PAST
Role of oxygen - the cause or the treatment
Many studies were conducted worldwide since 1951 to determine the exact mechanism of this disorder. Ophthalmic literature of the past reveals, anoxia in premature babies to be the prime causative factor for the development of RLF and hence in 1952 was called as anoxic retinopathy. A study by Szewczyk[3] revealed that this was the response of immature neural tissue to anoxia. He explained the mechanism would probably be due to low oxygen tension in the fetal blood of premature which causes retinal vessels to dilate initially, and still when the demand is not satisfied it leads to edema, transudation and haemorrhages. Campbell[4] first brought to notice the development of RLF in infants undergoing intensive oxygen therapy. A clinicopathologic study at the Women’s hospital Melbourne, Australia by Ryan[5] between 1948 and 1950 revealed 23 cases of RLF. It was noted that no case of RLF was reported prior to the introduction of an oxygen cot. The nursing staffs were giving oxygen liberally to all babies with this oxygen cot and hence there was increase in the incidence. Later on, from October 1950, oxygen was restricted to only babies with cyanosis and since then a fall in number of RLF was seen. With this study it was understood that, the normal human fetus is in a state of cyanosis, because pure arterial blood is not carried by any of the arteries. Since high oxygen concentrations are toxic to adults, similarly normal concentration is toxic to immature tissues. Hence it was concluded that RLF can be prevented by giving oxygen only to those premature babies who require it.
It was observed that anoxia might occur at the cellular level during oxygen therapy even though the environmental oxygen and the blood oxygen levels are increased. This paradoxical situation, which was called as “hypoxic-anoxia” will occur as a result of inactivation of oxidative enzymes from prolonged exposure to high oxygen levels.
Equal importance was also given to the rate of withdrawal from oxygen since it was noted that it minimizes the retinal damage induced by hyperoxia. A controlled nursery study by Bedrossian et al[6] reported a significantly higher incidence of RLF in infants who were rapidly withdrawn from an atmosphere of continuous oxygen as compared with a group where oxygen was gradually reduced.
Hence, it became necessary to monitor the oxygen and prior to the availability of arterial oxygen tension to measure, ophthalmoscopic monitoring of retinal vessel caliber was done. The following guidelines for oxygen therapy were recommended: (1) Oxygen should be given to premature infants proved to be hypoxic or strongly suspected; (2) When high concentrations of oxygen are required for significant periods, in addition to measuring the incubator oxygen level, arterial oxygen tension monitoring should also be done; (3) Ophthalmoscopic monitoring of retinal vasoconstriction should be done at regular intervals, and when marked constriction is detected, prompt reduction in the concentration of administered oxygen may prevent retinal damage; and (4) Even for a full term infant, retina is incompletely vascularised temporally, hence oxygen therapy should be cautiously administered and limited to specific indication only.
Pathogenesis
The effect of oxygen on the retina on the immature vasculature was described in two stages: (1) Primary stage or vasoconstrictive phase: This occurs during exposure to hyperoxia and there is also suppression of the normal anterior ward vascularisation of the retina. This mechanism of vasoconstrictive and obliterative effect of oxygen is seen predominantly in the developing retinal vessels. This inturn leads to suppression of vascular endothelial growth factor; and (2) Secondary stage or vasoproliferative phase: This occurs during the shifting from oxygen to room air, and involves dilatation and tortuosity of the existing larger vessels with neovascularisation and proliferation of new vessels into the vitreous. This is mainly due to the sudden surge in vascular endothelial growth factor levels.
Laboratory findings
In the 1950’s the kitten model was used in most experiments as its immature retinal vessels showed selective response to oxygen[7,8]. Smith et al[9] in the year 1994 demonstrated a good, easily replicable and measurable mouse model of oxygen induced retinopathy. One week old C57BL/6J mice were put in 75% oxygen chambers for 5 d and then brought back to normal atmospheric air. Vascular pattern was assessed using a fluorescein-dextran perfusion method. The abnormal neovascularization was measured by counting the nuclei of new vessels extending from the retina into the vitreous in 6 μ sagittal cross section[9]. Fluorescein-dextran angiography highlighted the entire retinal vasculature, including the neovascularization. Hyperoxia induced new vessels occurred at the junction between vascularised and avascular retina in the mid periphery[9]. Retinal neovascularisation was seen between day 17 and day 21, postnatally. Thus from this study it was concluded that, neovascularisation was seen after loss of patent vessels in the central retina with hyperoxia exposure. A shift from hyperoxia to room air causes relative ischemia in the non-perfused retina and the development of neovascularisation was seen at the interface of perfused and non-perfused retina.
