Published online Feb 8, 2017. doi: 10.5409/wjcp.v6.i1.103
Peer-review started: May 3, 2016
First decision: July 25, 2016
Revised: October 12, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: February 8, 2017
Processing time: 277 Days and 7.4 Hours
Vein of Galen malformation (VOGM) is a rare congenital vascular malformation caused by the maldevelopment of its embryonic precursor, the median prosencephalic vein of Markowski. VOGM results in neonatal morbidity and mortality, and premature delivery does not improve the outcome. We report a term female neonate in whom a vein of Galen malformation was diagnosed prenatally at 37 wk of gestation during a growth ultrasound and confirmed by fetal magnetic resonance imaging. Signs of cardiac decompensation were evident in the fetus. Multiple interventional radiology embolizations of the feeding vessels were performed successfully on days 7, 10, 12, 14 and 19. A review of the literature on the endovascular management of neonates with these malformations is presented herein.
Core tip: Vein of Galen malformation (VOGM) is a rare vascular anomaly that may present in the fetus or newborn, and may cause congestive heart failure. Historically, the management of VOGMs was neurosurgical, but outcomes were uniformly poor. Since the introduction of endovascular interventional techniques, the likelihood of a successful treatment is much greater and a cure is potentially achievable. We report herein a term female neonate with a VOGM that was sucessfully treated with multiple endovascular embolizations, and also present a review of the literature on the endovascular management of neonates.
- Citation: Puvabanditsin S, Mehta R, Palomares K, Gengel N, Silva CFD, Roychowdhury S, Gupta G, Kashyap A, Sorrentino D. Vein of Galen malformation in a neonate: A case report and review of endovascular management. World J Clin Pediatr 2017; 6(1): 103-109
- URL: https://www.wjgnet.com/2219-2808/full/v6/i1/103.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v6.i1.103
Vein of Galen malformations (VOGMs) are complex arteriovenous (AV) fistulas that occur infrequently. Their incidence is about one in 3 million population[1-3], and they represent less than 1% of the cerebral AV malformation. The majority of VOGMs are diagnosed in the neonatal period, and the remainder during early childhood. VGOMs was first reported in 1895 by Steinheil (cited by Dandy[4] in 1928). The reported lesion was, in fact, an arteriovenous malformation (AVM) of the diencephalon connected to a dilated vein of Galen. The majority of Vein of Galen Aneurysmal malformations (VGAMs) become symptomatic in the neonatal period and if left untreated have an almost 100% morbidity and mortality[5]. The first attempt to treat a VOGM was at the beginning of the last century, wherein bilateral internal carotid artery ligations were performed on an infant who had presented with intracranial hypertension[6]. At present, endovascular embolization is preferred for treating VOGMs[7,8]. The endovascular technique was developed in the early 1980s. Since then, more than 262 neonates with VOGMs have been treated using this method[7,8]. Because of the rarity of VOGMs, most publications are in the form of case reports. Our experience with a VOGM in a term infant with congestive heart failure is reported herein with a review the literature on endovascular embolization treatment in neonates.
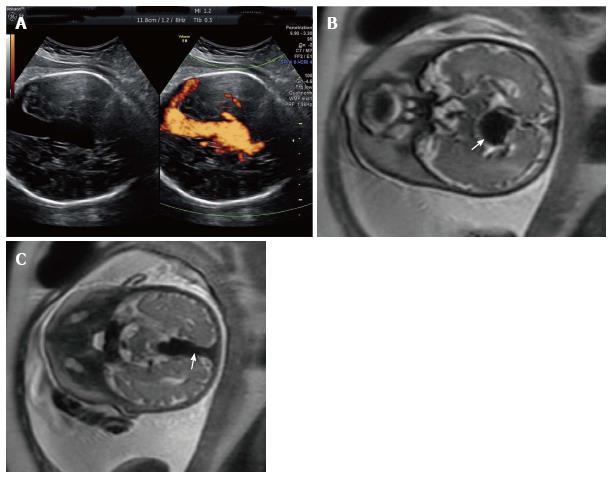
A 42-year-old G2P1001 presented for a routine growth ultrasound at 36 wk 5 d. The fetus was found to have a vein of Galen malformation, which had not been identified during the 20-wk anatomy scan (Figure 1A). A review of her medical history was significant for a prior uncomplicated full-term vaginal delivery. Her living child is alive and well, meeting all appropriate milestones. The patient denied any significant family history for congenital anomalies or social history for toxic environmental or occupational exposures. Prenatal care was otherwise uneventful. Fetal MRI confirmed the diagnosis of VOGM, demonstrating a persistent median prosencephalic vein, which measured up to 22 mm in the transverse dimension and 74 mm in length (Figure 1B and C). There was a network of feeding vessels (greater on the right as compared to the left) in the region of the medial temporal lobe, midbrain, and thalami, which likely represented feeder vessels emanating from the posterior cerebral arteries. Torcula and bilateral transverse sinuses were also enlarged. The lateral ventricles and cortical sulci were appropriate for the patient's gestational age. The infratentorial brain appeared normal, with no mass effect or midline shift.
