Published online Nov 8, 2015. doi: 10.5409/wjcp.v4.i4.160
Peer-review started: July 15, 2015
First decision: July 31, 2015
Revised: September 12, 2015
Accepted: September 29, 2015
Article in press: September 30, 2015
Published online: November 8, 2015
Processing time: 118 Days and 22.1 Hours
AIM: To study that inflammatory fibroid polyps (IFPs) in children are extremely uncommon tumors that may occur throughout the gastrointestinal tract.
METHODS: A systematic review of the pediatric literature and a report of a new case of IFP is also presented. The PubMed database was searched for original studies on pediatric IFPs since 1960, according to “Preferred reporting items for systematic reviews and meta-analyses” guidelines for systematic reviews.
RESULTS: Five studies were finally enclosed, encompassing 6 children with IFPs (mean age 64 mo). Tumors were located in the stomach (2 patients), in the small bowel (2 patients), in the rectum (1 patient) and in the colon (1 patient). Open surgery was performed in all patients and complete excision of the mass was achieved in all cases. All patients are alive and free of symptom. Authors described a further case of a 3-year-old boy with a large duodenal IFP, in whom the tumor was removed by “en block resection”. The presence of IFP throughout the gastrointestinal tract and its variable clinical appearances make it difficult to diagnose. An accurate pre-operative assessment is fundamental in order to differentiate IFP from other more aggressive gastrointestinal tumor, enabling unnecessary demolitive surgery.
CONCLUSION: When complete resection of the IFP is achieved, the prognosis is excellent.
Core tip: We present a new case of inflammatory fibroid polyp (IFP) occurring in a 3-year-old child who, to the best of our knowledge, is the first reported case of duodenal IFP in childhood. Below is a detailed systematic review of the literature on paediatric IFPs, focusing on etiopatogenesis, clinical presentation, diagnostic assessment and treatment.
- Citation: Righetti L, Parolini F, Cengia P, Boroni G, Cheli M, Sonzogni A, Alberti D. Inflammatory fibroid polyps in children: A new case report and a systematic review of the pediatric literature. World J Clin Pediatr 2015; 4(4): 160-166
- URL: https://www.wjgnet.com/2219-2808/full/v4/i4/160.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v4.i4.160
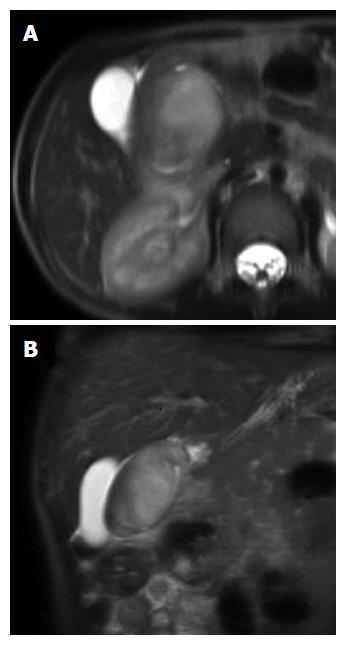
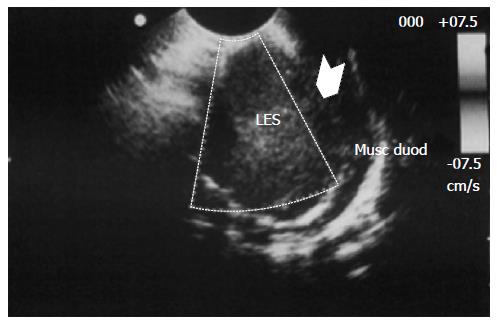
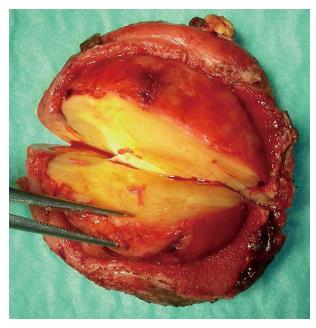
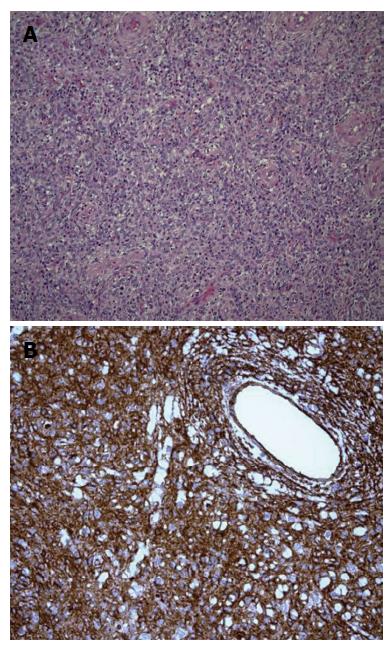