Epidemics in ROP
A variation in number of cases was seen in different era and in different countries. This was termed as epidemics in ROP. What actually triggered the beginning of first epidemic was unmonitored oxygen supplementation in the late 1940’s and 1950’s in Europe and North America[10,11]. After this incident, overuse of oxygen was stopped and careful administration of oxygen was recommended. Second epidemic was faced by the developed countries, in premature and low birth weight babies (< 1000 g at birth)[12]. India and the other developing countries come under the third epidemic which is characterized by severe ROP in bigger premature babies[13]. The reason again being lack of proper neonatal care and improper oxygen administration. Hence there is a need for strict guidelines of oxygen administration and monitoring and neonatologist play a major role in this aspect.
PRESENT
Classification
A committee for ROP classification was formed in 1984, which proposed an international classification of ROP (ICROP) by dividing the retina into three zones, extending from posterior to anterior retina and describing the extent of ROP in clock-hours of involvement[14]. However with the advances in retinal imaging techniques, a revised ICROP classification was put forth which described the zones better[15].
Zones
Three concentric zones, centered on the retina define the antero-posterior location of retinopathy.
Zone I: With optic disc as the center, and twice the distance from the disc to fovea, the circle formed is zone I. Using a 25 or 28 diopter (D)-condensing lens, when the nasal edge of the optic disc is kept at one edge, the temporal field of view is zone I extent.
Zone II: It starts from the edge of zone I and extends till the ora serrata nasally, with a corresponding area temporally.
Zone III: Zone III is the remaining crescent of retina temporally.
Extent of retinopathy
The extent of the ROP is documented by the number of clock hours involved. For the observer examining each eye, the temporal side of the right eye is 9 o’clock and that of the left eye is 3 o’clock and vice versa.
Stages of ROP
It denotes the degree of vascular changes. There are five stages.
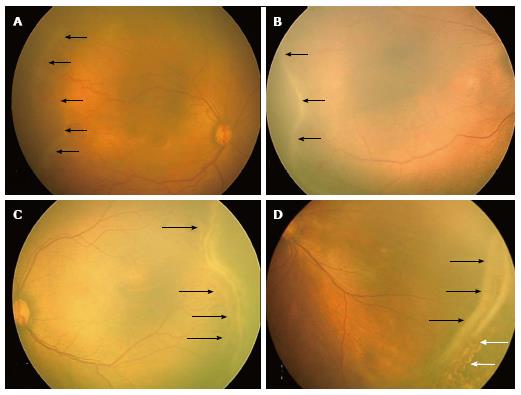
Stage 1 - demarcation line: A demarcation line is seen between the vascular and avascular retina. It is a thin structure that lies in the plane of the retina (Figure 1A).
Figure 1 RetCam fundus images showing retinopathy of prematurity stages 1, 2, 3 and 4A.
A: Fundus image of right eye showing stage 1 ROP with demarcation line (black arrows); B: Fundus image of right eye showing stage 2 ROP with ridge (black arrows); C: Fundus image of left eye showing stage 3 extra retinal fibrovascular proliferation (black arrows); D: Fundus picture of left eye showing stage 4A partial retinal detachment not involving the fovea (black arrows). Laser scars are shown with white arrows. ROP: Retinopathy of prematurity.
Stage 2 - ridge: The demarcation line grows to occupy a volume and has a height and width to form a ridge above the plane of retina (Figure 1B). Small tufts of new vessels also called as “popcorn” vessels may be seen posterior to the ridge.
Stage 3 - ridge with extra retinal fibrovascular proliferation: In this stage extraretinal fibrovascular tissue is seen arising from the ridge into the vitreous (Figure 1C). It may be continuous or non-continuous and is posterior to the ridge.
