Published online Feb 8, 2016. doi: 10.5409/wjcp.v5.i1.95
Peer-review started: July 27, 2015
First decision: August 26, 2015
Revised: September 25, 2015
Accepted: October 23, 2015
Article in press: October 27, 2015
Published online: February 8, 2016
Processing time: 188 Days and 2.9 Hours
AIM: To analyze the evolution in the management of airway infantile hemangioma (AIH) and to report the results from 3 pediatric tertiary care institutions.
METHODS: A retrospective study of patients with diagnosis of AIH and treated in 3 pediatric tertiary care institutions from 1996 to 2014 was performed.
RESULTS: Twenty-three patients with diagnosis of AIH were identified. Mean age at diagnosis was 6 mo (range, 1-27). Single therapy was indicated in 16 patients and 7 patients received combined therapy. Two therapeutic groups were identified: Group A included 14 patients who were treated with steroids, interferon, laser therapy and/or surgery; group B included 9 patients treated with oral propranolol. In group A, oral corticosteroids were used in 9 patients with a good response in 3 cases (no requiring other therapeutic option), the other patients required additional treatment options. Cushing syndrome was observed in 3 patients. One patient died of a fulminant sepsis. Open surgical excision and endoscopic therapy were performed in 11 patients (in 5 of them as a single treatment) with a response rate of 54.5%. Stridor persisted in 2 cases, and one patient died during the clinical course of bronchial aspiration. In group B, oral propranolol was used in 9 patients (in 8 of them as a single treatment) with a response rate of 100%, with an mean treatment duration of 7 mo (range, 5-10); complications were not observed.
CONCLUSION: Our experience and the medical literature support the use of propranolol as a first line of treatment in AIH.
Core tip: Through this study we want to highlight the importance of early use of propranolol in the treatment of airway infantile hemangioma. We also want to show our experience with other treatment options including corticosteroids, interferon and surgical and endoscopic treatments used before the propranolol era.
- Citation: Vivas-Colmenares GV, Fernandez-Pineda I, Lopez-Gutierrez JC, Fernandez-Hurtado MA, Garcia-Casillas MA, Matute de Cardenas JA. Analysis of the therapeutic evolution in the management of airway infantile hemangioma. World J Clin Pediatr 2016; 5(1): 95-101
- URL: https://www.wjgnet.com/2219-2808/full/v5/i1/95.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v5.i1.95
Even though infantile hemangiomas (IH) are the most common head and neck tumors during childhood, the airway is uncommon location, accounting for only 1.5% of all pediatric laryngeal lesions[1]. Symptoms at presentation of infantile hemangioma (AIH) are related to the grade of airway obstruction, which becomes more evident during periods of agitation, crying, or respiratory infections. Stridor, usually biphasic but more prominent during inspiration, is the most common presentation symptom. Diagnosis is performed by bronchoscopy image which typically reveals a unilateral, soft, submucous and reddish mass[2]. IHs are usually not present at birth; they proliferate during the first year of life, and then they involute. For AIH, the treatment goal is to provide an airway adapted for the development of these children. Multiple modalities, both medical and surgical, have been used for its treatment including external irradiation[3], tracheostomy[4], surgical resection[5], systemic or intralesional corticosteroids[6,7], laser vaporization[8] and interferon[9], but many have significant risks and complications. Until recently, the most common medical therapy for AIH was high-dose systemic corticosteroids, but this often results in significant well-known adverse effects including hypertension, irritability, and cushingoid appearance[10]. The introduction of propranolol by Leaute-Labreze in 2008 for the treatment of IH has revolutionized its management. Potential modes of actions for propranolol include vasoconstriction, a down-regulation of angiogenetic factors like vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and an up-regulation of apoptosis of capillary endothelial cells[11]. Our aim was to analyze the evolution in the management of AIH and to report the results from 3 pediatric tertiary care centers.