A fetal echocardiogram showed cardiomegaly with preserved biventricular systolic function. The superior vena cava (SVC) was moderately dilated. The right atrium was severely dilated whereas the left atrium was only mildly dilated and there was an aneurysmal patent foramen ovale (PFO) with right to left shunting. The right ventricle (RV) was moderately dilated with qualitatively good RV systolic function. There was marked reversal of flow in the distal aortic arch, apparently draining predominantly to the brachiocephalic artery.
Over the next 2 d, repeat sonographic evaluation demonstrated new polyhydramnios and abnormal Doppler studies. The fetus developed an abnormal fetal heart rate tracing with areas of minimal variability and non-reactivity. At this point, because of the massive VOGM causing a steal phenomenon, evidence of heart failure, worsening doppler studies, and fetal monitoring showing fetal compromise, the decision was made to proceed with delivery and to achieve optimization of management in the neonate. An elective uncomplicated primary cesarean section was performed because of fetal cardiac failure and breech presentation. The neonate had Apgar scores of 4 at 1 min and 8 at 5 min. Cord blood gas studies showed a pH of 7.30 and base deficit of -2.2.
Physical examination revealed a weight of 2675 g (25th centile), length 47 cm (30th centile), head circumference 33 cm (40th centile). No visible anomalies were noted at birth. The pertinent physical findings were cranial bruit and a grade 2/6 soft systolic heart murmur at the left sternal border.
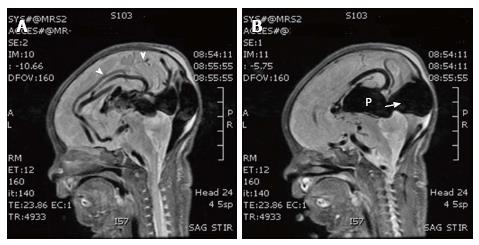
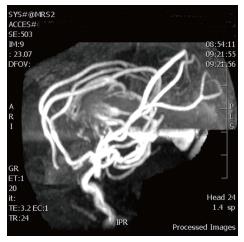
Chest radiography immediately after birth showed cardiomegaly. An echocardiogram performed at 1 h of life showed pulmonary hypertension, patent foramen ovale, dilated superior vena cava and reversal of flow in the descending aorta. Neurosonogram showed a large midline venous structure. MRI and magnetic resonance (MR) angiography showed VGOM and the vessels feeding the aneurysm (Figures 2 and 3). The feeding arteries are from bilateral middle cerebral arteries, bilateral anterior cerebral arteries and posterior cerebral arteries.