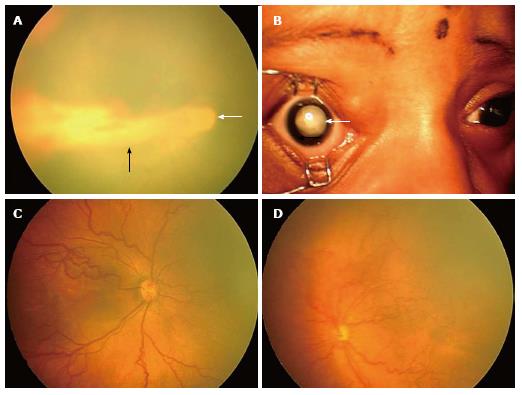
Stage 4 - subtotal retinal detachment: Here a partial detachment of the retina is seen which may be exudative or tractional. It is sub divided into the following: (1) Partial retinal detachment not involving the fovea (stage 4A) (Figure 1D); and (2) Partial retinal detachment involving the fovea (stage 4B) (Figure 2A).
Figure 2 RetCam fundus images showing retinopathy of prematurity stages 4B, 5, plus disease and aggressive posterior-retinopathy of prematurity.
A: Fundus picture of right eye showing stage 4B partial retinal detachment involving the fovea (black arrow). Optic disc is shown with a white arrow; B: Anterior segment picture of right eye showing stage 5 ROP with total retinal detachment (white arrow); C: Fundus picture of right eye showing dilated and tortuous vessels suggestive of plus disease; D: Fundus picture of left eye showing aggressive posterior ROP. ROP: Retinopathy of prematurity.
Stage 5 - total retinal detachment: Here a total retinal detachment is seen as child usually presents with leukocoria (white pupillary reflex) (Figure 2B).
Plus disease: It is an indicator of severity of the disease and is defined as venous dilation and arterial tortuosity of the posterior pole vessels (Figure 2C).
Pre-plus disease: It is defined as posterior pole vascular dilation and tortuosity which is more than normal but less than plus disease.
Aggressive posterior ROP: This refers to an uncommon, rapidly progressive, form of ROP previously referred to as “rush disease”. It is characterized by a posterior location, severe plus disease, and flat intraretinal neovascularization (Figure 2D). It can progress very fast to stage 5 ROP and blindness, if not intervened early. The flat neovascularization can be quite subtle and can easily confuse less experienced examiners.
Screening for ROP - present concept
Worldwide ROP is amongst the leading causes for childhood blindness[16]. Early detection and timely intervention to reduce this burden of blindness, makes screening an important aspect of ROP.
Screening is a process of identifying disease in the apparently normal subjects who are at risk by applying simple, safe, repeatable, sensitive and valid tests for disease detection. Due to lack of gold standard tests for ROP, the screening process may also be referred as “case detection initiative”[16]. The neonatal care for each country needs to be understood as ROP is diverse in presentation owing to the geographic variations, available infrastructure and altered temporal development of retinopathy in different locations in the retina[17]. Screening ultimately aims at reducing the incidence of ROP, prompt case detection and optimal treatment for ROP, thereby reducing the severity and overall burden of childhood blindness.
Eighty percent of infants with birth weight less than 1500 g born in the United Kingdom survive and the incidence of stage 3 ROP of approximately 8% to 10% has been reported[18]. Thus all babies having gestational age ≤ 31 wk or ≤ 1500 g are screened in United Kingdom[18]. American guidelines given by the American Academy of Pediatrics state that, infants with a birth weight ≤ 1500 g or gestational age of ≤ 30 wk and selected infants with a birth weight between 1500 and 2000 g or gestational age of more than 30 wk with an unstable clinical course, should be screened for ROP[19].
In many developing economies, larger babies with a birth weight between 1500 and 2000 g may also develop ROP[20]. Hence in counties like India, a birth weight ≤ 1750 g and/or gestational age of ≤ 34 wk may be used as a cut-off for ROP screening. Bigger babies with a gestational age of 34 to 36 wk gestation or a birth weight between 1750 and 2000 g should also be screened if child has a stormy neonatal course[20]. New Zealand has reported a reduced incidence in ROP due to progress in the screening and clinical management and recommends screening criteria of < 31 wk’ gestation or < 1250 g to be sufficient[21]. Other risk factors for ROP include severe respiratory distress syndrome, anemia, neonatal sepsis, thrombocytopenia, multiple blood transfusions and apnea. If these risk factors are not seriously taken into consideration, affected infants may inadvertently get excluded and hence careful review for risk factors should be taken by the pediatrician.