A retrospective study of all patients with diagnosis of AIH treated in 3 pediatric tertiary care institutions during a period of 18 years (1996-2014) was performed. Variables analyzed included gender, age at diagnosis, symptoms at presentation, lesion location, grade of airway obstruction (according to Cotton classification), treatment, complications and survival. In all the patients, diagnosis was obtained by endoscopic direct visualization of the lesion using a flexible fibrobronchoscope. Assessment of airway compromise was performed according to Cotton classification which divides airway obstruction into four categories[2] (grade I: Lesions have less than 50% obstruction, grade II: Lesions have 51% to 70% obstruction, grade III: Lesions have 71% to 99% obstruction and grade IV: Lesions have no detectable lumen or complete stenosis). Treatment option depended on symptoms at presentation, grade of respiratory impairment and time at diagnosis. Retrospective analysis divided patients into 2 groups according to the treatment received. Group A: Patients treated with oral corticosteroids (methylprednisolone at 2 mg/kg per day or dexamethasone at 0.5 mg/kg per day), interferon (1-3 × 106 U/m2, 3 times a week), endoscopic laser therapy (Diode laser) and/or surgery (open surgical excision through an anterior midline cricothyroidotomy, resection of the hemangioma and laryngotracheoplasty with costal cartilage graft if required). Group B: patients treated with oral propranolol, at a maximum dose was 2 mg/kg per day, divided in 2 doses. Prior to propranolol treatment initiation, an electrocardiogram, blood pressure and heart rate monitoring and serum glucose level were performed in all the patients. The response to each treatment was evaluated in all patients according to the clinical course and fibrobronchoscopic exam.
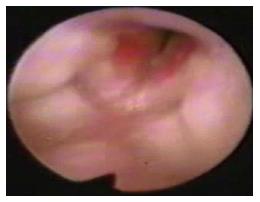
From 1996 to 2014, twenty-three patients with diagnosis of AIH were treated in 3 pediatric tertiary care hospitals (Table 1). There were 16 females and 7 males with a mean age at diagnosis of 6 mo (range, 1-27). All the patients were referred to our hospitals due to stridor and respiratory distress. Of the 23 patients, 14 were between the age range of 1-3 mo with impossibility for extubation. The remaining older patients, had sequelae from AIH as stridor and dyspnea by airway obstruction. Flexible fibrobronchoscope showed airway diameters ranging from 2.8 to 3.6 mm. Lesions showed a unique location in 18 patients (17 in subglottis and 1 in supraglottis). Five patients presented at joint location (1 in supraglottis and subglottis, 2 in glottis and subglottis and 2 in subglottis and trachea). Reduction of the cross-sectional area of the airway at the subglottic region prior to treatment initiation was observed in 22 patients: Grade I of Cotton classification in 10 patients, grade II in 4 patients and grade III in 8 patients. The patient with the supraglottis lesion did not have reduction of the cross-sectional area of the airway (Figure 1). Six patients had an associated facial hemangioma and 2 of them were diagnosed with PHACES syndrome (Figure 2). Single therapy was indicated in 16 patients, whereas 7 non-responder patients received combined therapy. In group A (n = 14), 4 patients had subglottic stenosis grade I, 3 grade II and 7 grade III. In 8 patients single therapy was indicated, 3 of them received exclusive oral corticosteroids (methylprednisolone at 2 mg/kg per day or dexamethasone at 0.5 mg/kg per day) for a mean time of 8 wk (range, 4-24) with complete response and without complications. Five patients underwent surgery exclusively. Four patients were treated with laryngotracheoplasty with graft and one with resection and cricothyroidotomy. Mean time of intubation after surgery was 7 d (range, 4-11 d) with need for reintubation in 1 case secondary to an increase of respiratory distress.
