Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.113328
Revised: September 16, 2025
Accepted: October 17, 2025
Published online: December 9, 2025
Processing time: 70 Days and 12.5 Hours
Hyponatremia is a prevalent and serious electrolyte imbalance in pediatric pneumonia and is linked to increased disease severity and adverse outcomes. Oral rehydration solution (ORS) is an available, inexpensive, safe, and ready-to-use oral solution that can supplement sodium in such cases.
To assess the impact of prophylactic sodium supplementation via ORS on clinical and hospital outcomes in infants and children admitted with pneumonia.
A randomized, interventional controlled trial was conducted on 140 infants and children admitted with pneumonia (70 per group). The primary outcome was hospital length of stay, with secondary outcomes including serum sodium and potassium levels, clinical respiratory scores, modified shock index, and nutritional/inflammatory markers. The hospital length of stay and both the laboratory and clinical parameters of the interventional and control groups were compared.
The hospital stay was longer in the control group than in the intervention group (P value = 0.001; effect size = 0.59). Clinical respiratory scores on day 4 were significantly lower in the intervention group than in the control group (P value = 0.001). Sodium levels were significantly lower in the control group than in the intervention group at discharge (P value = 0.002).
Prophylactic oral sodium supplementation through ORS may have a health-promoting effect on infants and children admitted with pneumonia.
Core Tip: Our study outlined the possible use of oral rehydration solution (ORS) to provide prophylactic sodium supplementation in infants and children with pneumonia. Sodium and potassium supplementation may improve clinical respiratory manifestations in infants and children with pneumonia. ORS supplementation may be a prophylactic therapy for prolonged hospitalization in infants and children with pneumonia.
- Citation: Atef Abdelsattar Ibrahim H, Agha M, Taha M. Effects of oral rehydration solution-based prophylactic sodium supplementation on clinical outcomes in pediatric pneumonia: A randomized controlled trial. World J Clin Pediatr 2025; 14(4): 113328
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/113328.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.113328
The World Health Organization (WHO) reported that pneumonia is a predominant cause of mortality in children under five years of age, accounting for approximately one-fifth of all juvenile fatalities worldwide[1].
Hyponatremia is the most prevalent electrolyte imbalance encountered in clinical practice and is a common finding in children with pneumonia[2-5].
Hyponatremia linked to childhood pneumonia is usually attributed to the syndrome of inappropriate secretion of antidiuretic hormone. This condition is characterized by hyponatremia and hypoosmolality and arises from the improper and persistent release or action of antidiuretic hormone[6].
Oral rehydration solution (ORS) is a first-line treatment for compensating for volume loss due to diarrhea and vomiting among children with gastroenteritis[7]. To maximize absorption, the ORS should contain both glucose and sodium to take advantage of the sodium-glucose cotransport in the gut. ORS are widely accessible as both premixed commercial solutions and powders that may be mixed with ordinary water[8]. It is more effective, safe, easy, and cost-efficient than intravenous therapy and is recommended by the WHO and the American Academy of Pediatrics[9].
Hyponatremia is a common electrolyte imbalance in pediatric pneumonia that is associated with greater disease severity and adverse outcomes. However, existing research does not provide evidence regarding prophylactic sodium administration to these patients and its effect on their clinical outcomes. Therefore, our objectives were to evaluate the possible prophylactic use of sodium supplementation via ORS as a novel supplement in infants and children with pneumonia.