ROP screening should start by 31 wk postconceptional age or 4 wk after birth, whichever is later[22]. In developing countries some babies may develop early aggressive posterior (AP)-ROP[23,24]. Thus, in developing countries, to enable early identification and treatment of AP-ROP, infants < 28 wk or < 1200 g birth weight should be screened relatively earlier at 2-3 wk of age[25].
Examination technique: The examination technique traditionally involves two steps namely the dilatation of pupil and indirect ophthalmoscopy preferably with a 28D lens. It is preferred to perform pupillary dilatation 45 min prior to commencement of the screening. Dilating drops used are a mixture of cyclopentolate (0.5%) and phenylephrine (2.5%) drops to be applied two to three times about 10-15 min apart. Alternatively, tropicamide (0.4%) may be used instead of cyclopentolate. Diluted cyclopentolate may also be used to reduce probable systemic adverse effects. Use of atropine is to be avoided. The neonatal nurse should be instructed to wife any excess drops from the eye lid to prevent systemic absorption and complications like tachycardia and hyperthermia. If the pupil is resistant to dilatation, it may indicate presence of persistent iris vessels (tunica vasculosa lentis) and must be confirmed by the ophthalmologist before applying more drops.
The United Kingdom guidelines do not mandate use of eye speculum (e.g., Barraquer, Sauer, Alfonso specula) and scleral depression (e.g., Flynn depressor) with topical anaesthesia. However, meticulous examination, warrants its use.
Present screening tools: ROP screening today follows a telemedicine approach which refers to use of information technology between participants who are geographically separated and offers a possible solution to screening challenges and aids effective management. There are no reports requiring on-site diagnostic examination by an ophthalmologist even if images have not appropriately identified severe retinopathy[26,27]. Retinal examination of infants at risk for ROP using the RetCam digital camera system using wide angle lens with interchangeable high magnification lenses allows photographic documentation permitting remote interpretation of images and is increasingly being used for telemedicine world over[28-31]. But this telescreening is advisable only in places where no ophthalmologist is available for bed side screening, as a recent review showed that digital imaging screening cannot replace indirect ophthalmoscopy[32].
Predictive factors for ROP progression which include postnatal weight gain, serum insulin-like growth factor 1 (IGF-1) levels, and quantifiable vessel changes in the retina can reliably be isolated and used to indicate presence or absence of disease. The Weight, IGF-1 levels, Neonatal, ROP (WINROP) study[33] carried out weekly measurements of IGF-1 levels and weekly weight from birth until 36 wk. WINROP correctly identified all infants with a low risk ROP and those requiring laser treatments for proliferative ROP on the basis of predictive factors.
The rapid advances in technologies and increasing knowledge about disease and genetics along with the growing need for efficient, effective, and timely ROP evaluations may completely transform the present diagnostic approach in near future.
Treatment for ROP - present concept
Although the ICROP classification gave a detailed classification of ROP, it never recommended when to treat ROP. Following are the treatment stages of ROP.
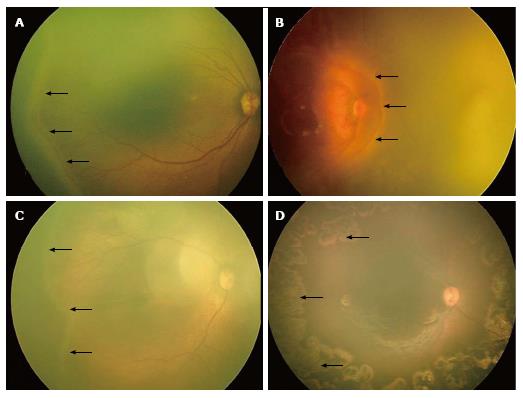
Threshold ROP: The cryotherapy for retinopathy of prematurity (CRYO-ROP) study[34] stated that treatment should be imparted to eyes with threshold disease, defined as stage 3 ROP in zone I or II, having five contiguous or eight discontiguous clock hours with plus disease (Figure 3A). This was the previous “cut off” for treatment.
Figure 3 RetCam fundus images showing threshold, type 1, type 2 retinopathy of prematurity and post laser regressed retinopathy of prematurity.