| Age (mo) | Gender | Symptoms at diagnosis | Lesion location | Degree of SGE (Cotton scale) | Treatment | Outcomes | |
| 1 | 2 | F | Stridor | Subglottic | II | Corticosteroids | Asymptomatic |
| 2 | 3 | F | Stridor | Glottis and subglottic | Interferon, corticosteroids | Asymptomatic | |
| 3 | 1 | M | Stridor | Subglottic | II | Corticosteroids | Asymptomatic |
| 4 | 1 | F | Stridor | Subglottis and trachea | I | Resection and cricothyroidotomy | Asymptomatic |
| 5 | 1 | M | Stridor, dyspnea | Supraglottis and subglottis | III | Laryngotracheoplasty | Dysphonia |
| 6 | 14 | M | Stridor, dyspnea | Subglottic | III | Corticosteroids, laser, resection and cricothyroidotomy | Asymptomatic |
| 7 | 27 | F | Stridor | Subglottic | II | Laser, laryngotracheoplasty | Exitus |
| 8 | 2 | F | Stridor, dyspnea | Subglottic | III | Laryngotracheoplasty | Dysphonia |
| 9 | 4 | F | stridor, dyspnea | Subglottic | III | Corticosteroids, laryngotracheoplasty | Dysphonia |
| 10 | 3 | F | Stridor, dyspnea | Subglottic | III | Laryngotracheoplasty | Transitory stridor |
| 11 | 5 | F | Stridor, dyspnea | Subglottic | III | Laryngotracheoplasty | Asymptomatic |
| 12 | 16 | M | Stridor, dyspnea | Glottis and subglottic | I | Corticosteroids | Asymptomatic |
| 13 | 5 | F | Stridor, dyspnea | Subglottic | III | Corticosteroids, resection and cricothyroidotomy | Transitory stridor |
| 14 | 3 | F | Stridor | Subglottis and trachea | I | Corticosteroids, laryngotracheoplasty | Asymptomatic |
| 14 | 19 | M | Stridor | Subglottic | I | Propranolol | Exitus |
| 16 | 5 | F | Stridor | Supraglottis | - | Propranolol | Asymptomatic |
| 17 | 26 | M | Stridor | Subglottic | I | Propranolol | Asymptomatic |
| 18 | 5 | F | Stridor, dyspnea | Subglottic | III | Corticosteroids, propranolol | Asymptomatic |
| 19 | 3 | F | Stridor | Subglottic | II | Propranolol | Asymptomatic |
| 20 | 3 | M | Stridor | Subglottic | I | Propranolol | Asymptomatic |
| 21 | 2 | F | Stridor | Subglottic | I | Propranolol | Asymptomatic |
| 22 | 2 | F | Stridor | Subglottic | I | Propranolol | Asymptomatic |
| 23 | 3 | F | Stridor | Subglottic | I | Propranolol | Asymptomatic |
Complete response was observed in 40% of the patients. Stridor persisted in 1 patient and dysphonia was observed in 2 cases. Complications included one pneumothorax after reintubation and 1 infection of surgical wound. The other 6 patients in this group A, received combined therapy with corticosteroids, interferon (in one patient at a dose of 1 × 106 U/m2, gradually increased to 3 × 106 U/m2, 3 times a week) and open surgery/laser due to a poor response to single therapy (three patients were treated with laryngotracheoplasty and graft, three patients with resection and cricothyroidotomy and 2 patients initially were treated with endoscopic therapy by Diodo laser, that were subsequently treated with surgical resection). Complications in this group included Cushing syndrome in 3 cases that required other therapeutic alternatives for poor response to steroids. Dysphonia was observed in 2 cases after surgical treatment, and in 2 cases reintubation was necessary secondary to increased respiratory distress.
