Published online Aug 8, 2016. doi: 10.5409/wjcp.v5.i3.325
Peer-review started: January 20, 2016
First decision: April 18, 2016
Revised: May 8, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: August 8, 2016
Processing time: 200 Days and 7.4 Hours
AIM: To ascertain United Kingdom adherence to European society of Paediatric Gastroenterology, Hepatology and Nutrition guidance (ESPGHAN).
METHODS: A national cross sectional questionnaire study of neonatal units across England was completed between January and March 2014. All 174 units in the country were attempted to be contacted to complete a telephone survey. This included all level 1, 2 and 3 units. They were initially contacted by phone and asking any senior member of the team about their current practice and procedures. The first ten telephone interviews were completed with two researchers present to ensure consistency of approach. If no response was received or no details were available, one further attempt was made to contact the unit. The results were recorded in a proforma and then collated and entered into a spreadsheet for analysis. Comparison to United Kingdom adherence to ESPGHAN guidance was completed.
RESULTS: Response rate was 53%. There was variation in use of all supplements. The survey collected data from 91 neonatal units (53% response rate). It was found that 10% of neonatal units had no fixed policy on supplements. The protocols regarding supplementation involved predominantly folic acid, vitamin A, vitamin D and iron, with much variation in doses and regimens. The criteria for prescribing supplements was largely based on age (47%) with only 7% using a weight targets to initiate supplements. Summary data regarding the appropriateness of each nutritional supplement for a variety of different weights are presented, as well as comparison to ESPGHAN guidance which suggests issues with both underdoing of Breast Fed infants and overdosing of infants on several artificial formulas which already contain significant amounts of these nutritional elements.
CONCLUSION: There is significant heterogeneity in neonatal policies when prescribing supplements to neonates. National policies which take international guidance into account are recommended.
Core tip: Nutritional supplementation in neonates is common in neonatal units, but there is no clear United Kingdom guidance. This study set out to ascertain United Kingdom practice with a national cross-sectional study with reference to European society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) nutritional guidance. Fifty-five percent of the 174 units in the country were contacted. There was variation in use of all supplements. Comparison to ESPGHAN guidance suggests issues with both underdoing of Breast Fed infants and overdosing of preterm infants on several artificial formulas which already contain significant amounts of nutritional elements. National policies which take international guidance into account are recommended, with similar research needed in other countries.
- Citation: Gordon M, Isaji S, Tyacke F. Significant variations in nutritional supplementation amongst neonates in the United Kingdom. World J Clin Pediatr 2016; 5(3): 325-329
- URL: https://www.wjgnet.com/2219-2808/full/v5/i3/325.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v5.i3.325
Nutritional needs of both preterm and term neonates are not the same as older children and are subject to rapid changes. A number of nutritional supplements have been studied in relation to prematurity, notably vitamin A, D, iron and folic acid - these form the basis of supplementation recommendations in neonates. Preterm infants have higher nutrient requirements than term infants but inappropriate or absent supplementation can be detrimental to their health[1]. Preterm infants have a low vitamin A status at birth[1]. Evidence shows vitamin A supplement significantly reduces the risk of chronic lung disease and reduces mortality, however excessive levels can lead to symptoms[2].
Preterm infants are susceptible to developing iron deficiency, particularly more premature infants and those being exclusively breastfed without supplementation. As iron plays a role in various tissue functions this would support the need supplementation in preterm infants[3]. Vitamin D is needed for bone health and low levels can cause rickets and seizures secondary to low calcium[4]. Folic acid is used for the prevention of anaemia of prematurity. Levels are high at birth but fall rapidly in the first few weeks of life more notably in the lowest birthweight neonates[5].
Nutritional supplements are almost ubiquitous for infants admitted to United Kingdom neonatal units. Compositions of vitamin supplements vary, for example, Dalavit and Abidec are both commonly used, but Dalavit contains nearly 4 times the amount of vitamin A[1] as Abidec. Doses of supplements should be adjusted according to the type of milk the infant is receiving. Breast milk is best for preterm and low birth weight babies - better long term health outcomes have been well documented, but higher doses of supplements or the addition of fortifiers is required in order to reach the recommended daily intake of vitamins and minerals.
There are currently no national guidelines on nutritional supplementation, but local protocols exist based on growth and nutrition studies and guidance provided by expert groups[6]. The aim of this study was to establish current practices in neonatal supplementation in neonatal units across England[6] and to compare these dosing regimens to guidance provided by European society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN)[7].
A national cross sectional questionnaire study of Neonatal units across England was conducted between January and March 2014[6]. This included all level 1, 2 and 3 units. They were initially contacted by phone and asking any senior member of the team about their current practice and procedures. Eligible staff included senior nurses, advanced neonatal nurse practitioners and senior medical staff.
Firstly, the existence of a local policy was established. Then, details of the supplements used, their brands, dosing, criteria for initiation and the impact of gestational age, weight and feeding type were recorded.
The first ten telephone interviews were completed with two researchers present to ensure consistency of approach and then further interviews were conducted by either researcher. If no response was received or no details were available, one further attempt was made to contact the unit.