Inflammatory fibroid polyp (IFP) is an uncommon benign lesion that may occur throughout the gastrointestinal tract, even if stomach is the most affected site[1,2]. IFP natural history is unknown but rapid growth of the lesion within few months has been reported. IFP is found in all age groups but its typical presentation is in the 5th to 7th decade of life[2]; occurrence in children is extremely rare, with only 6 reported cases[3-7]. We present a new case of IFP occurring in a 3-year-old child who the best of our knowledge is the first reported case of duodenal IFP in childhood. A detailed systematic review of the literature on pediatric IFPs, focusing on ethiopatogenesis, clinical presentation, diagnostic assessment and treatment is then provided. A 3-year-old boy was referred to our Centre because of asthenia and anorexia. Physical examination was unremarkable. Blood test revealed a microcytic hypochromic anemia and eosinophilia; stools were positive both for heme and Enterobius Vermicularis. Upper GI endoscopy showed a 3.7 cm peduncolated mass of the duodenum. Contrast enhanced Abdominal MRI detected homogeneous enhancement of the mass after contrast (Figure 1). The tumor was close to the pancreatic head, but the common bile duct and the papilla major were spared. Endoscopic ultrasound (EUS), showed an hypoechoic mass located into the submucosa (echo-layer 3), not completely separated from the muscularis propria (echo-layer 4). In some EUS scans, the lesion showed features suggesting origin from the muscular layer, thus mimicking gastrointestinal stromal tumors (GIST) features (Figure 2). Cytological analysis of EUS fine needle aspiration (FNA) was compatible with diagnosis of leiomyoma. At laparotomy the mass deformed the postero-lateral wall of the proximal duodenum; the sero-muscular wall at tumor level was longitudinally severed but the lack of a cleavage with the submucosa did not allow local tumor excision. Intraoperative histological examination showed mesenchymal spindle cells proliferation with an inflammatory eosinophilic infiltrate, without morphological features suggesting malignancy. The tumor was completely removed by partial en block duodenectomy (Figure 3), followed by a duodenal inverted Y plasty. Histological findings were compatible with diagnosis of IFP: stromal looking cells with marked inflammatory eosinophilic infiltrates was evident, with no presence of mitosis or necrotic areas (Figure 4). The lesion was immunoistochemically reactive to vimentin (Figure 4) and CD34, and negative to citocheratins AE1-AE3 and CAM-5.2, LCA, CD20, CD3, ALK1, CD21, CD23, CD35, CD117, S100, CD1a, muscular markers (desmin, smooth and striated muscle), neuro-endocrine markers (cromagranin, sinaptofisin, CD56), D2-40, EMA, Mieloperossidase, CD99, GFAP. Proliferative index Ki67 was 2%. H. pylori research was negative. The postoperative course was unremarkable, and the patient was discharged home on post-operative day-7. Radio-allergo-absorbent test 6 mo later was positive for cow’s milk protein allergy. At 4-year-follow-up the child is doing well.
