Published online Nov 8, 2013. doi: 10.5409/wjcp.v2.i4.70
Revised: September 25, 2013
Accepted: October 15, 2013
Published online: November 8, 2013
Processing time: 102 Days and 19.3 Hours
AIM: To compare the manifestations of chest tuberculosis (TB) in pediatric and adult patients based on contrast enhanced computed tomography of chest.
METHODS: This was a retrospective study consisting of 152 patients of chest TB including 48 children and 104 adults who had undergone contrast enhanced computed tomography of chest prior to treatment. The patterns and severity of parenchymal, mediastinal and pleural manifestations were analyzed and compared among different age groups.
RESULTS: Parenchymal changes observed include consolidation, air space nodules, miliary TB, cavitation, bronchiectasis and fibrosis and these were noted in 60% of children, 71% of adolescents and 76.9% of adults. These changes were more common in right upper lobe in all age groups. There was no significant difference in the frequency of these changes (except nodules) in different age groups. Centrilobular nodules were seen less commonly in children less than 10 years (P = 0.028). Pleural effusion was noted in 28 (18.42%) patients and pericardial effusion in 8 (5.3%) patients. No significant difference in the serosal involvement is seen among children and adults. Mediastinal adenopathy was seen 70% of children, 76.3% adolescents and 76.9% of adults and paratracheal nodes were seen most frequently. Nodes had similar features (except matting) among all age groups. Matting of nodes was seen more commonly in children (P = 0.014).
CONCLUSION: Pediatric chest tuberculosis can have severe parenchymal lesions and nodal involvement similar to adults. The destructive lung changes observed in children needs immediate attention in view of the longer life span they have and hence in formulating optimal treatment strategies.
Core tip: Primary tuberculosis in children was traditionally thought to be distinct from reactivation tuberculosis in terms of location, pattern and severity. On the contrary, aggressive forms of pulmonary tuberculosis akin to adult forms are increasingly seen in pediatric clinical practice especially in adolescents. Our study revealed that similar to older patients, children with tuberculosis are equally prone to develop significant destructive lung changes with severe sequelae. Having longer life expectancy the impact is much more severe in children. Moreover, the cavitating lesions with high bacterial load make them highly infective and pose an important threat to community health.
-
Citation: Veedu PT, Bhalla AS, Vishnubhatla S, Kabra SK, Arora A, Singh D, Gupta AK. Pediatric
vs adult pulmonary tuberculosis: A retrospective computed tomography study. World J Clin Pediatr 2013; 2(4): 70-76 - URL: https://www.wjgnet.com/2219-2808/full/v2/i4/70.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v2.i4.70
Pulmonary tuberculosis (TB) is a common lung infection worldwide with higher prevalence in developing countries. It continues to be a major medical and social problem with high morbidity and mortality. TB is second only to human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) as the greatest killer worldwide due to a single infectious agent. In 2011, 8.7 million people contracted tuberculosis out of which 1.4 million died from TB. About half a million children (0-14 years) fell ill with TB, and 64000 children died from the disease in 2011[1]. The annual risk of tuberculous infection in children in developing countries is 2%-5%. About 8%-20 % of deaths due to tuberculosis occur in children[2,3].
Tuberculosis in children is mostly related to primary infection and earlier studies stated that it present with various forms of relatively less aggressive primary tuberculosis[4]. Traditionally it was thought that manifestations of primary tuberculosis are distinct from reactivation tuberculosis in terms of location and pattern[5,6]. The most common form of pediatric TB, the classical primary complex consists of a focal parenchymal lesion typically in mid-lower zones with enlarged draining hilar/paratracheal node. Other presentations of primary TB include miliary TB[7], exudative pleuritis and tracheo-bronchial TB[8,9]. However contrary to the common notion, aggressive forms of pulmonary tuberculosis akin to adult forms are increasingly seen in pediatric clinical practice especially in adolescents[10-13]. A thorough review of available literature did not reveal any comparative studies with computed tomography (CT) scan in pediatric and adult tuberculosis. Our retrospective study is aimed to compare the pulmonary manifestations of TB in pediatric and adult population.