Response rate after surgery in patients with combined therapy was 66.6%. One patient died of a fulminant sepsis and other patient died during the clinical course of bronchial aspiration (Table 2).
| Evolution after treatment | Group A n = 14 | Group B Oral propranolol n = 9 | |||||
| Oral corticosteroids | INF | Open surgical resection and endoscopic therapy | |||||
| Single treatment n = 3 | Combined treatment n = 6 | Combined treatment n = 11 | Single treatment n = 5 | Combined treatment n = 6 | Single treatment n = 8 | Combined treatment n = 1 | |
| Asymptomatic | 3 (100) | 0 (0) | 0 (0) | 2 (40) | 4 (66.6) | 8 (100) | 1 (100) |
| Symptomatic | 0 (0) | 6 (100) | 1(100) | 3 (60) | 2 (33.3) | 0 (0) | 0 (0) |
| Dysphonia | 0 (0) | 0 (0) | 0 (0) | 2 (40) | 1 (16.6) | 0 (0) | 0 (0) |
| Stridor | 0 (0) | 5 (83.3) | 1 (100) | 1 (20) | 1 (16.6) | 0 (0) | 0 (0) |
| Complications | 0 (0) | 3 (50) | 0 (0) | 1 (20) | 1 (16.6) | 0 (0) | 0 (0) |
| Exitus | 0 (0) | 1 (16.6) | 0 (0) | 0 (0) | 1 (16.6) | 1 (12.5)2 | 0 (0) |
In group B (n = 9) patients were treated with propranolol at a maximum dose of 2 mg/kg per day, divided in 2 doses. Six patients presented with subglottic stenosis grade I, 1 grade II and 1 grade III; the lesion was localized in the supraglottis in 1 patient. In 8 patients, propranolol was used as monotherapy. One patient had previously received corticosteroids and endoscopic therapy with Diodo laser, without response. Response rate after propranolol therapy was 100%. Mean duration of treatment was 7 mo (range, 5-10), and complications were not observed. One patient died secondary to a congenital hypertrophic cardiomyopathy, not related to treatment with propranolol (Table 2).
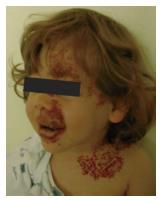
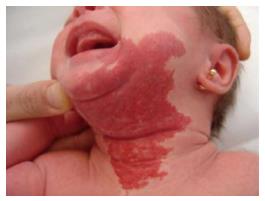
AIH is a challenging entity that usually presents with inspiratory and expiratory stridor at 3 or 4 wk after birth which becomes more evident during periods of agitation, crying, or respiratory infections. This delayed presentation after birth is secondary to the natural course of IH with a progressive growth during the early proliferative phase[12-14]. Most authors agree that IH shows a rapid growth until 6-12 mo of age followed by involution after 18 or more months[15]. Up to 50% of these patients have cutaneous IH[16], with a typical beard-area distribution and whose presence may guide to the clinical diagnosis of AIH in patients with respiratory symptoms (Figure 3). Some authors have described association of AIH with PHACES syndrome[17]. In our series, 6 (26%) of the patients had an associated facial hemangioma and 2 of them had a PHACES syndrome.
Management and treatment guidelines for the treatment of AIH are not well established and different treatment options have been reported[12,15,17]. There seems to be a consensus regarding tracheostomy as a therapeutic approach that currently seems to be abandoned by virtually all authors[18]. Systemic corticosteroids can be effective in halting further growth of AIH during the proliferative phase, with success rates ranging from 60% to 90%[19,20]. However, efficacy rates may be lower in large, function-threatening AIH, and adverse effects may be intolerable (Cushing syndrome, growth retardation, hypertension, and immunodeficiency), reported in 12.9% of the cases and verified in our experience[21-23]. Interferon was widely heralded for treatment in refractory AIH, but it has a significant risk of neurotoxic effects (spastic diplegia), especially in very young infants under 6 mo of age[24]. In our series, interferon was only used in a patient, without complications, but with poor response.
