod, Denmark) coated with anaesthetic gel (Instillagel, Farco-Pharma, Cologne, Germany) and fixed with a hypoallergenic, adhesive strip (Leukostrip, Smith and Nephew, Hamburg, Germany). Thereafter a urine test was performed and only in the absence of signs of acute UTI the examination was continued. The catheter was connected with a three-way valve. Target bladder capacity was calculated according to the following formula: Bladder volume (mL) = (Age in years + 2) × 30[18].
First, the bladder was filled up to approximately one third of its capacity with 0.9% saline solution. Then, 0.5 mL-1 mL of SH was slowly administered and the bladder was subsequently filled with saline solution until maximum bladder capacity was reached or the child started voiding. SH was always prepared according to the manufacturer’s recommendation and was applied in a sterile manner. Meanwhile, the distribution of the UCA was observed and recorded in bladder, ureters and kidneys by ultrasound during filling and voiding. In order to increase the reflux detection rate this procedure was repeated at least twice. The average amount of administered SH was 2.6 ± 1.2 mL per patient and individually adapted to achieve the best diagnostic contrast. During the contrast-specific examination mode the mechanical index was turned to a low level (0.4) to minimize possible ultrasound-related disruptions of the microbubbles[19]. All examinations were digitally recorded and performed/supervised by one specialized pediatric radiologist (in the tertiary care university hospital) and one trained pediatrician (in the secondary care hospital).
During the examination and until 30 min thereafter, all children were observed for any perineal skin or mucosal tissue reactions, generalized hypersensitivity or anaphylactoid reactions (i.e., urticaria, pruritus, nausea, vomiting, abdominal pain, respiratory problems). Parents were instructed to monitor their child for three days and inform a doctor in case of any adverse event. After three days, parents were contacted for a standardized telephone survey comprising six specific items (skin rash, itching, wheals, fever, shortness of breath, consultation of a doctor) with dichotomic answers. Furthermore, they were asked for any onset of unspecific symptoms (i.e., discomfort due to catheterization). Finally, a question concerning the willingness to have VUS with SH repeated, if clinically necessary for possible future examinations completed the survey.
Type, duration and onset of adverse events were recorded and classified as minor, moderate or severe event based on the World Health Organization Draft Guidelines for Adverse Event Reporting and Learning Systems[20].
Mann-Whitney-U test was used for statistical analysis. A P-value < 0.05 was considered statistically significant.
No signs of acute hypersensitivity, generalized allergy or anaphylactoid reaction were observed during the examination and over the ensuing 30 min. The parents of all 531 children participated in the standardized telephone survey. Some adverse event was reported in 22 children (4.1% with 20 cases in the tertiary and two in the secondary care hospital; mean age 2.6 years; range 1 mo 6.2 years).
The majority of adverse events noted by the parents (17/22) did not reveal any association with potential allergic reactions: In three cases (0.6%), parents reported a new onset dysuria and mild fever, most likely due to an acute episode of chronic UTI. In a 6.2-year-old girl, a medical consultation was attended and successful antibiotic therapy was performed. In a 1-mo-old boy, hospital admittance was necessary for successful treatment of multiple antibiotic resistant bacterial UTI. In a 3-year-old girl, symptoms were self-limited. Duration of symptoms in all three cases was longer than 24 h. In 5 children (0.9%), a discomfort due to bladder catheterization (4 females and 1 male, 2 mo-5.8 years) and in four cases (0.7%) symptoms of a common cold (3 females and 1 male, 1.3 years-4.5 years) were reported. In 5 children (4 females, 1 male; 1 mo-2.4 years; 0.9%), a self-limited, perineal erythema was described. In one case (male, 21 mo), a spell of pruritus was reported and self-limitation was observed within 48 h.