In this retrospective study we analyzed CT records of 152 patients of pulmonary tuberculosis who underwent CT scans from November 2010 to January 2013. The diagnosis of pulmonary tuberculosis was based on clinical/ and radiographic/and pathologic criteria. Patients were presented with clinical symptoms such as cough for more than two weeks, fever, weight loss, hemoptysis or anorexia. Along with clinical features two out of four of the following criteria had to be met: (1) A positive mantoux test; (2) History of contact with a sputum positive patient of tuberculosis; (3) Radiographic findings of mycobacterium tuberculosis such as primary complex, miliary disease, cavitary lesion, or hilar adenopathy; and (4) Isolation of AFB from sputum, gastric aspirate or broncho-alveolar lavage, lymph node aspirate[14-19]. Immunocompromised patients were excluded from the study. Informed consent and clearance from the local ethical committee was not required due to the retrospective nature of the study.
CT scans were performed either on Somatom Sensation 40 (Siemens, Erlangen, Germany) or Somatom Definition Flash (Siemens healthcare, Forchheim, Germany). Images were acquired after administering intravenous nonionic iodinated contrast [Iomeron 400 (Iomeprol, Bracco, Milano, Italy), Iohexol 300 (Omnipaque, GE Health care, Ireland)] which was injected by hand with an average delay of 50-70 s and thus providing venous phase images. Adult patients were given 60 mL of contrast and pediatric dose was calculated according to the body weight not exceeding 2 mL/kg.
Scanning was performed in adults with a collimation of 1.2 mm, a pitch of 1.4:1, a 512 × 512 matrix, field view of 38 cm, 120 kVp, and 100 mAs. In children images were acquired with a smaller field of view with 120 kVp and variable mAs (on Somatom Sensation 40) and variable kVp and mAs (on Somatom Definition Flash) according to the body thickness by tube current modulation.
After acquisition images were reconstructed in lung and mediastinal windows. Lung window images were reconstructed using sharp kernel (B60f) and a wide window width of 1500 HU with centre at -600 HU. Mediastinal window images are reconstructed using smooth kernel (B30f) and window width of 400 HU with centre at 40 HU. For both lung and mediastinal windows images were reconstructed with a thickness of 5mm in adults and 3 mm in children. HRCT images were reconstructed in lung window settings using ultra-sharp kernel (U80f) with section thickness of 1.5 mm. For HRCT reconstruction interslice gap was 10 mm in adults and 8 mm in children.
Images were reviewed by two radiologists, with 15 years and 7 years of experience in thoracic imaging and interpretations made by consensus. Images were qualitatively analyzed for the presence of parenchymal changes and lymph nodal involvement. Parenchymal changes such as consolidation, centrilobular nodules, miliary nodules, bronchiectasis, cavitation and fibrosis were assessed. For analyzing the zonal predominance and bulkiness of the disease both lungs were divided into upper, mid and lower zones. Lung field from apex to carina as upper zone, carina to the level of inferior pulmonary veins as mid zone and below as lower zone. Distribution of abnormalities and total number of zones involved as a measurement of bulkiness of the disease were assessed. Mediastinal and hilar lymph nodes were assessed for the size, necrosis, matting and calcification. Other findings like pleural and pericardial effusion were noted.
For descriptive statistics the study population was divided into three groups; group A: children(less than or equal to10 years), group B: adolescents (11-18 years) and Group C: adults (above 18 years). The incidence, pattern and severity of parenchymal changes and nodal involvement were compared among the groups. They were also divided as below and above 10 years; and below and above 18 years for the determination of statistical significance. Statistical analysis was done using stata statistical software (version 12.1). χ2 test and Fisher’s exact test were used for analysis. A P value of less than 0.05 was considered as significant.