Open surgical resection, first described by Sharp in 1945[25], showed a success rate of 98%. Bitar et al[23] operated on 50 patients with AIH who required a mean intubation or stenting period of 9 d, and carried a 10% complication rate, including subglottic stenosis, bleeding, and wound infections. Although in our experience we observed a global response rate in surgical patients of 54.5% (patients treated with single and combined therapy), we believe this therapeutic option is too invasive in the propranolol era. However, a role for surgical resection in combination with propranolol may exist for early emergency cases in which waiting for medical treatment response is not an option. Complications associated with AIH surgery include dysphonia (observed in 3 of our patients who underwent laryngotracheoplasty with graft). Open surgical resection should be considered only for selected cases, after failure of other treatments.
The CO2 laser was considered as the initial treatment of choice although it is not very specific for the treatment of these lesions and it has a limited effectiveness in coagulation of the hemangioma. Published series observed up to 20% of residual subglottic stenosis in patients treated with this technique. The neodymium laser (Laser Nd:YAG) is considered an useful laser coagulator although large lesions may cause damage to the surrounding tissues and probably increase the risk of subglottic stenosis[17,24]. Other authors prefer the potassium-titanyl-phosphate laser (KTP), arguing that it is absorbed mainly by hemoglobin, making it ideal for treating vascular lesions[26]. Saetti et al[20] carried out a retrospective medical records review of all patients treated for congenital subglottic hemangiomas, and they observed a success rate of 95% in patients treated with diode laser as primary treatment, with a complication rate of 9%. In our experience, the 2 patients treated with laser therapy required additional surgery for persistent symptoms.
Peridis et al[27] performed a meta-analysis, on the effectiveness of propranolol for the treatment of AIH in 36 patients. In a retrospective manner, they analyzed the effectiveness of propranolol vs steroids, CO2 laser or vincristine in predominantly case reports with relatively small sample sizes in each treatment group. It could be demonstrated that propranolol is the most effective treatment as compared to former treatments. In our series, we observed a response rate of 100% for patients treated with oral propranolol. No adverse reactions were documented. Lou et al[28], carried out a meta-analysis including 35 studies to identify studies which estimated the efficacy of propranolol therapy in infants with hemangiomas of all sites of the body. They evaluated the efficacy of propranolol vs other treatments. Sixteen studies with 45 IH cases and 45 controls compared the efficacy of propranolol with other treatment modalities in treating AIH. Heterogeneity was absent (Q = 5.00, I2 = 0.0%, P = 0.986). They observed that propranolol therapy is more effective in treating AIH (OR = 20.91, 95%CI: 7.81-55.96, P < 0.001). Potential risks associated with propranolol include bradycardia, hypotension, and hypoglycemia[29,30]. To reduce the rate of adverse reactions, Bajaj et al[17], gave some recommendations for the use of propranolol in infantile isolated subglottic hemangioma prior to treatment that included a detailed history and clinical examination, inspection of the whole body for hemangiomas, as well as cardiovascular and respiratory assessment. Pre-treatment tests should include electrocardiogram, heart rate and blood pressure monitoring.
Not only the effectiveness of propranolol in the management of AIH is important, but also its efficiency in terms of cost when it is compared to other therapeutic options which involve more use of hospital resources in these complex cases. Further cost-effectiveness studies are required to better define the exact cost of treating patients with AIH. The optimal duration of propranolol treatment is unknown, but it is currently accepted that the patients should remain on propranolol until the hemangioma enters the phase of involution, which usually occurs after the first year of life. Vlastarakos et al[31] performed a meta-analysis that included 17 studies with 61 patients treated with propranolol, observing a rate of relapse of 11.5% after withdrawal of propranolol. This was not observed in our patients cohort.
Though our retrospective study has several limitations that should be considered in the interpretation of our findings, including the small number of patients in the study cohort and the referral bias inherent in our status as referral centers for pediatric airway disorders, we consider propranolol is currently the first line treatment for symptomatic AIH, considering its efficacy and relatively mild side effects. The two cohorts, pre-propranolol group and propranolol group, were not randomized and other reasons beside propranolol might have contributed to the better outcome of the propranolol group. Pre-propranolol group contained more patients with advanced disease compared to propranolol group (Cotton grade II and III, 71% vs 22%). This could be explained by the fact that patients with more advanced disease may have a delayed referral to institutions with pediatric airway expertise. Early initiation of oral propranolol might avoid this advanced stage.