Only a minority of the observed adverse events (5/22) were suggestive of potential allergic reaction. All of them (0.9%, 4 females and 1 male, 5 mo-6.2 years) were minor adverse events with a subacute onset and self-limitation within 24 h. Perineal urticaria was reported in approximately 0.5% (n = 3, 2 females and 1 male, 6 mo-3.8 years) and an isolated, mild fever was seen in 0.4% (n = 2, 2 females, 2-6.2 years). In one case with isolated fever (female, 2 years) a medical consultation took place. No hospital admittance was necessary due to any reported potential allergic adverse event (Table 1).
| Adverse event | Severity | Total (n, %) | Age (range) | Gender (f/m, n) | Duration | Onset |
| Urinary tract infection | Minor/moderate | 3 (0.6%) | 1 mo-6.2 yr | f (2), m (1) | 24-72 h | Subacute |
| Perineal erythema | Minor | 5 (0.9%) | 1 mo-2.4 yr | f (4), m (1) | < 24 h | Subacute |
| 21 mo | 24-48 h | Subacute | ||||
| Discomfort due to catheterization | Minor | 5 (0.9%) | 2 mo-5.8 yr | f (4), m (1) | < 24 h | Subacute |
| Symptoms of a common cold | Minor | 4 (0.7%) | 1.3-4.5 yr | f (3), m (1) | < 24 h | Subacute |
| Hints for hypersensitivity | ||||||
| Perineal urticaria | Minor | 3 (0.5%) | 6 mo-3.8 yr | f (2), m (1) | < 24 h | Subacute |
| Isolated, mild fever | Minor | 2 (0.4%) | 2-6.2 yr | f (2) | < 24 h | Subacute |
In the present study cohort in 224/531 (42.2%) children a VUR was detected. In those cases with described adverse events in 8/22 (36.4%) children a VUR was observed. In those children with potential allergic reaction a VUR was detected in 2/5 (40%).
We compared the mean amount of administered SH between those children showing adverse events and those without by using a Mann Whitney-U test (group 1: Patients with adverse events, n = 22; group 2: Patients without adverse events, n = 509). There was no significant difference (P > 0.05) between the two groups.
Ninety-six point two percent (n = 511) of the parents stated that, if clinically necessary, they would prefer further VUS examinations with the use of SH for their child over an X-ray voiding study.
No severe allergic adverse events were noted in this prospective, two-center survey on adverse events after intravesical administration of SH. In 0.9% of the study population, subacute minor adverse events were reported, which may have been caused by self-limited minor allergic reactions.
Recent studies have demonstrated an equal or higher sensitivity of VUS when compared to VCUG, especially in cases of high grade VUR[3,8,9]. In a metaanalysis Darge et al[6] described a sensitivity of 57%-100%, a specificity of 85%-100%, positive/negative predictive values of 58%-100%/87%-100%, respectively, and a diagnostic accuracy of 78%-96%. Moreover Kis et al[13] examined a total of 183 children using VCUG and VUS in parallel. They detected VUR with VUS in 34.4% and with VCUG in 28.1%. Reflux was detected by both methods in 24.3%. VCUG missed cases of high-grade reflux whereas VUS missed only low-grade reflux. They suggest - based on their study results - that contrast-enhanced harmonic VUS using SH is superior to VCUG in the detection and grading of VUR.
To date, there are only few studies concerning the intravesical use of SH in children. Most of them focus on the diagnostic efficacy in VUS and experiences with adverse events are only additionally reported[9,13-15,20]. In case of additional VCUG with intravesical application of an iodinated contrast agent during the same session, sufficient correlation of adverse events to one of the administered contrast agents was not possible[9,13,15,20]. Duran et al[14] conducted a diagnostic study focusing on VUS with exclusive administration of SH. Thus, potential adverse events could be related to SH. None of the above noted studies (Table 2) reported any adverse event. In a single Pediatric Medical Center, Papadopoulou et al[17] focused on the safety profile of intracavity use of SH. They reported 37 cases (f/m = 18/19; 1 mo-8.9 years; 3.7%) of adverse events, which were mainly attributed to the bladder catheterization rather than to the administration of SH in a total of 1010 (f/m = 5/4; mean age: 2.9 years; range 15 d-17.6 years) children. Papadopoulou et al[17] described no signs of acute hypersensitivity or generalized allergic reaction, which is in line with the findings from our own study. The present study described a comparable frequency of adverse events, as noted in 4.1% of our patients.