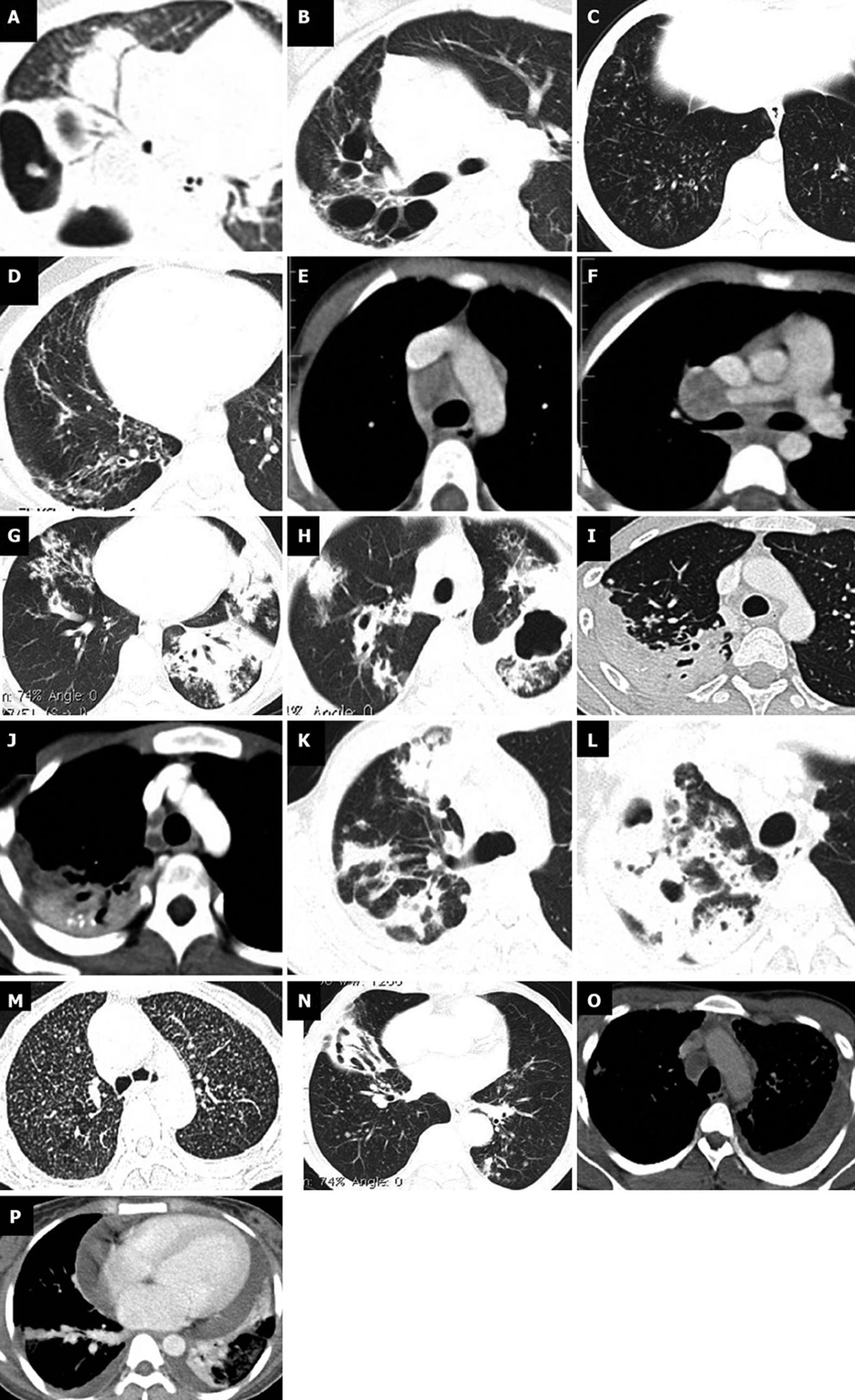
The study group included 80 males and 72 females patients ranging in age from 3 mo to 96 years (mean 30 years). They were comprised of 10 children, 38 adolescents and 104 adults. Incidence and pattern of parenchymal changes and lymph nodal involvement were analyzed and compared among different groups (Figure 1).