In conclusion, the management of AIH has evolved from surgical resection and systemic steroids to oral propranolol in the last 7 years. Bronchoscopy plays an important role in the diagnosis and evaluation of treatment response. Our experience as referral centers for pediatric airway disorders and the medical literature support the early use of propranolol as a first line of treatment in AIH due to its benefits in terms of effectiveness and efficiency. Surgical and/or endoscopic approach represent a second line therapeutic option for non-responder patients to propranolol. Management of children with AIH should be performed in pediatric institutions with expertise in both, vascular anomalies and airway disorders.
The management of airway infantile hemangioma has evolved from surgical resection and systemic steroids to oral propranolol in the last 7 years. The authors present an experience as referral centers for pediatric airway disorders, which is in accordance with the most recent published medical literature regarding the use of propranolol as a first line of treatment in airway infantile hemangioma.
Further research studies should be performed in order to investigate the role of other betablocker agents in the treatment of airway infantile hemangioma.
The authors’ status as referral centers for pediatric airway disorders and vascular anomalies has permitted us to obtain a greater experience in the management of these challenging patients.
Since the introduction of propranolol in 2008 for the treatment of infantile hemangioma, this agent has become first line treatment for lesions located in the airway. Historically, this has been a challenging site for the occurrence of infantile hemangioma, but propranolol treatment has dramatically changed the prognosis of these young patients. The study analyzes the evolution in the management of airway infantile hemangioma.
Infantile hemangioma is the most common vascular tumor in children that has a rapid growth phase (1-3 mo of age), followed by a slow growth phase (3-12 mo of age) and involution (1-10 years of age).
In the paper, the authors present a nice and conclusive overview on the clinical presentation and the treatment of airway infant hemangiomas, highlighting the revolutionary advance achieved by the introduction of propranolol in hemangioma treatment. The paper is well written, with good language, its content is conclusive and it is very important to distribute the beneficial experiences with propranolol.
P- Reviewer: Classen CF, Grizzi F, Inserra A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Holinger PH, Brown WT. Congenital webs, cysts, laryngoceles and other anomalies of the larynx. Ann Otol Rhinol Laryngol. 1967;76:744-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 166] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Cotton RT. Management of subglottic stenosis. Otolaryngol Clin North Am. 2000;33:111-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | New GB, Clark CM. Angiomas of the larynx: report of three cases. Ann Otol Rhinol Laryngol. 1919;28:1025-1037. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Suehs OW, Herbut PA. Hemangioma of the larynx in infants. Arch Otolaryngol. 1940;32:783-789. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Sharp HS. Haemangioma of the trachea in an infant; successful removal. J Laryngol Otol. 1949;63:413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Cohen SR. Unusual lesions of the larynx, trachea and bronchial tree. Ann Otol Rhinol Laryngol. 1969;78:476-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Shikhani AH, Jones MM, Marsh BR, Holliday MJ. Infantile subglottic hemangiomas. An update. Ann Otol Rhinol Laryngol. 1986;95:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Simpson GT, Healy GB, McGill T, Strong MS. Benign tumors and lesions of the larynx in children. Surgical excision by CO2 laser. Ann Otol Rhinol Laryngol. 1979;88:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 9. | Sherrington CA, Sim DK, Freezer NJ, Robertson CF. Subglottic haemangioma. Arch Dis Child. 