| Ref. | Total | gender (f/m) | Age (range) | + VCUG | Adverse events | Severity |
| Ascenti et al[15] | 80 | 44/36 | 3 mo-5 yr | Yes | None | None |
| Papadopoulou et al[9] | 228 | 105/123 | 6 d-13 yr | Yes | None | None |
| Kis et al[13] | 183 | 89/94 | 2 d-44 mo | Yes | None | None |
| Ključevšek et al[20] | 66 | 31/35 | 5 d-1 yr | Yes | None | None |
| Duran et al[14] | 295 | 153/154 | 13 d-18 yr | No | None | None |
| Papadopoulou et al[17] | 1010 | 563/447 | 15 d-17.6 yr | No | 37 | Minor |
| Present study | 531 | 478/53 | 1 mo-25.2 yr | No | 20 | Minor |
| 2 | Moderate |
Self-limited or successfully treated UTIs were observed in 0.6% that is in two children who had already been on prophylactic antibiotic therapy. Papadopoulou et al[17] reported UTIs with a lower frequency of only 0.1%, a discrepancy which may be explained by the standardized oral 3-d prophylactic antibiotic therapy prior to VUS administered to all their study participants. In the present study, and as recommended in the guidelines of the European Association of Urology/European Society for Pediatric Urology, children received a continuous oral antibiotic prophylaxis only in case of previously detected VUR (69% of all patients). In contrast to Papadopoulou et al[17], an additional short-time, prophylactic antibiotic therapy was not performed, which enables the detection of adverse events solely related to SH without being compounded by additional antibiotic therapy. The comparably small incidence of subsequent UTIs in both studies raises the question whether a prophylactic antibiotic therapy to prepare patients for the VUS examination is indeed beneficial, also keeping in mind the concerns about the spread of antimicrobial resistances across bacterial strains[21]. The results reported by Papadopoulou et al[17] in comparison to our own study suggest a VUS-related increase of 0.5% in mild UTI when no antibiotics are used as preparation.
The present study showed self-limited, subacute discomfort due to catheterization in five patients (0.9%). In contrast, Papadopoulou et al[17] described this in 2.6%. The difference could be related to the smaller number of male patients undergoing catheterization (f/m = 478/53) in the present study, boys being more susceptible for discomfort after catheterization due to anatomical conditions of the urethra and a possibly more difficult catheterization. This unequal sex ratio is related to the established diagnostic algorithm for the assessment of VUR in the participating study hospitals: The primary imaging modality in girls is VUS, whereas in boys, VCUG is routinely used on first referral due to a better delineation of the urethra in order to exclude urethral valves. For follow-up, VUS is the first choice in boys, as it is in girls.
A self-limited, perineal erythema was reported in five children, which was most likely related to a new onset of dermatitis (all children were diapered) or to disinfection in VUS procedure.
Unlike previous studies which reported no cases of allergic reactions, five unclear cases (three cases of urticaria and two cases of mild isolated fever) with hints for a potential allergic cause were observed: All of them were minor, subacute adverse events and self-limited. In four of five patients, the potential allergic reaction was reported by the parents during the telephone interview and suspected allergic aetiology was based solely on subjective parental assessment. In a 2-year-old girl with isolated fever, the parents consulted the family doctor who could not identify a clear reason for the fever episode. Therefore, a more detailed conclusion is not possible and the described symptoms have to be rated as possible hints for minor allergic reaction. Moreover, none of the parents contacted the study doctors at the onset of the described events. Maybe additional laboratory data and a physical examination after 24 h could be helpful to rule out these potential hints for an allergic reaction in future studies.
Contrary to recent studies[9,13-15,20] the average amount of used SH was higher (2.6 ± 1.2 mL vs 0.5-1.0 mL) which was most likely due to the higher mean age (4.9 years vs 5.1 mo-2.9 years). Therefore, a higher dose of SH was necessary to gain a sufficient bladder contrast. Moreover, at least two voiding cycles were performed in each patient and additional scans were done in unclear cases. In accordance to Papadopoulou et al[17] no dependency of adverse events from the amount of administered SH and children’s age were described.