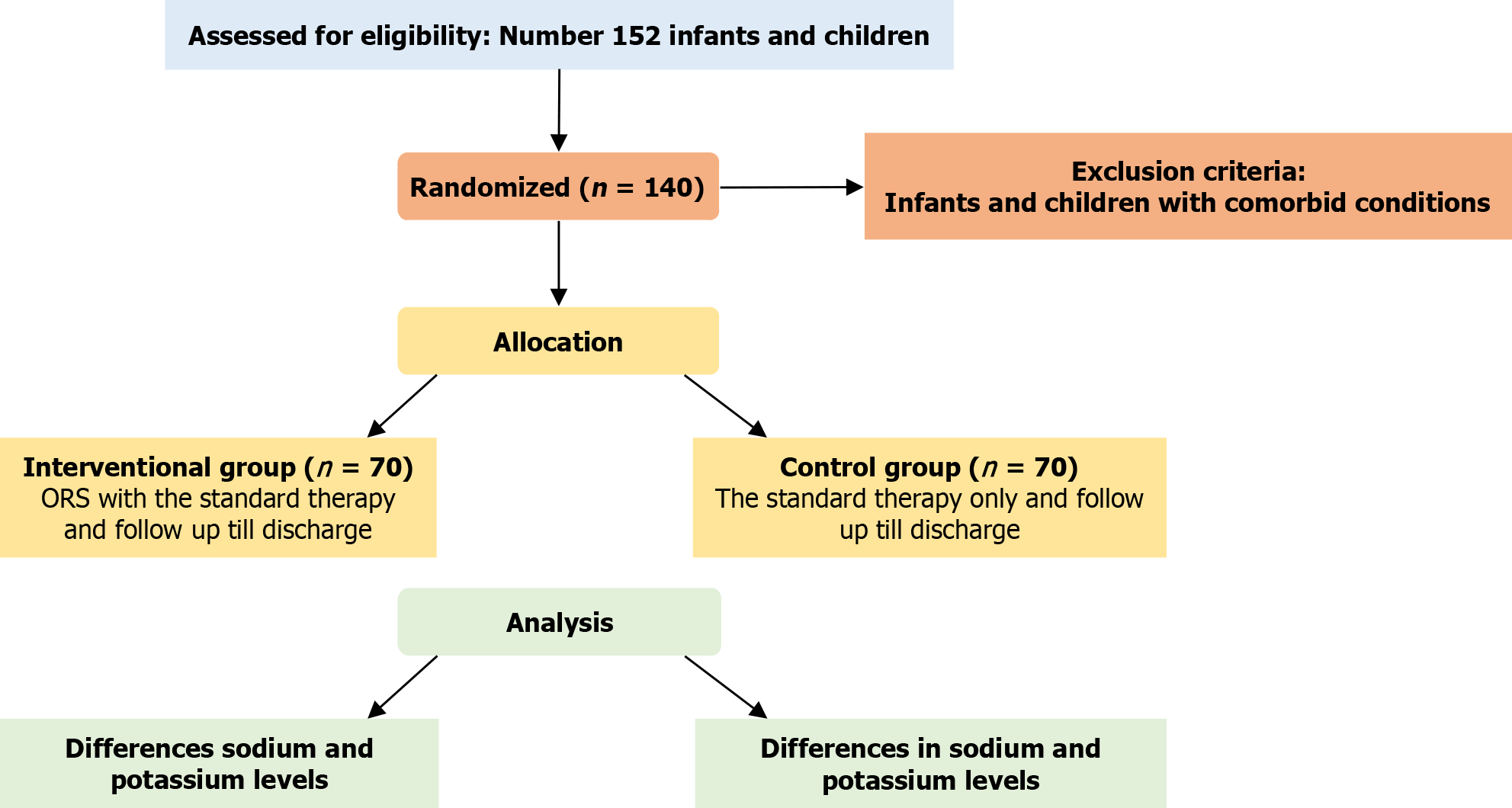
Our study was a randomized parallel controlled trial on infants and children admitted with pneumonia at Mataria Teaching Hospital conducted from April 2025 to July 2025. The flow chart of the study is shown in Figure 1.
Children of both sexes with pneumonia whose caregivers or parents disclosed approval to participate in the study and whose ages ranged from 1 month to 5 years were enrolled. Children with comorbidities that could affect sodium or fluid balance, including renal disease, heart failure, or severe malnutrition, and children with marked electrolyte abnormalities at baseline or those on sodium or potassium medications were excluded.