A: Fundus image of right eye showing “threshold ROP” (black arrows); B: Fundus image of right eye showing stage 3 ROP in zone 1 (black arrows) with plus disease suggestive of “type 1 ROP”; C: Fundus image of right eye showing stage 2 ROP in zone 2 (black arrows) without plus disease suggestive of “type 2 ROP”; D: Fundus picture of right eye showing laser scars (black arrows). ROP: Retinopathy of prematurity.
Pre-threshold ROP: The early treatment for retinopathy of prematurity (ETROP) study[35] redefined these guidelines. They defined the actively treatable and observational types of pre-threshold ROP as “type 1” (high-risk prethreshold ROP) and “type 2” ROP respectively. “Type 1 ROP” is defined as: (1) Any stage of ROP in zone I with plus disease (Figure 3B); or (2) Stage 3 in zone I without plus; or (3) Stages 2 or 3 in zone II with plus disease. These are the modified guidelines for treatment. “Type 2 ROP” is defined as stages 1 or 2 in zone I (Figure 3C) without plus, or stage 3 in zone II without plus. These can be observed and watched at one week or less follow-up. Cases having stages 1 or 2 in zone II require two weekly follow up, while stages 1 or 2 in zone III require three weekly follow-up[19].
Treatment modalities
Cryotherapy: This involves treatment of the avascular retina using of a cryoprobe in order to reduce unfavorable outcomes of ROP like retinal folds and retinal detachment. Cryotherapy however is stressful for the babies, requires general anesthesia and creates lot of periocular inflammation. It is therefore no more the treatment of choice.
Indirect laser photocoagulation: Laser photocoagulation of the peripheral retina using indirect delivery system has proved to be the gold standard, time tested and successful means of treatment since many years[33,36,37]. Laser photocoagulation using infra red diode laser forms a portable mode of treatment and can be performed in the nursery by skilled professionals (Figure 3D). The biggest advantage is that it can be done under topical anesthesia. However many institutions prefer general anesthesia for patient comfort. Laser ablation covers the relatively hypoxic retina into anoxic, thereby reducing stimulus for new vessel formation and disease progression. The ETROP study from its six years analysis confirmed that eyes with type 1 ROP benefited from laser treatment at high risk pre threshold stage[38]. This failure rate of 9.6%, was better than the results shown by the CRYO-ROP study.
Cryotherapy or laser photocoagulation, ablation has its own demerits and causes destruction of the retina amounting to significant visual field loss. Pharmacologic therapy is thus ushering a new era of ROP management.
Anti-vascular endothelial growth factors drugs: Anti-vascular endothelial growth factor (VEGF) drugs directly block the effects of VEGF, and a single intravitreal injection is less time consuming and less expensive as compared to lasers. Exceptionally successful results with anti-VEGF drugs in adult retinal vascular diseases led to its trial in paediatric retinopathy as a monotherapy as well as in combination with lasers. Intravitreal bevacizumab as an initial mono therapy was reported to cause regression of type 1 ROP in 88% cases with 9% requiring additional laser treatment and 1% requiring additional injection[39]. The BEAT-ROP (Bevacizumab Eliminates the Angiogenic Threat of ROP) study[40] is the only randomised trial done comparing anti-VEGF vs conventional laser. It suggested superiority of anti-VEGF treatment over conventional laser therapy for stage 3+ ROP in zone I. Superiority in severe ROP in zone II could not be established due to inadequate sample size. Safety is a major concern with use of anti-VEGF drugs in paediatric age group and this study could not prove it because of a short follow up. Recent studies also shown that that systemic VEGF levels remain suppressed for 8 wk after intravitreous bevacizumab injection[41].
Regarding the best approach, laser treatment is still the gold standard and anti-VEGF therapy should be tried only in selected cases.
Surgical management is reserved for advanced stages of ROP (stages 4 and 5). The stage of ROP and features specific to each eyes guide the choice of surgical technique. It is shown that best anatomical and visual outcome can be attained if surgical intervention is done at 4A ROP as it halts progression to worse stages[42]. The surgical options available for stage 4 ROP are lens sparing vitrectomy or scleral buckling. For stage 5, vitrectomy with lensectomy or open sky vitrectomy can be performed. Visual outcome for stages 4B and 5 is very poor and can lead to permanent visual impairment[42].