The results were recorded in a proforma and then collated and entered into a spreadsheet for analysis. Comparison to ESPGHAN guidance was completed.
The survey collected data from 91 neonatal units (53% response rate), with a representative sample of hospital size and level of neonatal care achieved[6]. It was found that 10% of neonatal units had no fixed policy on supplements. The protocols regarding supplementation involved predominantly folic acid, vitamin A, vitamin D and iron.
In regards to folic acid, when supplementing expressed breast milk (EBM), 36% of hospitals prescribed 50 μg of folic acid daily, whilst 37% of units prescribed no folic acid. For remaining units, the dose varied from 50 μg daily to 1 mg weekly of folic acid.
Similar results were obtained when looking at the vitamins A and D data. Dalavit and Abidec doses varied in each hospital. Two units had no fixed regime and was based on which supplement (Dalavit or Abidec) was available at the time of prescribing.
When considering iron supplementation[7], over 65% of units prescribed iron supplementation with various feeds types whereas 27% did not supplement with iron at all. Doses across the different units varied between 0.5 mL sytron once daily to 2.5 mL twice daily. Forty-six percent of units recognised that no additional iron supplementation is needed for babies receiving preterm formula. The criteria for prescribing supplements was largely based on age (47%) with only 7% of units interviewed using a weight based set of criteria to initiate supplements. A small number of hospitals had no fixed criteria, and certain hospitals (24%) used both age and weight.
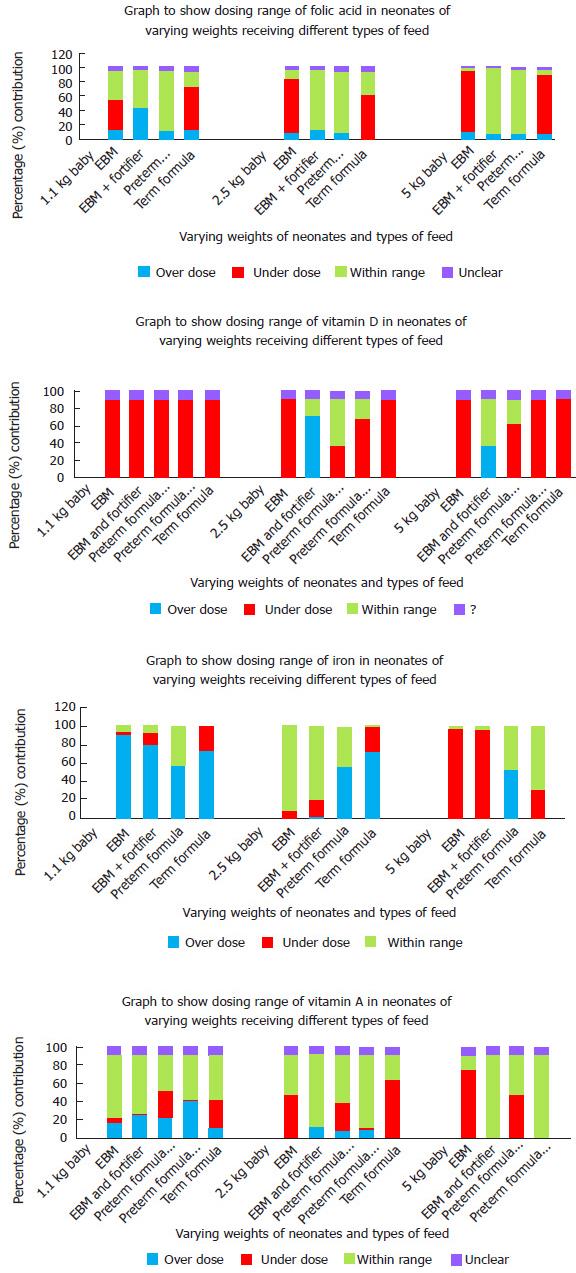
Summary data regarding the appropriateness of each nutritional supplement for a variety of different weights are presented in Figure 1. Table 1 demonstrates the amount of each of the supplements that are delivered purely through feeding with breast milk, fortified breast milk and with a variety of artificial milks.
| Type of feed (based on 180 mL of feed) | Vitamin A (μgRE) | Vitamin D (μg) | Folic acid (μg) | Iron (mg) |
| EBM | 104 | 0.07 μg/kg per day | 9 | 0.126 |
| EBM + 2 × 2.2 g Nutriprem (C and G) Breast Milk Fortifier | 522 | 9.1 μg/kg per day | 63 | Neg |
| EBM + 2 × 2 g SMA BMF | 486 | 13.68 μg/kg per day | 54 | Neg |
| SMA gold Prem 1 | 333 | 6.1 μg/kg per day | 52 | 2.5 |
| SMA Gold Prem 2 | 180 | 2.7 μg/kg per day | 27 | 2.16 |
| C and G Nutriprem 1 | 650 | 5.4 μg/kg per day | 63 | 2.9 |
| C and G Nutriprem 2 | 180 | 3.06 μg/kg per day | 36 | 2.16 |
| C and G 1 | 97.2 | 2.16 μg/kg per day | 23.4 | 0.954 |
| Aptamil 1 | 97.2 | 2.16 μg/kg per day | 23.4 | 0.954 |
| SMA 1 | 119 | 2.2 μg/kg per day | 19.8 | 1.2 |
| HiPP 1 | 126 | 2.16 μg/kg per day | 18 | 0.9 |
| Neocate LCP | 100.8 | 2.16 μg/kg per day | 15.84 | 1.8 |
| ESPGHAN recommendation | 400-1000 µg RE/kg per day | 20-25 μg/d | 35-100 µg/kg per day | 2-3 mg/kg per day (from 2-6 wk) |
Dosing of all nutritional additives varied greatly across the country[6]. Only a small proportion of units actually achieved dosing within ESPGHAN recommended limits in all supplements[7]. More than 80% of units are in-fact overdosing smaller infants iron potentially causing toxicity.