The inclusion and exclusion criteria according to the population, intervention, comparison, and outcome framework[10] were as follows:
Population: Infants and children admitted with pneumonia at Mataria Teaching Hospital.
Intervention: Impact of ORS supplementation on clinical and hospital outcomes.
Comparison: Both groups were compared in terms of differences in hospital length of stay, clinical respiratory scores, and sodium and potassium levels.
Outcome: The primary outcome is the impact of ORS supplementation on hospital length of stay.
Parents/Legal guardians provided informed written consent before participation in the study. When culturally or legally needed, the permission of both parents was obtained in line with institutional policy.
Demographic and clinical characteristics, including age, sex, admission diagnosis, presence of underlying disease, vital signs, anthropometric measurements, and nutritional status, were recorded.
Eligible interventional infants and children with pneumonia were supplemented with WHO-compliant ORS (Pharco Pharmaceuticals, Egypt), which contained 75 mmol/L (0.52 g/sachet) Na+, 20 mmol/L (0.3 g/sachet) K+, 75 mmol/L glucose, with an osmolarity of 245 mOsm/L. The sachet was dissolved in 200 mL of water according to the ma
Throughout the intervention phase, adherence to ORS administration was closely monitored. Each dose was observed and documented, and vomiting or refusal events, as well as the exact amount consumed, were noted. An independent investigator reviewed daily adherence reports to ensure compliance.
Follow-up laboratory tests and clinical assessments were performed throughout hospitalization to observe and document potential adverse ORS-related effects, including feeding intolerance, overhydration, electrolyte disturbances (hypernatremia or hyperkalemia), and aspiration.
Children who were assigned to the control group received standard hospital management along with a placebo (sterile water).
In accordance with institutional protocols, all patients in both groups received standard supportive care, which included intravenous fluids, oxygen supplementation, and antibiotics.
Children were followed up until discharge or death. The primary outcome, length of hospital stay, was defined prospectively as the time from hospital admission to discharge (measured in days). Discharge criteria were standardized as follows: Stable vital signs for at least 12 hours, adequate oral intake, and oxygen saturation ≥ 92% on room air.
Secondary outcome measures included the impact of ORS supplementation on other hospital outcomes, such as the modified shock index and clinical respiratory score.
Clinical diagnosis of pneumonia: Clinical findings of tachypnea, fever, and reduced oxygen saturation, in addition to radiological findings suggestive of pneumonia[13].
The clinical respiratory score and modified shock index were calculated as previously described[14-16].
Hyponatremia and hypokalemia: Were defined using reference ranges as previously described[11].
The length of hospital stay was prospectively defined as the interval (in days) from the time of admission to the time of discharge.
Nutritional and inflammatory markers: The prognostic nutritional index was obtained as follows: 10 × serum albumin (g/dL) + 0.005 × lymphocyte count (mm3)[17]. The neutrophil-to-lymphocyte ratio was determined as the absolute neutrophil count divided by the absolute lymphocyte count, while the platelet-to-lymphocyte ratio was calculated as the platelet count divided by the absolute lymphocyte count[18,19].
Quality improvement services: All participants who received ORS were monitored for any adverse events that emerged, such as hypernatremia, hyperkalemia, feeding intolerance, aspiration, or overhydration risk. ORS seems to be a safe adjuvant therapy, as no complications or adverse events emerged. In addition, compliance was monitored to ensure adherence. To remove any potential risk of aspiration, enteral nutrition was started using nasogastric tubes according to Consensus guidelines from the American Society for Parenteral and Enteral Nutrition, Society of Critical Care Medicine, and European Society of Pediatric and Neonatal Intensive Care[20,21].
An independent investigator randomly allocated the enrolled children to either the intervention group or the control group at a 1:1 ratio using a computerized program (GraphPad QuickCalcs). Sequentially numbered, opaque, and sealed envelopes that were prepared and administered independently of the clinical team were used to guarantee allocation concealment. The intervention was hidden from the outcome assessor and data analysts.