Periodic follow up and the burden of visual morbidity then become the prime concerns after the retinopathy is adequately treated. Visual rehabilitation can be achieved only through an integrated coordination between the pediatricians, ophthalmologists, paramedicals and parents. With the advances in screening tools, it may be hoped that occurrence of severe retinopathy or severe visual morbidity from ROP may be reduced in future.
FUTURE
Future trends in screening of ROP
Newer predictor of ROP: Timely screening of ROP is crucial for early management and improved outcomes. Current screening guidelines use only two most important risk factors gestational age and birth weight, and not the post-natal factors. However only approximately 10% of the premature babies screened need treatment[43]. Various neonatal scoring systems such as clinical risk index for babies, scores for neonatal acute physiology (SNAP), and SNAP-perinatal extension-II have also been attempted to predict ROP, but none showed sufficient power to predict severe ROP[44]. Thus there is a need for improvement of the current screening protocols by developing new better predictors to reduce the number of ROP screening examinations[44].
Low weight gain proportion: Currently, low weight gain by six weeks of life after premature birth is being accepted as a risk factor for causing ROP. Proportion of the weight gain is defined as the weight at 6 wk of life minus the birth weight divided by the birth weight. Low weight gain proportion, i.e., weight gain less than 50% of the birth weight in the first 6 wk of life is being considered superior to birth weight and gestational age alone as predictors for severe ROP[45,46]. In order to develop an efficient clinical prediction model, Binenbaum et al[47] found that a birth weight-gestational age-weight gain model could reduce the need for examinations by 30% in a high-risk cohort, while still identifying all infants requiring laser therapy.
WINROP algorithm: A surveillance algorithm WINROP was developed by Löfqvist et al[33] to detect infants at risk for developing severe ROP. WINROP is based on the weekly measurement of body weight and serum IGF-1 level from birth until postconceptional age of 36 wk. In their first prospective study, which included 50 preterm infants, the WINROP algorithm could identify all preterm babies diagnosed with severe ROP later. Since then WINROP algorithm has been validated in different cohorts of many countries with sensitivity ranging from 85% to 100%[48-51]. These studies have validated WINROP algorithm as a useful ROP screening tool that can be used to focus care on those at high risk for ROP. Currently, WINROP is being tested in a large multi-center multinational trial to validate it as universal screening tool.
ROPScore: ROPScore is based on birth weight, gestational age, weight gain and blood transfusions from birth to 6th week of life and use of oxygen. Eckert et al[44] initially analyzed 16 variables and established this score after linear regression. The study with 474 patients, and the area under the receiver operating characteristic curve for the score were 0.77 and 0.88 to predict any stage and severe ROP respectively. They concluded ROPScore as a promising tool which maybe more predictable than birth weight and gestational age in predicting the occurrence of ROP in very low birth weight preterm infants. Also, the score is easy enough to be routinely used by ophthalmologists or the nursing staff during screening for ROP.
IGF-1: Apart from use of IGF-1 in WINROP algorithm, the usefulness of IGF-1 level was evaluated in a prospective study by Pérez-Muñuzuri et al[52] They studied 74 premature newborn babies and concluded that determination of IGF-1 serum levels in the 3rd week post-partum, is a good prognostic tool to identify babies that are at a high risk of developing ROP.
Plasma soluble E-selectin: Elevated plasma soluble E-selectin (sE-selectin) levels have been found to have an association with ROP and have been reported as independent risk predictor for ROP by Pieh et al[53]. They concluded that a score based on the gestational age of the preterm child and sE-selectin plasma levels would improve prediction of ROP. Increase of 10 ng/mL increases the ROP risk by 1.6 fold. For this purpose, plasma concentrations should be assessed 2 to 3 wk after birth, in premature infants.
Thus in the future, new screening tools would be developed with a hope to reduce the burden of ROP screening on the ophthalmologist and also reduce these stressful examinations on the preterm babies. Further studies are needed to validate the usefulness of these predictors. Once validated, these post-natal variables can be used successfully for early prediction of severe ROP.