For this systematic review we adhered to Preferred reporting items for systematic reviews and meta-analyses guidelines[8,9]. The PubMed database was searched for studies on IFP that were published since 1960. The date of the last search was December 2014. Inclusion criteria were English articles that reported original data on IFPs in pediatric setting. Eligible study designs were case report, case series and review. We omitted reports in which titles or abstracts indicated that they were on adult population (> 18 years) and they not clearly reported the method of diagnosis and treatment. We then evaluated the full text of the passed articles. Titles and abstracts of identified publications were checked and reviewed against the predefined inclusion criteria, and afterward, the full text articles was similarly assessed for eligibility.
Two independent authors extracted information related to the study. Methodological quality of the studies was assessed with the level of evidence and the strength of guideline recommendations in diagnosis scales[8]. For each study, data were extracted for age at presentation, sex, clinical presentation, size and location, diagnostic assessment, treatment, pathological examination and outcome.
The initial PubMed search yielded 126 potentially relevant articles. Finally, 5 eligible articles met all the inclusion criteria and were enclosed in the review, encompassing a total of 6 cases. All reviewed studies were case reports (classes of evidence III and rating scales of evidence E)[8].
In this series, IFPs affected both sex equally. Mean age of presentation was 64 (SD 2.16) mo, with a different distribution throughout the GI tract: in 2 patients (33%) IFP arose in the stomach, in 2 (33%) in the small bowel, and in remaining 2 patients, one IFP was in the rectum and one in the colon. In 2 patients (33%) the polyp was pedunculated, in 2 (33%) was sessile, and endoexophytic in the remaining 2 (Table 1).
| Ref. | Sex | Age (yr) | Clinical/laboratory | Diagnostic tools | Location | Size (cm) | Morphology | Treatment | Histology/immunohistochemistry | Follow-up |
| Samter et al[3] | Male | 4 | Acute abdomen | X-ray | Transverse colon | 3.5 × 3.5 × 3 | Peduncolated | Resection of transverse colon (7 cm), including a perforated diverticulum proximal to an obstructive intraluminal polyp | Granulation tissue containing an infiltrate of plasma cells Europhiles, lymphocytes and eosinophils; the predominant cells were stellate and spindle-shaped cells with plump vesicular nuclei an even chromatin distribution; numerous blood vessels | Alive and free from disease |
| Samter et al[3] | Female | 8 | Vomiting, diarrhea, cherry colored stools, palpable mass in RLQ1, hypochromic anemia | X-ray | Jejunum | 5 × 3.7 × 3 | Sessile | Resection of jejunum (12 cm) | Closely packed collagen fibers, and spindle-shaped cells with vesicular nuclei; inflammatory infiltrated composed by neutrophils, lymphocytes and eosinophils | Alive and free from disease |
| Persoff et al[4] | Male | 3 | Intermittent crampy abdominal pain and vomiting | X-ray Upper gastrointestinal series | Ileum | 3 | Peduncolated | Resection of ileum, including an ileo-ileal intussusception on intraluminal polyp | Proliferating edematous fibro vascular tissue diffusely infiltrated with inflammatory cells, many of with eosinophils, that involved all layers of the bowel wall except the mucosa | Alive and free from disease |
| Pollice et al[5] | Male | 8 | Recurrent enterorrhagy, anemia | Colonoscopy | Rectum | 3 × 1.5 | Sessile | Resection of rectum (7 cm) | Fibrous connective tissue with collagenous fibers arranged in large bundles; the inflammatory infiltrates consisted mainly of plasma cells and lymphocytes; numerous thin walled capillaries, sometimes dilated | Alive and free from disease |
| Schroeder et al[6] | Female | 5 | Epigastric pain, weakness, splenomegaly, hypocromic anemia | Ultrasound, | Stomach | Several cm | Endoexophytic | Subtotal gastrectomy | Not reported | Alive and free from disease |
| CT | ||||||||||
| Chongsrisawat et al[7] | Female | 4 | Fever, arthralgia and severe anemia (Hb 3.9 g/dL) | Upper gastrointestinal series | Stomach | 5 × 8 | Sessile | Partial gastrectomy | Proliferative fibroblast and blood vessels admixed with mixed inflammatory cell infiltrated in stroma | Alive and free from disease |
| Our patient | Male | 3 | Asthenia, anorexia, hypochromic anemia, eoshinophylia | Upper GI endoscopy MRI EUS with FNA | Duodenum | 3.7 | Sessile | Partial “en bloc” with the tumor duodenectomy | Inflammatory infiltrates rich in eosinophils and stromal spindle-shaped cells, with no evidence of mitosis and necrotic areas | Alive and free from disease |
Abdominal pain is the most common referred symptom, although physical examination was generally unremarkable in this series, except for in an 8-year-old girl who displayed a palpable mass in right lower quadrant[3]. Chronic anemia was the most common sign, present in 4 patients (67%). Recurrent enterhorragy and vomiting were also frequent. Chongsrisawat et al[7] reported a 4-year-old girl with atypical presentation, with chronic fever, arthralgia of knees and ankles, iron deficiency anemia, and hypoalbuminemia. One patient presented intermittent crampy abdominal pain and vomiting, and ileo-ileal intussusception was subsequently diagnosed[4]. Samter et al[3] reported one case of peritonitis caused by perforation of colonic IFP.