Clinical trial registration: The trial was registered with Clinical Trial Registration ID: NCT06951347 in April 2025 (https://clinicaltrials.gov/study/NCT06951347). Registration preceded the first patient enrollment in accordance with CONSORT/ICMJE requirements.
The trial was powered for the primary endpoint, which is the length of hospital stay. We opted for a medium effect size (d = 0.5) using G*Power software (version 3.1.9.2), a conservative estimate, balancing detectability and feasibility. The alpha was set as 0.05, the power (1 - β) was set as 0.80, and the case-to-control ratio was set as 1:1. Therefore, the minimum sample size needed was 64 for each group, with a total of 128 children. To compensate for possible dropouts, the sample size was rounded to 140 (70 per group).
Numerical variables are presented as the mean, standard deviation, median, and interquartile range and were analyzed using the Shapiro-Wilk and the Kolmogorov-Smirnov normality tests. Categorical variables are presented as frequencies and percentages. The normally distributed numerical variables were compared via a t test (independent). With respect to the variables that were not normally distributed, a comparison of numerical variables was carried out via the Mann-Whitney U test. Two-sided P values ≤ 0.05 were considered significant. All the statistical analyses were performed through the computer program IBM SPSS (Statistical Package for the Social Science; IBM Corp, Armonk, NY, United States), release 25 for Microsoft Windows. Mediation analysis[22] was carried out using JASP 0.19.3 software.
Our study was conducted on 140 infants and children with pneumonia admitted to Mataria Teaching Hospital to outline the impact of ORS supplementation on clinical and laboratory outcomes. Tables 1 and 2 illustrate the sociodemographic and clinical criteria of the enrolled study participants.
| Age (months) | |
| Median (IQR) | 4 (8.8) |
| Min–Max | 1-59 |
| Gender distribution | |
| Males | 74 (52.9) |
| Females | 66 (47.1) |
| Hospital length of stay (days) | |
| Median (IQR) | 9 (7) |
| Min-Max | 5-35 |