Telescreening: Timely referral by pediatricians and meticulous examination by an experienced ophthalmologist is the gold standard for ROP screening. Digital retinal imaging is emerging as an important tool for ROP screening. Non-ophthalmologists like the neonatal nurses and technicians are being trained to use these digital imaging devices effectively. Pediatric ophthalmologists’ services can be extended to the remote areas by electronic transfer of the images captured by these paramedical staffs[54]. Daniel et al[55] validated that remote evaluation of the digital retinal images by trained technicians taken by them can reduce referral warranted ROP. The result of these studies suggest that telescreening provides future strategies for outreach ROP screening and will allow access of diagnostic expertise to underserved areas in developing as well as developed countries[56].
Optical coherence tomography: It is an imaging tool which gives cross sectional images of the retina and has been extensively used in adults. Although it is not widely used in ROP, this technology is already providing new insights at a cellular and subcellular level into normal retinal development, the acute ROP process, and its long-term sequelae[57].
Newer therapeutic modalities for ROP
Anti-VEGF, systemic propranolol, IGF-1 replacement, granulocyte colony stimulating factor, Jun kinase inhibitor and omega-3 polyunsaturated fatty acid supplementation are the newer preventive strategies being evaluated through insights into the molecular pathogenesis of ROP in animal studies[58]. Newer emerging therapeutic options have the potential to complement current therapies and improve treatment outcomes. However, any new therapeutic option must be thoroughly evaluated before existing treatment paradigms can be modified, as these newer agents are mostly systemically administered and may have unknown widespread side effects.
Anti-VEGF: Recently various anti-VEGF agents are being evaluated as promising treatment modality for various stages of ROP. Bevacizumab is the most widely used anti-VEGF for treatment of acute ROP since 2007, and evidences from case reports and small studies suggest that intravitreal bevacizumab monotherapy may be a viable first-line treatment for select cases of ROP[58]. Other anti-VEGF agents are also being evaluated as adjunctive or alternate therapy. A recent study showed that administration of intravitreal pegaptanib along with laser is useful therapy with stable regression of ROP in 90% of eyes compared to 61% of only laser treated eyes[59]. Recently one prospective nonrandomized interventional case series study has evaluated 1-year outcomes of intravitreal aflibercept injection in 26 eyes with type 1 prethreshold ROP and found favorable anatomical and visual outcome in 96% and 80% eyes[60]. The efficacy of ranibizumab and bevacizumab for the regression of ROP have been compared and found similar in retrospective studies. However, high myopia was more prevalent in the bevacizumab-treated eyes, while reactivation rate was significantly higher following treatment with ranibizumab, probably due to shorter half-life[61,62]. Lutty et al[63] studied the effect of VEGF trap on normal retinal vascular development and oxygen-induced retinopathy in dog and concluded that it inhibits the retinal neovascularisation, however dose selection is an important variable as higher doses also inhibit vasculogenesis or retinal revascularization.
In future, intravitreal anti-VEGF injection may become the first choice treatment replacing laser therapy for zone I stage 3 ROP or cases with media opacity, if efficacy and safety are validated. Also anti-VEGF can be considered as an adjunctive therapy in patients treated with laser photocoagulation or vitrectomy.
Propranolol: Reports of use of systemic propranolol for an effective treatment of infantile hemangioma resulted in exploration of anti-angiogenic role of propranolol in ROP. A study on oxygen-induced retinopathy in a mouse model showed that propranolol decreases VEGF overproduction in the hypoxic retina. In addition, beta-AR blockade has no effect on VEGF levels in the heart, brain or lungs, as VEGF expression is these organs is independent of hypoxia[64]. Based on these findings, the safety and efficacy of propranolol in newborns with ROP (PROP-ROP) study[65], was conducted. It evaluated safety and efficacy of oral propranolol given to preterm newborns infants having early stages of ROP. In this study, 26 preterm babies with stage 2 ROP treated with oral propranolol (0.25 or 0.5 mg/kg per 6 h) showed less progression to stage 3 or stage 3 plus and a 100% relative reduction of risk for progression to stage 4. However serious adverse effects like bradycardia and hypotension were observed in about 20% of infants treated with propranolol, and the study was halted due to increased mortality in the treatment arm[65]. Recently, an experimental study reported failure of propranolol treatment to suppress retinopathy development in mice[66]. Thus, the safety of oral propranolol to the vulnerable premature infants is uncertain, and further studies including animal as well as prospective clinical trial are needed.