Although we report on a two-center study, the evaluation of a large heterogeneous cohort of children is limited by an unequal distribution between the participating hospitals with the tertiary care hospital contributing far more patients. Nevertheless, there was a comparable amount of observed adverse events in both institutions (4.1% in the tertiary and 4.5% in the secondary care hospital) with potential hints for an allergic reaction only reported in the tertiary care hospital.
In contrast to SH, Levovist® was approved for the intravesical use in children in Europe until its withdrawal. Therefore the evaluation of its safety-profile in clinical practice within studies focusing on Levovist® was only secondary. In 2013 Darge et al[12] published a review on safety of CE-US in children for non-cardiac applications. In this meta-analysis of eight studies[19,22-28], 17 of 1062 children presented with minor adverse events after intravesical administration of Levovist®[19,22], most likely related to the placement of the catheter and with a comparable frequency, as reported from VUS using SH.
Zerin et al[29] described postprocedural symptoms in 35.1% of the children (n = 228) undergoing VCUG, radionuclide cystography or diuretic renal scintigraphy. The frequency of postprocedural symptoms was nearly identical in the VCUG group and the two other groups. Dysuria was the most common symptom (32.9%). Symptoms disappeared within 24 h in 40%. They concluded that most postprocedural symptoms could be attributed to the discomforting, minimally invasive procedure of bladder catheterization itself as well as its psychological impact on the children, rather than the contrast agent. Weese et al[30] report 2 cases (0.3%) of anaphylactoid reactions during VCUG or retrograde pyelography in a retrospective review of 783 patients.
This survey revealed that 96.2% of parents would favor further radiation-free VUS examinations utilizing SH for detection of potential vesicoureteral reflux. The remaining 3.8% disagreeing to further VUS examinations felt uncomfortable due to its off-label-use.
In summary, the off-label-use of SH for radiation-free assessment of VUR provides a good safety profile. The present study did not reveal any acute severe allergic adverse event. Minor-to-moderate adverse events were observed in 4.1% and had hints for an allergic cause in less than 1% of the study population. Accordingly, the off-label-use had a high level of acceptance among the interviewed parents. The study results underline the demand for an approval of SH in pediatric applications.
The role of vesicoureteral reflux (VUR) in the pathophysiology of renal damage is controversially discussed. Voiding urosonography (VUS) entails the intravesical administration of an urosonography contrast agent (US-CA) for the diagnosis of VUR. VUS is now recognized as a practical, radiation-free modality with comparable or higher sensitivity than voiding cystourethrography (VCUG). First generation US-CA consisted of stabilized galactose-based air-filled microbubbles. Until its withdrawal in 2011, Levovist® (Bayer-Schering, Berlin, Germany) was approved for pediatric application and was commonly used for VUS. Since that time, sulfur hexafluoride (SH, SonoVue®, Bracco, Milan, Italy) remains as the only US-CA available in the European Union. To date, it has not been approved for the use in children. SH is a second-generation US-CA composed of a stabilized aqueous suspension of sulfur hexafluoride microbubbles with a phospholipid shell. Due to the clinical demand it has gained widespread off-label acceptance in Europe.
To date there are only few studies evaluating the safety profile of intravesical application of SH in children. These studies mainly address diagnostic efficacy of VUS using SH and only additional limited data concerning adverse reactions were reported on the side. The aim of this prospective two-center-study was to evaluate the risk profile of SH in VUS based on a large cohort of children with suspected or proven VUR.
To date there is no prospective multicenter study focusing on SH related adverse events in VUS. This is the first two-center-study which evaluates the risk profile of SH in VUS based on a large cohort of children with suspected or proven VUR. The authors did not address diagnostic efficacy of VUS using SH, also they did not focus on a comparison to VCUG. Therefore the authors performed no additional VCUG with intravesical application of an iodinated contrast agent during the same session to gain sufficient correlation of adverse events to the administered SH. In a single Pediatric Medical Center, Papadopoulou et al focused on the safety profile of intracavity use of SH. They reported 37 cases of adverse events, which were mainly attributed to the bladder catheterization rather than to the administration of SH in a total of 1010 children. The present two-center-study described a comparable frequency of adverse events. But in contrast to Papadopoulou et al, in this study a minority of the observed adverse events were suggestive of potential allergic reaction. Tough none of the observed events were acute or severe and no hospital admittance was necessary.