| Na on admission (mmol/L) | |
| Median (IQR) | 136 (3) |
| Min-Max | 135-144 |
| Na on day 2 (mmol/L) | |
| Median (IQR) | 135 (3) |
| Min-Max | 127-150 |
| Na on day 4 (mmol/L) | |
| Median (IQR) | 135 (3) |
| Min-Max | 127-148 |
| Na on discharge (mmol/L) | |
| Median (IQR) | 136 (3) |
| Min-Max | 128-148 |
| K on admission (mmol/L) | |
| Median (IQR) | 4.9 (1.0) |
| Min-Max | 3.0-6.3 |
| K on day 2 (mmol/L) | |
| Median (IQR) | 4.8 (1.2) |
| Min-Max | 3.1-6.5 |
| K on day 4 (mmol/L) | |
| Median (IQR) | 5 (1.2) |
| Min-Max | 3.2-6.4 |
| K on discharge (mmol/L) | |
| Median (IQR) | 4.8 (1) |
| Min-Max | 2.5-6.1 |
| Modified shock index on admission | |
| Median (IQR) | 2 (0.3) |
| Min-Max | 1.4-2.4 |
| Modified shock index on day 4 | |
| Median (IQR) | 1.8 (0.3) |
| Min-Max | 1.2-2.5 |
| Modified shock index on discharge | |
| Median (IQR) | 1.7 (0.4) |
| Min-Max | 1.2-2.7 |
| Respiratory score on admission | |
| Median (IQR) | 8 (2) |
| Min-Max | 5-12 |
| Respiratory score on day 4 | |
| Median (IQR) | 5 (4) |
| Min-Max | 2-12 |
| Fasting time in hours | |
| Median (IQR) | 24 (18) |
| Min-Max | 6-48 |
| Weight Z score | |
| Median (IQR) | -1.1 (2.4) |
| Min-Max | -3.0:2.0 |
| Height Z score | |
| Median (IQR) | -1.1 (1.2) |
| Min-Max | -3.0:0.8 |
| BMI Z score | |
| Median (IQR) | -0.7 (2.7) |
| Min-Max | -3.0:3.0 |
| NLR on admission | |
| Median (IQR) | 1.3 (1.4) |
| Min-Max | 0.03-27.2 |
| NLR on discharge | |
| Median (IQR) | 1.0 (0.9) |
| Min-Max | 0.09-12 |
| PNI on admission | |
| Median (IQR) | 62.0 (23.3) |
| Min-Max | 27.5-248 |
| PNI on discharge | |
| Median (IQR) | 58.0 (14.7) |
| Min-Max | 29.5-102 |
| PLR on admission | |
| Median (IQR) | 81.5 (85.1) |
| Min-Max | 13.3-419 |
| PLR on discharge | |
| Median (IQR) | 88.2 (75.2) |
| Min-Max | 11.9-548 |
To remove any potential bias, matching between cases and controls was carried out. There were no significant differences except for the potassium levels at admission, which were lower in the intervention group than in the control group, as shown in Table 3. However, this difference may underscore the value of ORS