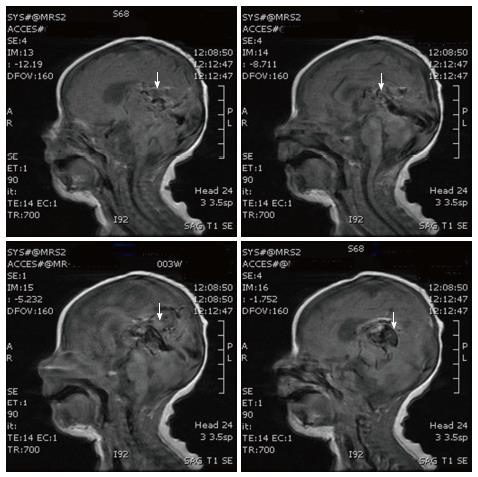
Over the first few days, the infant gradually became tachypneic and had a hyperdynamic precordium. Medical treatment of cardiac failure (furosemide, digoxin, and milrinone) was begun on the second day of life. Since the patient showed signs of cardiac decompensation despite the cardiac failure therapy, embolization with N-butyl cyanoacrylate (NBCA) was performed via the umbilical artery catheter at 7 d of age. The patient was intubated and placed on a conventional ventilator at 10 d of age. Trans-femoral embolizations were performed on the 10th, 12th and 14th day of life, and finally trans-axillary artery embolization was performed at 19 d of age. A repeat neurosonogram done on the 19th day of life showed intraventricular hemorrhage and mild post hemorrhagic hydrocephalus, which however, did not require any intervention. The patient was extubated on the 20th day of life, MRI and MR angiography performed on the following day showed that the dilatation of the Vein of Galen had decreased markedly (Figure 4). The infant was discharged at 53 d of age. When seen at 6 mo of age in the follow-up clinic, the patient was developing normally.
The vein of Galen is located under the cerebral hemispheres and drains the anterior and central regions of the brain into the sinuses of the posterior cerebral fossa. The median prosencephalic vein of Markowski usually regresses during the 11th week of gestation, and by 3 mo of gestation, its posterior part joins the internal cerebral veins and basal veins to form the vein of Galen. The term “vein of Galen malformation” is a misnomer because the dilated vein seen in the location of the vein of Galen is the prosencephalic vein. An arteriovenous (AV) shunt between this vein and the arteries supplying the brain induces hemodynamic changes because of excess blood flow from these arteries into the VOGM, and the heart having to work harder[9]. Hydrocephalus is the most common finding associated with a diagnosis of VOGM[7,10,11].
VGAMs are AVFs supplied by a variety of feeder vessels that drain into the aberrantly persistent fetal median prosencephalic vein of Markowsky[10], an embryonic precursor of the vein of Galen. The development of a VGAM may be an acquired event between the 6th and 11th week of gestation[10]. VGAMs have been classified in various ways, based on their complexity, type of supplying arteries, location of fistula, or degree of venous ectasia (Table 1)[1,6,10]. Lasjaunias classified VGAMs into two types, namely the choroidal and mural type[10,12]. In the choroidal type, multiple fistulas communicate with the median vein of the prosencephalon via an arterial network in the choroid fissure. The feeder vessels generally come from the anterior and posterior choroidal, and anterior cerebral, and at times, also the quadrigeminal and thalamoperforating arteries. The choroidal type is the most severe form of VGAM, which frequently presents with high-output cardiac failure, macrocephaly with loud bruits, and dilated orbital veins due to multiple high-flow fistulas with less restriction of outflow[10,13]. In the mural type of VGAM, single or multiple fistulas are present in the wall of the dilated median vein of the prosencephalon, generally at its inferolateral margin. The feeder vessels arise from the quadrigeminal or posterior choroidal arteries or both, and could be either unilateral or bilateral. The mural type has less fistulas and more restriction of outflow, which leads to greater dilation of the median vein of the prosencephalon but protects the heart from high-output failure. It manifests in late infancy as macrocephaly, hydrocephalus, seizures, subarachnoid hemorrhage, developmental delay, and failure to thrive[10,11]. Vein of Galen aneurysmal malformations (VGAMs) and vein of Galen aneurysmal dilations (VGADs) are the most frequently seen arteriovenous malformations in infants and fetuses. VGAD is an enlargement of the true vein of Galen and not its embryonic precursor.