Considering the diagnostic assessment, peripheral blood eosinophilia was never reported in this series. Although abdominal X-ray may provide some clue of the disease, gastrointestinal endoscopy and EUS was the preferred method for IFP diagnosis in adult population.
Most of the studies in adult population agreed that complete resection is the treatment of choice for IFPs. Nevertheless in this pediatric series all the masses were larger than 3 cm of diameter, and no endoscopic polypectomy was performed (Table 1). Open surgery was performed in all patients, and complete excision of the mass was achieved in all cases (Table 1). The 2 patients with gastric IFP underwent partial gastrectomy. The patients with IFP arising in the small bowel underwent resection and primary anastomosis. The patient with colonic IFP underwent colonic resection and primary colo-colonic anastomosis[7]. The remnant patient underwent resection (7 cm) of the rectum. All but one studies[6] reported data on pathological examination. A variable grade of inflammatory infiltrate was evident in all patients, while eosinophilic infiltrate was present in three (50%). At follow-up all patients are alive and present no symptom recurrence.
IFP was firstly described by Vanek[1] in 1949. Although at least 1000 IFP cases have been reported in literature, only 6 cases have been described in pediatric patients (Table 1)[3-7]. Both sexes appear equally affected. Mean age of presentation was 5 ± 2 years (range 3-8), IFPs were about equally distributed throughout the GI tract: 29% respectively into the stomach, small bowel and colon-rectum and 13% into the duodenum. That differs from adults in whom 70% of cases were found in the stomach[2-8].
IFPs present in a variety of ways depending on their size, location and adjacencies. Polyps in the stomach usually cause pyloric obstruction, while IFPs located on the small bowel cause chronic episodes of colicky abdominal pain, lower gastrointestinal bleeding, anemia and intermittent episodes of intestinal bowel intussusceptions[1-3]. In pediatric population the most frequent symptom was asthenia and anemia related to the mass bleeding, but they are unspecific findings[1-5]. Rupture of colonic IFP has been described in a 4-year-old boy[3]. Physical examination is generally not conclusive (Table 1).
Etiopathogenesis of IFPs is still largely unknown. An allergic hypothesis has been firstly proposed, on the base on eosinophilic infiltrate[1]. The role of parasite or chronic H. Pylori infection has been also reported[8-11]. Although IFPs have been regarded as inflammatory and reactive phenomenon occurring in response to unknown irritants or allergic factors[1], recent data show that the spindle cells express platelet-derived growth factor receptor alpha (PDGFRA), and the majority of IFP harbor activating PDGFRA mutations configuring IFPs as true mesenchymal tumors[12].