supplementation, as no further significant differences were detected at days 2 or 4 or at discharge, as shown in Table 4. Sodium levels at discharge were significantly lower in the control group. In addition, the fasting time was shortened, and the clinical respiratory score was significantly lower on day 4 in the intervention group, as shown in Table 4.
| Interventional (n = 70) | Control (n = 70) | P value | |
| Age in months | |||
| Median (IQR) | 5 (9.5) | 4 (4.6) | 0.1461 |
| Min-Max | 1.3-4.8 | 1-59 | |
| Modified shock index on admission | |||
| Median (IQR) | 1.9 (0.3) | 2.0 (0.3) | 0.0511 |
| Min-Max | 1.4-2.4 | 1.5-2.4 | |
| Respiratory score on admission | |||
| Median (IQR) | 8 (2) | 8 (2) | 0.9511 |
| Min-Max | 5-12 | 5-12 | |
| Sodium on admission (mmol/L) | |||
| Median (IQR) | 136 (2) | 137 (3) | 0.0611 |
| Min-Max | 135-142 | 135-144 | |
| Potassium on admission (mmol/L) | |||
| Mean (SD) | 4.6 (0.7) | 4.9 (0.7) | 0.0382,a |
| Min-Max | 3.0-6.0 | 3.3-6.3 | |
| Weight Z score | |||
| Median (IQR) | -1.0 (2.16) | -1.2 (2.4) | 0.1631 |
| Min-Max | -3.0:2.0 | -3.0:1.8 | |
| Height Z score | |||
| Mean (SD) | -1 (0.7) | -1.1 (0.9) | 0.4942 |
| Min-Max | -3.0:0.6 | -3.0:0.8 | |
| BMI Z score | |||
| Median (IQR) | -0.7 (2.5) | -1.1 (2.7) | 0.2581 |
| Min-Max | -3.0:3.0 | -3.0:2.0 | |
| NLR | |||
| Median (IQR) | 1.0 (1.4) | 1.0 (1.8) | 0.1981 |
| Min-Max | 0.1-27.2 | 0.03-24 | |
| PNI | |||
| Median (IQR) | 60.0 (20.6) | 64.7 (24.0) | 0.6941 |
| Min-Max | 45-126 | 27.5-248 | |
| PLR | |||
| Median (IQR) | 84.4 (87.1) | 74.8 (84.4) | 0.4261 |
| Min-Max | 26.6-419 | 13.3-375.3 |
| Interventional (n = 70) | Control (n = 70) | P value | |
| Na on day 2 (mmol/L) | |||
| Median (IQR) | 135 (3) | 136 (5) | 0.7951 |
| Min-Max | 131-150 | 127-143 | |
| Na on day 4 (mmol/L) | |||
| Median (IQR) | 135 (3) | 135 (5) | 0.4131 |
| Min-Max | 129-145 | 127-148 | |
| Na on discharge (mmol/L) | |||
| Median (IQR) | 137 (3) | 135 (4) | 0.0021,a |
| Min-Max | 132-141 | 128-148 | |
| K on day 2 (mmol/L) | |||
| Mean (SD) | 4.7 (0.7) | 4.8 (0.7) | 0.3722 |
| Min-Max | 3.1-6.5 | 3.4-6.2 | |