| Classification system | |
| Litvak | |
| Category A | Aneurysms of the great vein of Galen |
| Category B | Racemorse conglomeration of blood vessels in the cerebral structures |
| Category C | Transitional types of midline AV shunts |
| Lasjaunias | |
| Type I | Choroidal type |
| Type II | Mural type |
| Yasargil | |
| Type I | Pure AVF between leptomeningeal arteries and feeders from P3, segments of posterior cerebral arteries and vein of Galen |
| Type II | Feeders from the thalamo-perforating vessels and from P1 and P2 segments of the posterior cerebral arteries |
| Type III | Mixture of type I and II |
| Type IV | |
| IV A | Aneurysmal dilation of the vein of Galen resulting from shunting from an adjacent thalamic AVM |
| IV B | Similar to type IV A with the AVM being mesencephalic instead of thalamic |
| IV C | Thalamomesencephalic or mesodiencephalic plexiform malformation along with an adjacent and separate cisternal AVF to the vein of Galen |
| Secondary enlargement of vein of Galen | |
| Vein of Galen dilation | Malformations that drain pial or dural shunts into the true vein of Galen or its tributary associated with the dilation of the vein of Galen |
| Vein of Galen varix | Dilation of the vein of Galen in the absence of AV shunt |
The mortality rates of the patients (all ages) who were embolized during the 1980s were 17%, and in the 1990s and 2000s they were 12%. The complication rates during the 1980s were 45%, and in the 1990s and 2000s they were approximately 35%[7]. Post-embolization complications included cerebral hemorrhage/hematoma (37%), cerebral ischemia (6%), macrocephaly or hydrocephalus, leg ischemia (3%), vessel perforation (3%), pulmonary embolism, and non-target embolization. Over the 1980s, 1990s, and 2000s, good clinical outcome rates of the patients that were embolized were 49%, 70% and 70%, respectively[7].
A systemic review (1987-2014) of endovascular embolization was performed for VOGMs that included 667 subjects[7], of which 44% were neonates at the time of treatment. In the cases with a neonatal diagnosis, the most common presenting symptoms (94%) were cardiovascular and respiratory distress due to the high-flow AV shunts of VOGMs[7,14]. Our case supports the findings. A study showed that 23%-70% of the children with VOGMs who received endovascular embolizations were neurologically normal at short-term follow-up[15,16]. A recent review of outcomes of neonatal cases published over the past 15 years revealed poor prognosis for neonates who did not receive interventional embolization for severe cardiac failure secondary to VGAM[16,17].
Transarterial embolization is the preferred route in most specialized centers. However, it best controls heart failure when there are only a small number of arterial feeders. When there are many small arterial pedicles, it often is impossible to occlude more than a few at a time in the unstable neonate. In such cases, transvenous embolization may help achieve some control of the heart failure. A combination of the transvenous and transarterial embolization (kissing microcatheter technique) is another feasible endovascular option with a differing success rate[18]. The outcome of neonates with VGAM has improved tremendously due to advances in endovascular treatment. Table 2 summarizes the clinical details and outcomes of 47 neonates who were treated with endovascular embolization since the beginning of the 21st century[5,16-23].
| Diagnosed | Gestational age (wk) | Birth weight (g) | Symptoms | Total sessions | Outcome | Ref. |
| Antenatal (31 wk) | N/A | N/A | CCF | Multiple | Normal | Mitchell et al[19] 2001 |
| Postnatal | N/A | N/A | CCF | Multiple | Normal | |
| Postnatal | N/A | N/A | CCF | 1 | Death (2 d) | |
| Postnatal | N/A | N/A | CCF | Multiple | Normal | |
| Antenatal (36 wk) | Full term | N/A | CCF | 3 | Death (24 d) | Frawley et al[17] 2002 |
| Postnatal | Full term | N/A | CCF | 3 | Normal | |
| Postnatal | Full term | N/A | CCF | 1 | Death (2 d) | |
| Postnatal | Full term | N/A | CCF | 4 | Psychomotor delay | |
| Postnatal | Full term | N/A | CCF | 4 | Normal | |
| Postnatal | Full term | N/A | CCF | 1 | Normal | |