We compared the parenchymal changes among pediatric, adolescent and adult patients. Parenchymal lesions were noted in 6 (60%) children, 27 (71%) adolescents and 80 (76.9%) adults (Table 1). Parenchymal changes were more common in right upper followed by right middle zone in all age groups. Higher incidence of changes noted in upper and middle zones than lower zones. Left lower zone is least commonly involved in patients older than 10 years. Among the patients with parenchymal disease, multiple zones (≥ 3) were seen to be involved in 3 (30%) children, 16 (42.1%) adolescents and 45 (56.25%) adult patients (Table 1). The average number of zones involved in children, adolescent and adult patients were 2.83, 3.33 and 3.32 respectively.
| Children (n = 10) | Adolescents (n = 38) | Adults (n = 104) | ||
| Incidence of lung and nodal involvement | Parenchymal | 6 (60) | 27 (71) | 80 (76.9) |
| Lesions | ||||
| Mediastinal | 7 (70) | 29 (76.3) | 74 (71.2) | |
| nodes | ||||
| Zonal distribution of parenchymal changes | Right upper | 5 (50) | 19 (50) | 58 (55.77) |
| Left upper | 2 (20) | 15 (39.47) | 45 (43.27) | |
| Right middle | 4 (40) | 19 (50) | 54 (51.92) | |
| Left middle | 1 (10) | 16 (42.11) | 43 (41.35) | |
| Right lower | 3 (30) | 12 (31.58) | 35 (33.65) | |
| Left lower | 2 (20) | 9 (23.68) | 30 (28.85) | |
| Pattern of parenchymal changes | Consolidation | 3 (30) | 16 (42.11) | 35 (33.65) |
| Centrilobular nodulesa | 2 (20) | 24 (63.16) | 67 (64.42) | |
| Miliary nodules | 1 (10) | 0 (0.00) | 3 (2.88) | |
| Bronchiectasis | 1 (10) | 8 (21.05) | 17 (16.35) | |
| Fibrosis | 1 (10) | 7 (18.42) | 14 (13.46) | |
| Cavitation | 3 (30) | 10 (26.32) | 23 (22.33) | |
| Lymph nodal distribution | Paratracheal | 7 (70) | 22 (57.59) | 57 (54.81) |
| Precarinal | 6 (60) | 9 (23.68) | 27 (25.96) | |
| Subcarinal | 7 (70) | 17 (44.74) | 40 (38.46) | |
| Hilar | 5 (50) | 13 (34.21) | 27 (25.96) | |
| AP window | 0 (0) | 9 (23.68) | 12 (11.54) | |
| Characteristics of lymph nodes | Lymphadenopathy | 7 (70) | 21 (55.26) | 50 (48.08) |
| Necrosis | 6 (60) | 17 (44.74) | 43 (41.35) | |
| Mattinga | 5 (50) | 11 (28.95) | 16 (15.38) | |
| Calcification | 4 (40) | 10 (26.32) | 25 (24.04) | |
| Pleural and pericardial effusion in the patients | Pleural effusion | 2 (20) | 5 (13.16) | 21 (20.19) |
| Pleural loculation | 1 (10) | 2 (5.3) | 10 (9.6) | |
| Pericardial effusion | 0 (0) | 1 (2.63) | 7 (6.73 ) |
Among the 152 patients we studied, consolidation was found in 54 (35.53%), centrilobular nodules in 93 (61.18%), bronchiectasis in 26 (17.11%), miliary in 4 (2.63%), fibrosis in 22 (14.47%) and cavitation in 36 (23.84%) patients. There was no statistically significant difference in the incidence of consolidation, miliary nodules, bronchiectasis, cavitation and fibrosis among different age groups studied. Centrilobular nodules were seen less commonly in children (P = 0.028) (Table 1).
Mediastinal lymphadenopathy was seen in 7 (70%), 29 (76.3%) and 74 (71.2%) respectively in children, adolescents and adult patients (Table 1). Among the mediastinal lymph nodes right paratracheal is the most commonly involved followed by subcarinal in all age groups. Involvement of multiple nodal groups (≥ 2) was seen in 28 (58%) of younger patients (≤ 18 years) and 51 (49%) older patients. In less than 10 years category all the 7 (70%) children with significant lymphadenopathy had involvement of multiple nodal groups. Involvement of multiple nodes (≥ 2 groups) was more commonly seen in children less than 10 years. The average number of nodal groups involved in children, adolescent and adult patients were 3.57, 2.52 and 2.23 respectively. Lymph node matting was seen more commonly in children (Table 1).