In summary, the off-label-use of SH for radiation-free assessment of VUR provides a good safety profile. The study results underline the demand for an approval of SH in pediatric applications.
VUR is a condition in which urine flows retrograde from the bladder into the ureters/kidneys. VUS is a radiation-free imaging modality and has become an established alternative to the most common radiological modality for detection of VUR that is fluoroscopic VCUG. SH (SonoVue®) is a second-generation US-CA composed of a stabilized aqueous suspension of sulfur hexafluoride microbubbles with a phospholipid shell.
The authors conducted a survey on adverse events of SH in VUS in children. This paper is well-written and has valuable information.
| 1. | Moorthy I, Easty M, McHugh K, Ridout D, Biassoni L, Gordon I. The presence of vesicoureteric reflux does not identify a population at risk for renal scarring following a first urinary tract infection. Arch Dis Child. 2005;90:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Zaffanello M, Franchini M, Brugnara M, Fanos V. Evaluating kidney damage from vesico-ureteral reflux in children. Saudi J Kidney Dis Transpl. 2009;20:57-68. [PubMed] |
| 3. | Darge K. Voiding urosonography with US contrast agents for the diagnosis of vesicoureteric reflux in children. II. Comparison with radiological examinations. Pediatr Radiol. 2008;38:54-63; quiz 126-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Darge K. Voiding urosonography with ultrasound contrast agents for the diagnosis of vesicoureteric reflux in children. I. Procedure. Pediatr Radiol. 2008;38:40-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Darge K. Voiding urosonography with US contrast agent for the diagnosis of vesicoureteric reflux in children: an update. Pediatr Radiol. 2010;40:956-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Darge K, Grattan-Smith JD, Riccabona M. Pediatric uroradiology: state of the art. Pediatr Radiol. 2011;41:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Veldhoen S, Sauer A, Gassenmaier T, Petritsch B, Herz S, Blanke P, Derlin T, Bley TA, Wirth C. Contrast-enhanced voiding urosonography phantom study: intravenous iodinated and gadolinium-based contrast agents may cause false-negative results in assessment of vesicoureteral reflux in children. Pediatr Radiol. 2015;45:862-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Nakamura M, Wang Y, Shigeta K, Shinozaki T, Taniguchi N, Itoh K. Simultaneous voiding cystourethrography and voiding urosonography: an in vitro and in vivo study. Clin Radiol. 2002;57:846-849. [PubMed] |
| 9. | Papadopoulou F, Anthopoulou A, Siomou E, Efremidis S, Tsamboulas C, Darge K. Harmonic voiding urosonography with a second-generation contrast agent for the diagnosis of vesicoureteral reflux. Pediatr Radiol. 2009;39:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Schlief R. Developments in echo-enhancing agents. Clin Radiol. 1996;51 Suppl 1:5-7. [PubMed] |
| 11. | Schneider M, Arditi M, Barrau MB, Brochot J, Broillet A, Ventrone R, Yan F. BR1: a new ultrasonographic contrast agent based on sulfur hexafluoride-filled microbubbles. Invest Radiol. 1995;30:451-457. [PubMed] |
| 12. | Darge K, Papadopoulou F, Ntoulia A, Bulas DI, Coley BD, Fordham LA, Paltiel HJ, McCarville B, Volberg FM, Cosgrove DO. Safety of contrast-enhanced ultrasound in children for non-cardiac applications: a review by the Society for Pediatric Radiology (SPR) and the International Contrast Ultrasound Society (ICUS). Pediatr Radiol. 2013;43:1063-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Kis E, Nyitrai A, Várkonyi I, Máttyus I, Cseprekál O, Reusz G, Szabó A. Voiding urosonography with second-generation contrast agent versus voiding cystourethrography. Pediatr Nephrol. 2010;25:2289-2293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Duran C, del Riego J, Riera L, Martin C, Serrano C, Palaña P. Voiding urosonography including urethrosonography: high-quality examinations with an optimised procedure using a second-generation US contrast agent. Pediatr Radiol. 