| Antenatal (38 wk) | Full term | N/A | CCF | 3 | Normal | |
| Antenatal (32 wk) | Full term | N/A | CCF | 4 | Death (39 d) | |
| Postnatal | 36 | N/A | CCF | 1 | Death (24 h) | Jones et al[20] 2002 |
| Postnatal | Full term | N/A | CCF | Multiple | Normal | |
| Antenatal (28 wk) | N/A | N/A | CCF | 1 | Death (29 h) | Maheshwari et al[23] 2003 |
| Postnatal | Full term | 3400 | CCF | 1 | Death (7d) | Mathew et al[21] 2013 |
| N/A | Full term | N/A | CCF | 2 | Normal | McSweeney et al[22] 2010 |
| N/A | Full term | N/A | CCF | 1 | Normal | |
| N/A | Full term | N/A | CCF | 1 | Normal | |
| N/A | Full term | N/A | CCF | 4 | Psychomotor delay | |
| N/A | Full term | N/A | CCF | 3 | Psychomotor delay | |
| N/A | Full term | N/A | CCF | 5 | Psychomotor delay | |
| N/A | Full term | N/A | CCF | 1 | Death | |
| N/A | Full term | N/A | CCF | 1 | Death | |
| Antenatal (35 wk) | Full term | 3290 | CCF | 1 | Normal | Karadeniz et al[16] 2011 |
| Postnatal | N/A | N/A | CCF | 2 | Normal | Meila et al[18] 2012 |
| Antenatal | N/A | N/A | CCF | 4 | Normal | |
| Postnatal | N/A | N/A | CCF | 6 | Death (2 yr) | |
| Postnatal | N/A | N/A | CCF | 2 | Psychomotor delay | |
| Postnatal | N/A | N/A | CCF | 6 | Psychomotor delay | |
| Antenatal | N/A | N/A | CCF | 3 | Normal | |
| Postnatal | N/A | N/A | CCF | 4 | Normal | |
| Antenatal | N/A | N/A | CCF | 3 | Psychomotor delay | |
| Postnatal | 36 | 2897 | CCF | 5 | Normal | Berenstein et al[5] 2012 |
| Postnatal | 34 | 1810 | CCF | 1 | Death | |
| Antenatal | Full term | 4165 | CCF | 2 | Normal | |
| Antenatal | 36 | 3409 | CCF | 4 | Normal | |
| Postnatal | Full term | 3400 | CCF | 6 | Normal | |
| Postnatal | Full term | 2930 | CCF | 6 | Hemiparesis | |
| Antenatal | Full term | 2386 | CCF | 2 | Normal | |
| Antenatal | Full term | 4204 | CCF | 6 | Psychomotor delay | |
| Antenatal | 36 | 2825 | CCF | 3 | Psychomotor delay | |
| Antenatal (36 wk) | Full term | 2675 | CCF | 5 | Normal | The present report |
Due to the natural history of disease progression, prenatal diagnosis of VGAM is often not made until the third trimester when imaging studies (including a fetal MRI) are done. Progressive fetal cardiac dysfunction implies that the high flow lesion may not respond to treatment[24]. Melting brain syndrome is the term used to describe an advanced stage of a hydrodynamic disorder where cerebral blood flow is reduced because of the venous hypertension, and the brain parenchyma (mainly white mater) is progressively destroyed. It has been associated with all types of AV fistulas (including VOGMs), and can occur in fetuses, neonates and infants[13,25,26], but it has not been observed in adults.
Fetal MRI is now the preferred modality for diagnosing fetal central nervous system (CNS) abnormalities because of its advantages over ultrasonography[2,27,28]. Prenatal MRI and MR angiography can identify the prognostic factors (cardiac failure, polyhydramnios, pericardial and pleural effusion, ascites, fetal hydrops, and brain injury) that affect prenatal counseling and aid in delineating the blood supply to the lesion for planning postnatal management[2,27-29]. Yuval et al[15], while enumerating the prognostic features for VGAMs have suggested that neonates with cardiomegaly, dilated vena cava or jugular vein, retrograde aortic flow and multiple feeder arteries may have a better prognosis if there is immediate intervention. However, others have a more gloomy view based on the factors associated with a poorer outcome[20,24,30]. Fetal MRI may play an important role by identifying additional findings that are unrelated to the primary pathology but may be important for planning the type of delivery, e.g., placenta previa, umbilical cord wrapped around the neck, and uterine fibroids. Evidence of progressive fetal cardiac dysfunction is a grave sign and may suggest that the high flow lesion may not respond to therapy[24]. Prenatal diagnosis, fetal MRI, and fetal echocardiography provide an opportunity to plan the delivery of the fetus at a tertiary care center where immediate and definitive care can be provided by a multidisciplinary team.