| K on day 4 (mmol/L) | |||
| Median (IQR) | 4.9 (1.2) | 5.0 (1.2) | 0.4691 |
| Min-Max | 3.3-6.0 | 3.2-6.4 | |
| K on discharge (mmol/L) | |||
| Median (IQR) | 4.9 (0.8) | 4.8 (1.0) | 0.7371 |
| Min-Max | 3.5-6.1 | 2.5-6.0 | |
| Modified shock index on day 4 | |||
| Median (IQR) | 1.8 (0.3) | 1.8 (0.3) | 0.6911 |
| Min-Max | 1.2-2.5 | 1.3-2.4 | |
| Respiratory score on day 4 | |||
| Median (IQR) | 4 (2) | 6 (4) | 0.0011,a |
| Min-Max | 2-12 | 2-12 | |
| Modified shock index on discharge | |||
| Median (IQR) | 1.8 (0.4) | 1.6 (0.4) | 0.2951 |
| Min-Max | 1.2-2.7 | 1.2-2.5 | |
| Fasting time (hours) | |||
| Mean (SD) | 23.1 (11.5) | 28.2 (12.2) | 0.0122,a |
| Min-Max | 6-48 | 6-48 | |
| NLR | |||
| Median (IQR) | 1.0 (0.5) | 0.9 (1.2) | 0.9951 |
| Min-Max | 0.1- 3.7 | 0.1- 12 | |
| PNI | |||
| Median (IQR) | 58.7 (17.9) | 58 (12.6) | 0.6861 |
| Min-Max | 29.5-102 | 39.5-96 | |
| PLR | |||
| Median (IQR) | 92.0 (79.7) | 84.4 (69.8) | 0.2451 |
| Min-Max | 18-548 | 11.9-301.7 |
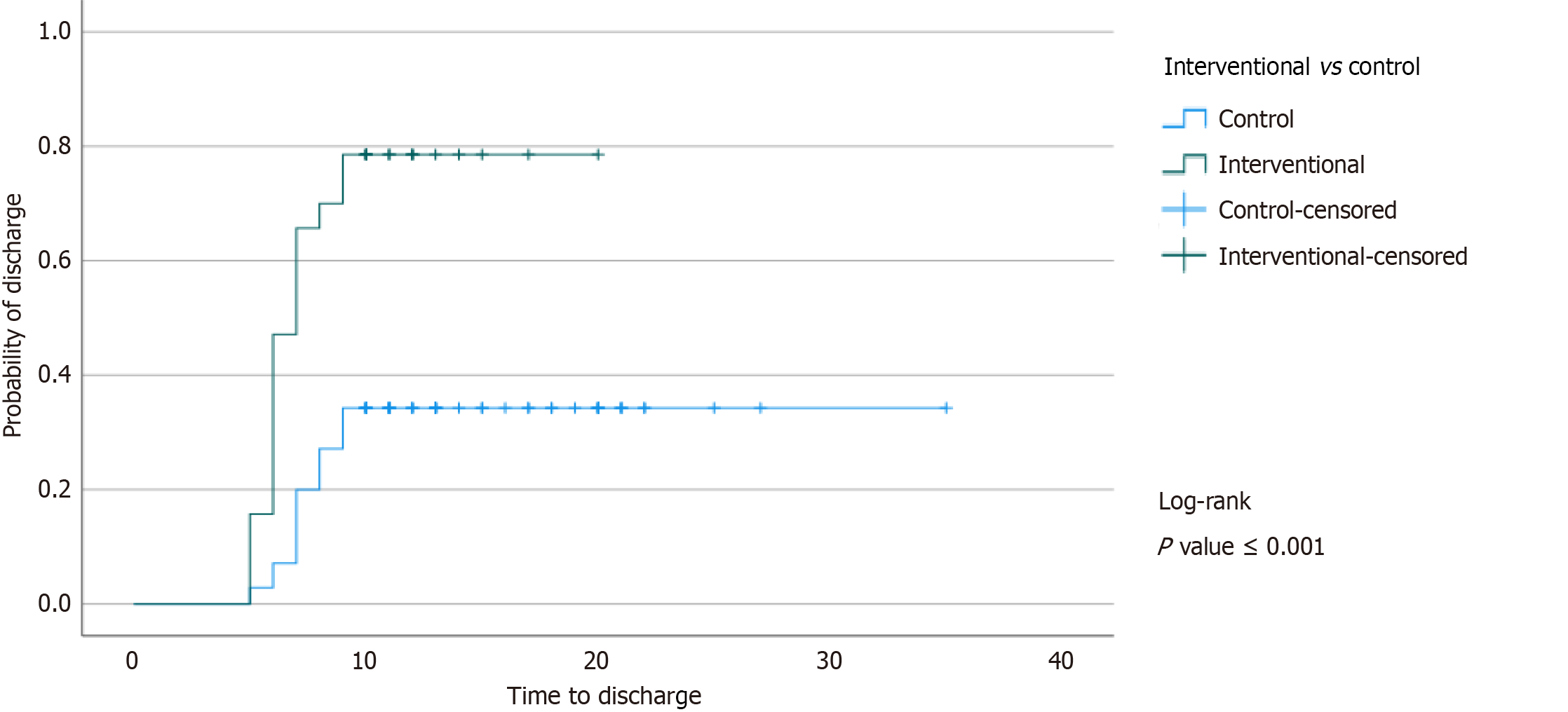
We were interested in determining the effect of ORS supplementation on hospital outcomes, as illustrated in Table 5. The hospital stay was significantly shorter in the intervention group. A Kaplan-Meier curve for the length of hospital stay illustrates a significantly shorter time to discharge in the interventional group compared to the control group; P value < 0.001, as shown in Figure 2.
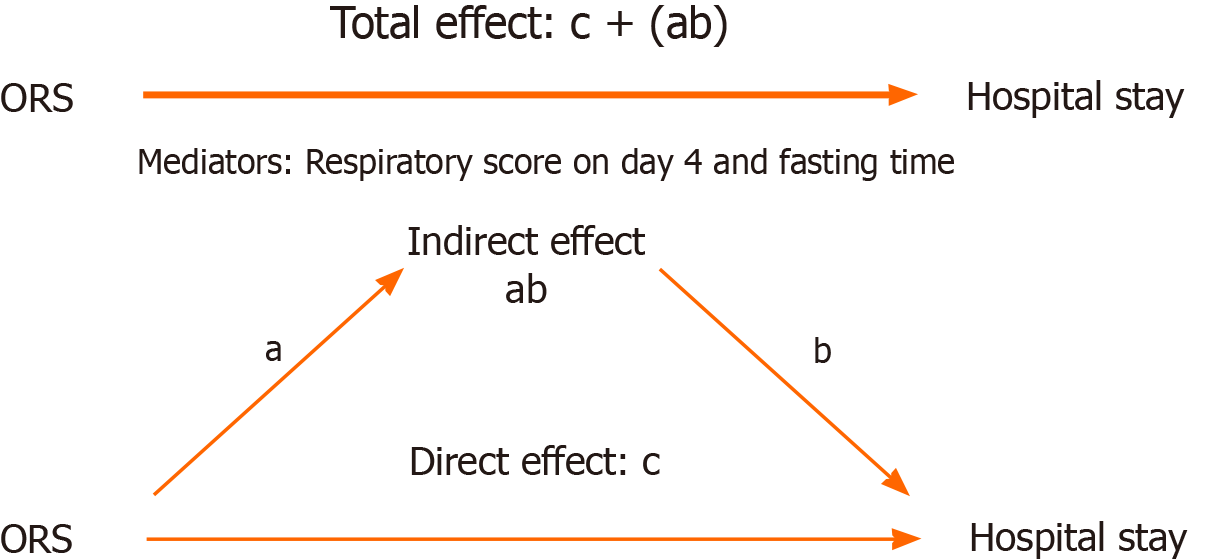
Similarly, we were interested in determining the differences in sodium and potassium levels as “on discharge – on admission”, as shown in Table 6. Furthermore, the direct effect of ORS supplementation on hospital stay was tested via a median analysis in the presence of mediators. The direct and total effects of ORS supplementation were significant, as shown in Table 7. The path diagram is shown in Figure 3. As illustrated, the total effect was the sum of the direct effect of ORS and the indirect effect of the mediators. As shown, the direct effect was significant, and the total effect was still significant and not affected by the mediators.