In 4 (40%) patients below 10 years lymphadenopathy was the only finding. Similarly 11 (29 %) patients between 10 to 18 years and 24 (23%) patients above 18 years had only lymphadenopathy.
Pleural effusion was noted in 28 (18.42%) patients and 13 of them showed loculation. Pericardial effusion was present in 8 (5.3%) patients. No significant difference in the serosal involvement is seen among children and adults (Table 1).
Tuberculosis continues to be a major medical, social and economic problem worldwide with its high morbidity and mortality. Children constitute about 5%-10% of patients suffering from tuberculosis worldwide. World Health Organization (WHO) data shows that in the year 2011 itself about half a million children fell ill with tuberculosis and about 64000 died from the disease[1].
The pathologic form of pulmonary tuberculosis is classically classified as primary and post primary or secondary tuberculosis and is depended on the sensitivity of infected host. Primary tuberculosis presenting with hilar/paratracheal adenopathy with or without focal parenchymal changes in mid-lower zones is thought to be predominant form of tuberculosis in children. However contrary to the traditional belief incidence of adult form of TB is increasingly seen in pediatric patients in terms of location and severity. Previous authors have already questioned the validity of the terminologies (primary and secondary tuberculosis) in recent literature[10,11].
Our study included 152 patients including 48 children (below 18 years). Previous studies on pediatric tuberculosis used varying age criteria for children (Table 2). We also have separately analyzed data of children below 10 years and adolescents (11-18 years).
| Ref. | Age (yr) | Mod | No | Cons | Nodu | Mil | Cavity | Bects | Node | Plefn |
| Leung et al[4] | < 16 | X-ray | 191 | 69% | NS | NS | NS | NS | 92% | 6% |
| Kim et al[21] | < 14 | CT | 41 | 49% | 29% | 17% | 7% | NS | 83% | 17% |
| Khatami et al[22] | < 15 | X ray | 30 | 43.30% | NS | NS | NS | NS | 90% | 6.70% |
| Koh et al[10] | 15-19 | X-ray | 90 | 25% | 96% | NS | 45% | 0 | 2% | 0 |
| Mukund et al[24] | < 17 | CT | 91 | NS | NS | NS | NS | NS | 96.70% | NS |
| Our study | < 18 | CT | 48 | 39.60% | 54% | 2% | 27% | 18.80% | 75% | 14.60% |
Mediastinal lymphadenopathy is the predominant finding in primary tuberculosis either alone or in association with lung lesions[20]. Our study showed mediastinal adenopathy in 7 (70%), 29 (76.3%) and 74 (71.2%) respectively in children, adolescents and adult patients. Compared to previous studies slightly lower incidence of adenopathy is observed in our study (Table 2). Although there was no significant difference in the incidence of adenopathy in different age groups, there was a definite trend in the extensiveness of nodal involvement. Involvement of multiple nodal groups was seen significantly more common in children. Mediastinal adenopathy was the only finding in 31.3% of children (group A and B) against 23% of older patients (group C). This is different from data of Kim et al[21] and Khatami et al[22] who showed isolated nodal involvement in 7% and 10% respectively. Paratracheal followed by subcarinal were the most common locations of nodal involvement in all the groups and is similar to a recent data from Andronikou et al[23] and Mukund et al[24].
Necrotic nodes characterized by inhomogeneous attenuation or low attenuation centre and enhancing peripheral rim were seen in 48% of children (group A and B) and 41% of adults (group C)[25]. Necrotic nodes were shown in 65% of children in a study by Mukund et al[24] which is comparable. Matted nodes were seen in 50% of young children (group A) against 19% of older patients (groups B and C) (P = 0.02). Nodal calcification was seen in 40%, 26.3% and 24% of children, adolescents and adults respectively. Nodal calcification was seen in 12 % and 28.4% of children in studies by Kim et al[21] and Mukund et al[24] respectively. Hence apart from higher incidence of matting seen in young children no other significant difference in nodal characteristics is seen in different age groups.