In summary, we report herein the case of a term neonate with a prenatally diagnosed VOGM, dilated SVC and right atrium, cardiomegaly, reversal of flow in the descending aorta, and polyhydramnios. Our case had extensive feeders arising from the anterior, middle, and posterior cerebral arteries. Additionally, we have provided a review of the literature regarding endovascular intervention during the neonatal period, and outcomes.
We thank Meghan S Mehta for her assistance with the literature search and the editing of the manuscript. We also thank Sylvia Sutton-Thorpe, and acknowledge the help of Chrystal and Christina Puvabanditsin for supporting this effort and preparing the manuscript.
A 2675-g term female neonate was diagnosed prenatally as vein of Galen malformation.
The infant developed congestive heart failure with signs of decompensation despite medical intervention.
MRI and MR angiography showed vein of Galen malformation. Chest radiography showed cardiomegaly.
Five endovascular embolizations with N-butyl cyanoacrylate (NBCA) was performed via the umbilical artery, femoral artery and axillary artery.
The development of the endovascular technique began in the early 1980s. Since then, more than 262 neonates with vein of Galen malformation have been reported using this method.
Endovascular treatment has transformed this previous bleak outlook of the infants with vein of Galen malformation and a cure is now potentially possible.
This entity is rare intracranial arterivenous malformation and can be fatal in neonates. Endovascular treatment has had a significant impact in outcome.
The authors presented their experience in a rare case of cerebral arteriovenous malformation that cause cardiovascular instability in a newborn infant. Generally, the manuscript is well prepared and interesting.
| 1. | Bhattacharya JJ, Thammaroj J. Vein of galen malformations. J Neurol Neurosurg Psychiatry. 2003;74 Suppl 1:i42-i44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Kwong Y, Cartmill M, Jaspan T, Suri M. Fetal MRI demonstrating vein of Galen malformations in two successive pregnancies--a previously unreported occurrence. Childs Nerv Syst. 2015;31:1033-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Paternoster DM, Manganelli F, Moroder W, Nicolini U. Prenatal diagnosis of vein of Galen aneurysmal malformations. Fetal Diagn Ther. 2003;18:408-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Dandy WE. Cerebrospinal fluid: absorption. Chicago: American Medical Association; 1929; P 2012. |
| 5. | Berenstein A, Fifi JT, Niimi Y, Presti S, Ortiz R, Ghatan S, Rosenn B, Sorscher M, Molofsky W. Vein of Galen malformations in neonates: new management paradigms for improving outcomes. Neurosurgery. 2012;70:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Alvarez H, Garcia Monaco R, Rodesch G, Sachet M, Krings T, Lasjaunias P. Vein of galen aneurysmal malformations. Neuroimaging Clin N Am. 2007;17:189-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Yan J, Wen J, Gopaul R, Zhang CY, Xiao SW. Outcome and complications of endovascular embolization for vein of Galen malformations: a systematic review and meta-analysis. J Neurosurg. 2015;123:872-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Lasjaunias P, Hui F, Zerah M, Garcia-Monaco R, Malherbe V, Rodesch G, Tanaka A, Alvarez H. Cerebral arteriovenous malformations in children. Management of 179 consecutive cases and review of the literature. Childs Nerv Syst. 1995;11:66-79; discussion 79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 9. | Vein of Galen malformation. Great Ormond Street Hospital. Available from: http: //www.gosh.nhs.uk/medical-information-0/search-medical-conditions/vein-galen-malformation. |
| 10. | Mortazavi MM, Griessenauer CJ, Foreman P, Bavarsad Shahripour R, Shoja MM, Rozzelle CJ, Tubbs RS, Fisher WS, Fukushima T. Vein of Galen aneurysmal malformations: critical analysis of the literature with proposal of a new classification system. J Neurosurg Pediatr. 2013;12:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | O’Brien MS, Schechter MM. Arteriovenous malformations involving the Galenic system. Am J Roentgenol Radium Ther Nucl Med. 1970;110:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Lasjaunias P, Ter Brugge K, Berenstein A. Vein of Galen aneurismal malformation. in: Surgical Neuroangiography 3. Clinical and Interventional Aspects in Children, ed 2. Berlin: Springer 2006; 105-226. [DOI] [Full Text] |