2012;42:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Ascenti G, Zimbaro G, Mazziotti S, Chimenz R, Fede C, Visalli C, Scribano E. Harmonic US imaging of vesicoureteric reflux in children: usefulness of a second generation US contrast agent. Pediatr Radiol. 2004;34:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Riccabona M. Application of a second-generation US contrast agent in infants and children--a European questionnaire-based survey. Pediatr Radiol. 2012;42:1471-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Papadopoulou F, Ntoulia A, Siomou E, Darge K. Contrast-enhanced voiding urosonography with intravesical administration of a second-generation ultrasound contrast agent for diagnosis of vesicoureteral reflux: prospective evaluation of contrast safety in 1,010 children. Pediatr Radiol. 2014;44:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Darge K, Troeger J, Duetting T, Zieger B, Rohrschneider W, Moehring K, Weber C, Toenshoff B. Reflux in young patients: comparison of voiding US of the bladder and retrovesical space with echo enhancement versus voiding cystourethrography for diagnosis. Radiology. 1999;210:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Ključevšek D, Battelino N, Tomažič M, Kersnik Levart T. A comparison of echo-enhanced voiding urosonography with X-ray voiding cystourethrography in the first year of life. Acta Paediatr. 2012;101:e235-e239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Wang HH, Gbadegesin RA, Foreman JW, Nagaraj SK, Wigfall DR, Wiener JS, Routh JC. Efficacy of antibiotic prophylaxis in children with vesicoureteral reflux: systematic review and meta-analysis. J Urol. 2015;193:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Berrocal T, Gayá F, Arjonilla A, Lonergan GJ. Vesicoureteral reflux: diagnosis and grading with echo-enhanced cystosonography versus voiding cystourethrography. Radiology. 2001;221:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Valentini AL, Salvaggio E, Manzoni C, Rendeli C, Destito C, Summaria V, Campioni P, Marano P. Contrast-enhanced gray-scale and color Doppler voiding urosonography versus voiding cystourethrography in the diagnosis and grading of vesicoureteral reflux. J Clin Ultrasound. 2001;29:65-71. [PubMed] [DOI] [Full Text] |
| 24. | Mentzel HJ, Vogt S, John U, Kaiser WA. Voiding urosonography with ultrasonography contrast medium in children. Pediatr Nephrol. 2002;17:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Galia M, Midiri M, Pennisi F, Farina R, Bartolotta TV, De Maria M, Lagalla R. Vesicoureteral reflux in young patients: comparison of voiding color Doppler US with echo enhancement versus voiding cystourethrography for diagnosis or exclusion. Abdom Imaging. 2004;29:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Papadopoulou F, Tsampoulas C, Siomou E, Tzovara J, Siamopoulou A, Efremidis SC. Cyclic contrast-enhanced harmonic voiding urosonography for the evaluation of reflux. Can we keep the cost of the examination low? Eur Radiol. 2006;16:2521-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Piscitelli A, Galiano R, Serrao F, Concolino D, Vitale R, D’Ambrosio G, Pascale V, Strisciuglio P. Which cystography in the diagnosis and grading of vesicoureteral reflux? Pediatr Nephrol. 2008;23:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Kljucevsek D, Kljucevsek T, Kersnik Levart T, Kenda RB. Ureteric jet Doppler waveform: is it a reliable predictor of vesicoureteric reflux in children? Pediatr Nephrol. 2009;24:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Zerin JM, Shulkin BL. Postprocedural symptoms in children who undergo imaging studies of the urinary tract: is it the contrast material or the catheter? Radiology. 1992;182:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Weese DL, Greenberg HM, Zimmern PE. Contrast media reactions during voiding cystourethrography or retrograde pyelography. Urology. 1993;41:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Classen CF, Sangkhathat S, Watanabe T S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