The typical parenchymal change in primary tuberculosis is focal consolidation (Ghon’s focus) seen classically in mid and lower zones. Cavitation, fibrosis and bronchiectasis are not commonly seen in primary tuberculosis. In our study, right upper zone was most commonly involved in all age groups and is comparable to the data by Koh et al[10] in which upper zone predominance is seen in 49% of patients. 68.75% of children (group A and B) showed lung parenchymal changes of tuberculosis against 76.9% of older patients (group C). Cavitation was seen in 30% of young children and 26% adolescents. Cavitating pneumonia was seen in 7% of children in the study by Kim et al[21] and 45% of adolescents by Koh et al[10]. The recognition of cavitating disease in children is important because the presence of cavities correlate with organism load, drug resistance, treatment outcome and infectivity[11]. Bronchiectasis was seen in 18.8 % children (group A and B) against 16.4% in adults (Table 1).
There was no significant statistical difference in the incidence of consolidation, miliary disease, bronchiectasis, fibrosis and cavitation among different age groups. Centrilobular nodules were less commonly seen in children. When we compared the incidence of nodules in children (group A) against older patients (group B and C) significantly lower incidence is noted in young children (P = 0.014).
Our study showed comparable frequency of pleural effusion, empyema and pericardial effusion in all age groups. It showed 20% and 13% incidence of pleural effusion in children and adolescents (Table 1). Kim et al[21] observed pleural effusion in 17% of children with tuberculosis.
To conclude, children with pulmonary tuberculosis are equally prone to develop significant destructive changes in the lung with severe sequelae similar to older patients. The impact is much more severe in cases of children because they have longer life expectancy. Moreover, the cavitating lesions with high bacterial load make these children highly infective and pose an important hazard to community health, than previously thought. The similar location and aggressiveness of parenchymal changes observed in children, blurs the boundary defining primary and reactivation tuberculosis. Hence the need for revision of these terminologies demands urgent attention.
First, our study included only those patients who had undergone CT scan at a tertiary institution and hence may not represent the exact patient population at the primary care level. However as all the patients in different age groups had been filtered in the same way the comparisons should remain valid. Secondly, we had only ten patients in below 10 years category and hence further studies including more number of children will help in strengthening the observations.
Tuberculosis is a contagious lung disease caused by mycobacterium tuberculosis. It is widely prevalent in developing countries and is a major threat to community health. Tuberculosis in children is traditionally believed to be less severe and less contagious as compared to adult disease. However recent evidences suggest that the distinction is far from truth and children are presenting with severe disease in presentation and outcome. This retrospective study is done to compare the disease characteristics based on imaging [computed tomography (CT) of chest] among children and adults who were presented to a tertiary care centre.
Tuberculosis in children can present in various forms ranging from subclinical infection to destructive parenchymal disease or extensive miliary disease. Early recognition of children having severe disease is essential in the management of disease and in prevention of spread. Although chest radiography is the primary imaging modality in chest tuberculosis, CT is advisable in children presenting with atypical and severe manifestations. Studies on this background are essential in planning and modifying the strategies in the management of pediatric tuberculosis.
Present study has shown that the disease can strike children with similar aggressiveness as in adults and can very much be a source of infection in the community. Hence it is important to take adequate measures to prevent disease spread along with optimal treatment planning.
Early recognition of severe manifestations of pediatric chest tuberculosis is beneficial in optimal treatment planning. Despite the higher radiation exposure involved CT of chest is the best investigation to get an accurate estimate of the disease in atypical and complicated cases.
CT stands for CT which is a radiological investigation using X-rays to produce tomographic images of the body. In the chest using different post-processing techniques CT provides much greater information than radiography.