| 13. | Berenstein A, Niimi Y. Youmans Neurological Surgery. H. Richard Winn, MD. The Netherlands: Elsevier Saunders 2011; . |
| 14. | Rodesch G, Hui F, Alvarez H, Tanaka A, Lasjaunias P. Prognosis of antenatally diagnosed vein of Galen aneurysmal malformations. Childs Nerv Syst. 1994;10:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Yuval Y, Lerner A, Lipitz S, Rotstein Z, Hegesh J, Achiron R. Prenatal diagnosis of vein of Galen aneurysmal malformation: report of two cases with proposal for prognostic indices. Prenat Diagn. 1997;17:972-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Karadeniz L, Coban A, Sencer S, Has R, Ince Z, Can G. Vein of Galen aneurysmal malformation: prenatal diagnosis and early endovascular management. J Chin Med Assoc. 2011;74:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Frawley GP, Dargaville PA, Mitchell PJ, Tress BM, Loughnan P. Clinical course and medical management of neonates with severe cardiac failure related to vein of Galen malformation. Arch Dis Child Fetal Neonatal Ed. 2002;87:F144-F149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Meila D, Hannak R, Feldkamp A, Schlunz-Hendann M, Mangold A, Jacobs C, Papke K, Brassel F. Vein of Galen aneurysmal malformation: combined transvenous and transarterial method using a “kissing microcatheter technique”. Neuroradiology. 2012;54:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Mitchell PJ, Rosenfeld JV, Dargaville P, Loughnan P, Ditchfield MR, Frawley G, Tress BM. Endovascular management of vein of Galen aneurysmal malformations presenting in the neonatal period. AJNR Am J Neuroradiol. 2001;22:1403-1409. [PubMed] |
| 20. | Jones BV, Ball WS, Tomsick TA, Millard J, Crone KR. Vein of Galen aneurysmal malformation: diagnosis and treatment of 13 children with extended clinical follow-up. AJNR Am J Neuroradiol. 2002;23:1717-1724. [PubMed] |
| 21. | Mathews AZ, Ibhanesebhor S, Richens T, Manjunatha CM. Heart failure in the new born; vein of Galen aneurysmal malformation. BMJ Case Rep. 2013;2013:pii: bcr0320126132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | McSweeney N, Brew S, Bhate S, Cox T, Roebuck DJ, Ganesan V. Management and outcome of vein of Galen malformation. Arch Dis Child. 2010;95:903-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Maheshwari PR, Pungavkar SA, Narkhede P, Patkar DP. Images in radiology. Vein of Galen aneurysmal malformation: antenatal MRI. J Postgrad Med. 2003;49:350-351. [PubMed] |
| 24. | Wagner MW, Vaught AJ, Poretti A, Blakemore KJ, Huisman TA. Vein of galen aneurysmal malformation: prognostic markers depicted on fetal MRI. Neuroradiol J. 2015;28:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Chow ML, Cooke DL, Fullerton HJ, Amans MR, Narvid J, Dowd CF, Higashida RT, Halbach VV, Hetts SW. Radiological and clinical features of vein of Galen malformations. J Neurointerv Surg. 2015;7:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Lasjaunias PL, Chng SM, Sachet M, Alvarez H, Rodesch G, Garcia-Monaco R. The management of vein of Galen aneurysmal malformations. Neurosurgery. 2006;59:S184-S94; discussion S184-S94;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Kośla K, Majos M, Polguj M, Antosik-Biernacka A, Stefańczyk L, Majos A. Prenatal diagnosis of a vein of Galen aneurysmal malformation with MR imaging - report of two cases. Pol J Radiol. 2013;78:88-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Deloison B, Chalouhi GE, Sonigo P, Zerah M, Millischer AE, Dumez Y, Brunelle F, Ville Y, Salomon LJ. Hidden mortality of prenatally diagnosed vein of Galen aneurysmal malformation: retrospective study and review of the literature. Ultrasound Obstet Gynecol. 2012;40:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Brunelle F. Brain vascular malformations in the fetus: diagnosis and prognosis. Childs Nerv Syst. 2003;19:524-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Has R, Günay S, Ibrahimoglu L. Prenatal diagnosis of a vein of galen aneurysm. Fetal Diagn Ther. 2003;18:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sangkhathat S S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