IGF: IGF plays an important role in fetal development during pregnancy. The levels IGF-1 rise significantly during the 3rd trimester of pregnancy, and it controls VEGF-mediated vascular growth in the retina[67]. However, IGF-1 levels fall rapidly after preterm birth, and prolonged period of low IGF-1 in preterm children have been reported to be associated with development of ROP. Conversely, normal vessel development occurs, if the IGF-1 levels are sufficient after birth[68]. Recent studies have shown that intravenous administration of recombinant IGF-1 (rhIGF-1) with its binding protein 3 (rhIGFBP-3) to premature infants increases the serum concentrations of IGF-1 and IGFBP-3, and is found to be safe[69,70]. Can et al[71] studied the effect of early aggressive parenteral nutrition (APN) vs conservative nutrition and found that IGF-1 levels were higher in the APN group.
Granulocyte colony stimulating factor: The potential role of granulocyte colony stimulating factor (G-CSF), a biologic cytokine commonly used to increase leukocyte count in neutropenic patients, is currently being evaluated to prevent ROP[72]. In a retrospective review of 213 neonates who received G-CSF for non-ophthalmic indications, Bhola et al[73] studied 50 infants with birth weight < 1500 g and gestational age < 32 wk. Only 10% of the infants who received G-CSF required laser compared to 18.6% in the control group. However the observed difference was not statistically significant. Another retrospective study determined the vitreous level of 27 types of cytokines in eyes with ROP, and levels of 6 cytokines including G-CSF were found significantly higher (P < 0.05) in eyes with ROP compared to the control group[74]. Recently, in an animal study of oxygen-induced retinopathy, G-CSF significantly reduced the vascular obliteration (P < 0.01) and neovascular tissue formation (P < 0.01) mainly by increasing levels of IGF-1[75]. The results of these studies suggest a potential role of G-CSF in ROP prevention, however further studies are needed to establish the same, and to determine the dose required, side effects and safety.
Omega-3 polyunsaturated fatty acids: Like IGF-1, omega-3 and 6 polyunsaturated fatty acids (PUFAs) are non-oxygen-regulated angiogenic factors, which are transferred from mother to the fetus in the third trimester of pregnancy. Consequently, premature newborns lack the maternal supply of PUFAs[72]. The mouse model studies of ROP have shown that omega-3 PUFA supplementation as well as an increased retinal omega-3 and omega-6 PUFA ratio result in a protective effect against pathologic retinal neovascularisation[76,77]. The protective action of omega-3 PUFA is considered to be mediated through the suppression of tumor necrosis factor-alpha[78]. These lipids supplementation can be provided through total parenteral nutrition in premature infants, and this may be an interesting therapeutic approach for ROP prevention. However, larger studies are required to establish the safety and efficacy of omega-3 PUFA supplementation therapy in premature babies.
Gene therapy: Association of mutations and polymorphism of various genes (e.g., Norrin, Frizzled 4, Lrp5) with severity of ROP or failure of treatment has been investigated in a number of small studies[79]. A recent study performed about the genetic and environmental influences on ROP in 257 infants including 38 monozygotic twins, 66 dizygotic twins, and 153 simple births found the heritability of ROP to be 0.73[80]. Interestingly, Good et al[81] demonstrated in a rat model of ROP that local gene transfer into retinal blood vessels was possible using recombinant viruses carrying genes of interest. They also found that adenovirus vector was specific to the inner retinal blood vessels and does not appear in deeper neural retina, when compared to other vectors like retroviruses and herpes virus[81]. Though no significant association between genetic abnormality and ROP has been reported till now, targeting the expression and regulation of various cytokines and growth factors involved in the pathogenesis of ROP by gene therapy appears as a promising future treatment method to restore an anti-angiogenic state[72].
Medico legal implications
Screening for ROP needs to be initiated timely after birth to prevent blindness. It is the responsibility of the caring pediatrician to initiate screening by referring to an ophthalmologist and it is the responsibility of the ophthalmologist to do correct screening and treatment. This has immense medico legal implications because if a child goes blind due to missed or late screening then the pediatrician and the ophthalmologist are at a very high risk of getting into a law suit[82,83].