Peripheral blood eosinophilia has been reported in adults[10,11], this finding was also observed in our patient, but it was never described in the other children of the series (Table 1). Ultrasonography (US) and computed tomography scan are usually requested; however, they fail to differentiate IFP from other more common lesions[4]. At GI Tract Endoscopy IFPs are seen as protruding intraluminal masses with a smooth and often ulcerated mucosa. As most IFPs are submucosal lesions, it is almost impossible to obtain a completely diagnostic endoscopic biopsy. EUS is the gold standard for diagnosis of IFPs in adults. The tumour presented as a hypoechogenic and homogeneous mass, located within the second or third sonographic layer of the GI tract wall, with an intact fourth layer. Ours was the first case in which EUS was performed in a child with IFP. However, EUS findings in our patient were wrongly suggestive of leiomyoma rather than IFP, due to the strong adherence of the proliferating mass to the muscular layer. These findings led us to perform FNA in order to confirm clinical suspicion and to rule out a GIST; unfortunately, as reported in the literature, the result of biopsy was non-diagnostic.
The striking features of IFPs are the characteristic arrangement of fibrous and vascular elements (“perivascular onion skinning”) associated with marked inflammatory eosinophilic infiltrate[2,8]. IFPs were suggested to show different appearance according to the different part of GI tract from which they arise. Gastric IFPs seem to arise at the base of the lamina propria, extending through and disrupting the muscularis mucosa. Ileal IFPs are intramural proliferations that push against the muscularis mucosae, eventually disrupting it and extending into the mucosa often ulcerating it. They obliterate the submucosa and muscularis propria often invading the mesentery. Gastric IFPs have less stromal edema and therefore they appear more solid in comparison with the ileal ones. Furthermore, gastric IFPs typically have also a prominent perivascular orientation of the different cells and eosinophils infiltration is more prominent than in ileal IFPs[13]. For duodenal IFPs it has been suggested they display the same histological feature of gastric IFPs; they arise in the lower layer of the lamina propria, causing splitting fraying and atrophy of the muscle wall layer[14,15]. That was observed also in our case in which the tumor was composed of stromal looking cells, embedded in a mixed matrix intermixed with marked inflammatory infiltrates extremely rich in eosinophils granulocytes. Nevertheless, in IFPs the degree of eosinophilic infiltration is variable and of doubtful significance. The different histological patterns and the variable degree of stromal eosinophilic infiltration have been correlated with the evolutive stages of the lesion. Both IFPs of the GI tract and the eosinophilic gastroenteritis have been also considerate as variants of the same disease[10,11]. Immunohistochemical investigations give little help in the diagnosis of IFPs, mainly by excluding mesenchymal and myogenic tumors that should be considered in differential diagnosis[8-10].
Complete resection is the treatment of choice for IFPs, in order to relieve symptoms and resolve diagnostic uncertainty. In adults, accessible pedunculated IFPs can be removed by endoscopy (endoscopic submucosal dissection is required), since most of the polyps are smaller than 2 cm[13,16-18]. This is uncommon in children in whom all polyps were larger than 2 cm, making endoscopic polypectomy impracticable, as in our case. As IFPs arise from the submucosa and may be sessile, endoscopic resection may result in perforation or incomplete resection in larger lesions. Surgeons should be aware of the existence of this rare benign entity, which may mimic spindle cell tumours such as GIST, leiomyoma, leiomyosarcoma and schwannoma[2-17]. EUS with fine needle biopsy might improve the accuracy of the preoperative diagnosis of duodenal IFPs, enabling local “en bloc resection” and avoiding unnecessary pancreatoduodenectomy[18]. According to the limited pediatric experience, when complete excision of the tumour is achieved, the prognosis is excellent. Unlike adults patients with IFPs, recurrence or metastazisation in children was never reported.
Inflammatory fibroid polyp (IFP) is an uncommon benign lesion that may occur throughout the gastrointestinal tract, even if stomach is the most affected site. IFP natural history is unknown but rapid growth of the lesion within few months has been reported.
IFP is found in all age groups but its typical presentation is in the 5th to 7th decade of life; occurrence in children is extremely rare, with only 6 reported cases.
The authors present a new case of IFP occurring in a 3-year-old child who the best of their knowledge is the first reported case of duodenal IFP in childhood. A detailed systematic review of the literature on pediatric IFPi is then provided.
The study provide a detailed systematic review of the literature on pediatric IFPs, focusing on ethiopatogenesis, clinical presentation, diagnostic assessment and treatment.