1997;76:458-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | O-Lee TJ, Messner A. Subglottic hemangioma. Otolaryngol Clin North Am. 2008;41:903-911, viii-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010;163:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 348] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Choa DI, Smith MC, Evans JN, Bailey CM. Subglottic haemangioma in children. J Laryngol Otol. 1986;100:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Goldsmith MM, Strope GL, Postma DS. Presentation and management of postcricoid hemangiomata in infancy. Laryngoscope. 1987;97:851-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Willshaw HE, Deady JP. Vascular hamartomas in childhood. J Pediatr Surg. 1987;22:281-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Hoeve LJ, Küppers GL, Verwoerd CD. Management of infantile subglottic hemangioma: laser vaporization, submucous resection, intubation, or intralesional steroids? Int J Pediatr Otorhinolaryngol. 1997;42:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Gray SD, Johnson DG. Head and neck malformations of the pediatric airway. Semin Pediatr Surg. 1994;3:160-168. [PubMed] |
| 17. | Bajaj Y, Kapoor K, Ifeacho S, Jephson CG, Albert DM, Harper JI, Hartley BE. Great Ormond Street Hospital treatment guidelines for use of propranolol in infantile isolated subglottic haemangioma. J Laryngol Otol. 2013;127:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Filston HC. Hemangiomas, cystic hygromas, and teratomas of the head and neck. Semin Pediatr Surg. 1994;3:147-159. [PubMed] |
| 19. | Adams DM, Lucky AW. Cervicofacial vascular anomalies. I. Hemangiomas and other benign vascular tumors. Semin Pediatr Surg. 2006;15:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Saetti R, Silvestrini M, Cutrone C, Narne S. Treatment of congenital subglottic hemangiomas: our experience compared with reports in the literature. Arch Otolaryngol Head Neck Surg. 2008;134:848-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Rahbar R, Nicollas R, Roger G, Triglia JM, Garabedian EN, McGill TJ, Healy GB. The biology and management of subglottic hemangioma: past, present, future. Laryngoscope. 2004;114:1880-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Enjolras O, Riche MC, Merland JJ, Escande JP. Management of alarming hemangiomas in infancy: a review of 25 cases. Pediatrics. 1990;85:491-498. [PubMed] |
| 23. | Bitar MA, Moukarbel RV, Zalzal GH. Management of congenital subglottic hemangioma: trends and success over the past 17 years. Otolaryngol Head Neck Surg. 2005;132:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Pransky SM, Canto C. Management of subglottic hemangioma. Curr Opin Otolaryngol Head Neck Surg. 2004;12:509-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Al-Sebeih K, Manoukian J. Systemic steroids for the management of obstructive subglottic hemangioma. J Otolaryngol. 2000;29:361-366. [PubMed] |
| 26. | Madgy D, Ahsan SF, Kest D, Stein I. The application of the potassium-titanyl-phosphate (KTP) laser in the management of subglottic hemangioma. Arch Otolaryngol Head Neck Surg. 2001;127:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Peridis S, Pilgrim G, Athanasopoulos I, Parpounas K. A meta-analysis on the effectiveness of propranolol for the treatment of infantile airway haemangiomas. Int J Pediatr Otorhinolaryngol. 2011;75:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Lou Y, Peng WJ, Cao Y, Cao DS, Xie J, Li HH. The effectiveness of propranolol in treating infantile haemangiomas: a meta-analysis including 35 studies. Br J Clin Pharmacol. 2014;78:44-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Rosbe KW, Suh KY, Meyer AK, Maguiness SM, Frieden IJ. Propranolol in the management of airway infantile hemangiomas. Arch Otolaryngol Head Neck Surg. 2010;136:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Maturo S, Hartnick C. Initial experience using propranolol as the sole treatment for infantile airway hemangiomas. Int J Pediatr Otorhinolaryngol. 2010;74:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Vlastarakos PV, Papacharalampous GX, Chrysostomou M, Tavoulari EF, Delidis A, Protopapas D, Nikolopoulos TP. Propranolol is an effective treatment for airway haemangiomas: a critical analysis and meta-analysis of published interventional studies. Acta Otorhinolaryngol Ital. 2012;32:213-221. [PubMed] |