The study of pediatric vs adult pulmonary tuberculosis is very good. The study highlights the incidence of severe manifestations in pediatric chest tuberculosis, the early detection of which is beneficial in optimal treatment planning.
| 1. | WHO Tuberculosis Fact sheet N°104. Reviewed on February 2013. Available from: http: //www.who.int/mediacentre/factsheets/fs104/en/index.html. Accessed June 15, 2013. |
| 2. | Kabra SK, Lodha R, Seth V. Some current concepts on childhood tuberculosis. Indian J Med Res. 2004;120:387-397. [PubMed] |
| 3. | Walls T, Shingadia D. Global epidemiology of paediatric tuberculosis. J Infect. 2004;48:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Leung AN, Müller NL, Pineda PR, FitzGerald JM. Primary tuberculosis in childhood: radiographic manifestations. Radiology. 1992;182:87-91. [PubMed] |
| 5. | Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161:1376-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1094] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 6. | Fonseca-Santos J. Tuberculosis in children. Eur J Radiol. 2005;55:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Hussey G, Chisholm T, Kibel M. Miliary tuberculosis in children: a review of 94 cases. Pediatr Infect Dis J. 1991;10:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 55] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Milković D, Richter D, Zoricić-Letoja I, Raos M, Koncul I. Chest radiography findings in primary pulmonary tuberculosis in children. Coll Antropol. 2005;29:271-276. [PubMed] |
| 9. | Van Dyck P, Vanhoenacker FM, Van den Brande P, De Schepper AM. Imaging of pulmonary tuberculosis. Eur Radiol. 2003;13:1771-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Koh WJ, Jeong YJ, Kwon OJ, Kim HJ, Cho EH, Lew WJ, Lee KS. Chest radiographic findings in primary pulmonary tuberculosis: observations from high school outbreaks. Korean J Radiol. 2010;11:612-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Marais BJ, Parker SK, Verver S, van Rie A, Warren RM. Primary and postprimary or reactivation tuberculosis: time to revise confusing terminology? AJR Am J Roentgenol. 2009;192:W198; author reply W199-W200. [PubMed] |
| 12. | Marais BJ, Gie RP, Hesseling AH, Beyers N. Adult-type pulmonary tuberculosis in children 10-14 years of age. Pediatr Infect Dis J. 2005;24:743-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Agrons GA, Markowitz RI, Kramer SS. Pulmonary tuberculosis in children. Semin Roentgenol. 1993;28:158-172. [PubMed] |
| 14. | Escreet BC, Cowie RL. Criteria for the diagnosis of pulmonary tuberculosis. S Afr Med J. 1983;63:850-854. [PubMed] |
| 15. | Campbell IA, Bah-Sow O. Pulmonary tuberculosis: diagnosis and treatment. BMJ. 2006;332:1194-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Vallejo JG, Ong LT, Starke JR. Clinical features, diagnosis, and treatment of tuberculosis in infants. Pediatrics. 1994;94:1-7. [PubMed] |
| 17. | Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. Lancet Infect Dis. 2008;8:498-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 350] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 18. | Shingadia D, Novelli V. Diagnosis and treatment of tuberculosis in children. Lancet Infect Dis. 2003;3:624-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Marais BJ, Pai M. Recent advances in the diagnosis of childhood tuberculosis. Arch Dis Child. 2007;92:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | McAdams HP, Erasmus J, Winter JA. Radiologic manifestations of pulmonary tuberculosis. Radiol Clin North Am. 1995;33:655-678. [PubMed] |
| 21. | Kim WS, Moon WK, Kim IO, Lee HJ, Im JG, Yeon KM, Han MC. Pulmonary tuberculosis in children: evaluation with CT. AJR Am J Roentgenol. 1997;168:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Khatami A, Sabouri S, Ghoroubi J, Rassouli N, Abdollah GF. Radiological Findings of Pulmonary tuberculosis in infants and young children. Iran J Radiol. 2008;5:231-234. |
| 23. | Andronikou S, Joseph E, Lucas S, Brachmeyer S, Du Toit G, Zar H, Swingler G. CT scanning for the detection of tuberculous mediastinal and hilar lymphadenopathy in children. Pediatr Radiol. 2004;34:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Mukund A, Khurana R, Bhalla AS, Gupta AK, Kabra SK. CT patterns of nodal disease in pediatric chest tuberculosis. World J Radiol. 2011;3:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Moon WK, Im JG, Yeon KM, Han MC. Mediastinal tuberculous lymphadenitis: CT findings of active and inactive disease. AJR Am J Roentgenol. 1998;170:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
P- Reviewers: Abdel-Aziz M, Al-Haggar M S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