IFP was firstly described by Vanek in 1949. Etiopathogenesis of IFPs is still largely unknown. An allergic hypothesis has been firstly proposed, on the base on eosinophilic infiltrate. The role of parasite or chronic H. pylori infection has been also reported. Now IFP is considered a benign reactive phenomenon similar to granulomata, occurring in response to unknown irritant or allergic factors. Recently mutations in platelet-derived growth factor receptor alpha have been identified in adult patients with IFP.
The manuscript is well written and very interesting.
| 1. | Vanek J. Gastric submucosal granuloma with eosinophilic infiltration. Am J Pathol. 1949;25:397-411. [PubMed] |
| 2. | Savargaonkar P, Morgenstern N, Bhuiya T. Inflammatory fibroid polyp of the ileum causing intussusception: report of two cases with emphasis on cytologic diagnosis. Diagn Cytopathol. 2003;28:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 3. | Samter TG, Alstott DF, Kurlander GJ. Inflammatory fibroid polyps of the gastrointestinal tract. A report of 3 cases, 2 occurring in children. Am J Clin Pathol. 1966;45:420-436. [PubMed] |
| 4. | Persoff MM, Arterburn JG. Eosinophilic granuloma causing intussusception in a three year old child. Am J Surg. 1972;124:676-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Pollice L, Bufo P. Inflammatory fibroid polyp of the rectum. Pathol Res Pract. 1984;178:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Schroeder BA, Wells RG, Sty JR. Inflammatory fibroid polyp of the stomach in a child. Pediatr Radiol. 1987;17:71-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Chongsrisawat V, Yimyeam P, Wisedopas N, Viravaidya D, Poovorawan Y. Unusual manifestations of gastric inflammatory fibroid polyp in a child. World J Gastroenterol. 2004;10:460-462. [PubMed] |
| 8. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9207] [Cited by in RCA: 8336] [Article Influence: 521.0] [Reference Citation Analysis (2)] |
| 9. | Cooper HM, Hedges LV, Valentine JC, editors . The handbook of research synthesis and meta-analysis. 2nd ed. New York, NY, Russell Sage, 2009. . |
| 10. | Trillo AA, Rowden G. The histogenesis of inflammatory fibroid polyps of the gastrointestinal tract. Histopathology. 1991;19:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Wille P, Borchard F. Fibroid polyps of intestinal tract are inflammatory-reactive proliferations of CD34-positive perivascular cells. Histopathology. 1998;32:498-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Schildhaus HU, Cavlar T, Binot E, Büttner R, Wardelmann E, Merkelbach-Bruse S. Inflammatory fibroid polyps harbour mutations in the platelet-derived growth factor receptor alpha (PDGFRA) gene. J Pathol. 2008;216:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Rossi P, Montuori M, Balassone V, Ricciardi E, Anemona L, Manzelli A, Petrella G. Inflammatory fibroid polyp. A case report and review of the literature. Ann Ital Chir. 2012;83:347-351. [PubMed] |
| 14. | Kolodziejczyk P, Yao T, Tsuneyoshi M. Inflammatory fibroid polyp of the stomach. A special reference to an immunohistochemical profile of 42 cases. Am J Surg Pathol. 1993;17:1159-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Benjamin SP, Hawk WA, Turnbull RB. Fibrous inflammatory polyps of the ileum and cecum: review of five cases with emphasis on differentiation from mesenchymal neoplasm. Cancer. 1977;39:1300-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Liu TC, Lin MT, Montgomery EA, Singhi AD. Inflammatory fibroid polyps of the gastrointestinal tract: spectrum of clinical, morphologic, and immunohistochemistry features. Am J Surg Pathol. 2013;37:586-592. [PubMed] |
| 17. | Abboud B. Vanek’s tumor of the small bowel in adults. World J Gastroenterol. 2015;21:4802-4808. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Dehghani SM, Romano C, Watanabe T S- Editor: Qiu S L- Editor: A E- Editor: Jiao XK