In general, overdosing of supplements was seen in smaller babies. Larger babies are more commonly receiving doses within the recommended limits. However, the criterion was seen to be based on either birth weight, gestational age or both. ESPGHAN recommends that the infant’s dry weight should be used when calculating the dose of supplements[5]. This would mean weighing the baby on a regular basis and adjusting doses accordingly. This practice was not being done in any unit surveyed; doses calculated from birth seem to remain static until discontinued.
Whilst there is clearly no national policy on this issue, there are local networks that carry guidance. Whilst it was outside the scope of this study to investigate these in great detail, the local network in Greater Manchester included a total of 8 units surveyed. Not only did the dose of vitamin A vary but units were also using different brands. Supplementing with folic acid was completely absent in one hospital but the use of iron was consistent. This highlights that current practice is clearly leading to massive variations in both strategy and outcome for babies. With such wide variation in dosing and differing criteria for initiation there is great potential for causing harm to infants, from either insufficient or excessive supplementation. Consistent dosing and one policy for all feed types are also not ideal and can put smaller babies in particular at risk.
Table 1 highlights certain dosing issues that could become tenants of a national policy. It is clear that neonates on preterm formula generally do not need further vitamin A, folic acid or iron supplementation, but require vitamin D. Neonates on EBM will require all additional supplementation, but those on fortifier will only require iron supplements. It seems that iron supplementation is not indicated for any babies on artificial formulas, as changing requirements have been considered in the changing constituents of preterm vs term formulations. It is also important to assess whether the supplements need to continue on discharge as both requirements and content of formulas change with age.
These principles and the huge variation in practical prescribing that have been highlighted by this study support the need for a standardised supplementation regime based on available evidence, with arrangements to update regular to consider changes in artificial formulas and fortification. This will allow the nutritional needs of infants to be met in an appropriate and safe manner. Further research is indicated to assess if similar problems exists in other countries.
There is significant heterogeneity in neonatal policies when prescribing supplements to neonates. National policies which take international guidance into account are recommended. Further research is indicated to assess if similar problems exists in other countries.
Nutritional requirements amongst preterm and term neonates differ from older infants and change rapidly. Preterm infants have higher nutrient requirements than term infants but inappropriate or absent supplementation can be detrimental to their health.
There are currently no national guidelines on nutritional supplementation, although there is international guidance from ESPGHAN.
This study confirms that despite international guidance that is evidence informed, practice across the country does not align to this or any prescribed guidance. Indeed, practice varies significantly and this potential means neonates may be under or overdosing on supplements.
It is advised that readers consider the evidence base of their local guidance and how this compares to national and international guidance.
ESPGHAN: European society of Paediatric Gastroenterology, Hepatology and Nutrition.
In the paper, the authors present a useful and interesting study regarding nutritional supplementation in preterm babies in the United Kingdom.
| 1. | Larmour K, Shaw V. Nutrition: enteral nutrition for the preterm infan (2016). Available from: http//www.gosh.nhs.uk/health-professionals/clinical-guidelines/nutrition-enteral-nutrition-preterm-infant. |
| 2. | Mactier H, Weaver LT. Vitamin A and preterm infants: what we know, what we don’t know, and what we need to know. Arch Dis Child Fetal Neonatal Ed. 2005;90:F103-F108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Rao R, Georgieff MK. Iron therapy for preterm infants. Clin Perinatol. 2009;36:27-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126:1040-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 646] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 5. | Roberts PM, Arrowsmith DE, Rau SM, Monk-Jones ME. Folate State of Premature Infants. Arch Dis Child. 1969;44:637-642. |
| 6. | Gordon M, Isaji S, Karlsen F. Nutritional supplementation amongst preterm and term neonates. Proceedings of the 48th annual meeting of the European society of Paediatric gastroenterology, hepatology and nutrition. 2015-05-06. Available from: http//espghan.org/uploads/media/ESPGHAN_A4_Abstract_2015_v2.pdf. |
| 7. | Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, Domellöf M, Embleton ND, Fusch C, Genzel-Boroviczeny O. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1012] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Al-Haggar M, Classen CF, Yu ZW S- Editor: Qiu S L- Editor: A E- Editor: Wu HL