| Interventional (n = 70) | Control (n = 70) | P value | |
| Differences in sodium and potassium levels (on discharge–on admission) | |||
| Difference of Na | |||
| Median (IQR) | 0 (3.0) | -1.5 (3.2) | < 0.0011,a |
| Min-Max | -7:5 | -12:9 | |
| Difference of K | |||
| Mean (SD) | 0.1 (0.7) | -0.2 (0.8) | 0.0132,a |
| Min-Max | -1.2:1.8 | -2.2:1.7 | |
| Differences in sodium and potassium levels (on day 4–on admission) | |||
| Difference of Na | |||
| Median (IQR) | -0.5 (3.2) | -2.0 (5.0) | 0.0331,a |
| Min-Max | -8:10 | -12:12 | |
| Difference of K | |||
| Mean (SD) | 0.1 (0.77) | -0.01 (0.78) | 0.1182 |
| Min-Max | -2.3:2.5 | -1.7:2.3 | |
| Differences in sodium and potassium levels (on day 2–on admission) | |||
| Difference of Na | |||
| Median (IQR) | -1 (2) | -1 (4) | 0.0861 |
| Min-Max | -10:14 | -11:5 | |
| Difference of K | |||
| Median (IQR) | 0.05 (1) | 0 (0.7) | 0.4961 |
| Min-Max | -1.4:2.2 | -2:1.4 | |
| The outcome is an improvement in hospital length of stay | |||||
| Estimate | Std. Error | Z value | aP value | 95%CI lower level | 95%CI upper level |
| A: Direct effect of Na supplementation | |||||
| -3.3 | 0.54 | -6 | < 0.001a | -4.4 | -2.2 |
| B: Indirect effect: In the presence of mediators | |||||
| Mediator 1: Respiratory score on day 4 | |||||
| 0.423 | 0.224 | -1.8 | 0.059 | -0.86 | 0.015 |
| Mediator 2: Fasting time | |||||
| -1.2 | 0.516 | -2.4 | 0.014a | -2.28 | 0.26 |
| C: Total effect: Direct + indirect | |||||
| -5.02 | 0.792 | -6.3 | < 0.001a | -6.5 | -3.4 |
To exclude any potential bias, we carried out a regression analysis to check whether the baseline potassium level was a significant covariate as it was significantly lower in the intervention group at the start of the study. The results indicated that baseline potassium level was not a significant covariate, as shown in Table 8.
| Hospital length of stay in days | |||||
| Variable | Univariate analysis | Multivariate analysis | |||
| B | CI | P value | CI | P value | |
| Interventional (ORS) vs control1 | -5.09 | -6.6:-3.4 | < 0.001a | -6.37:-3.4 | < 0.001a |
| Respiratory scores on admission | 1.3 | 0.86:1.9 | < 0.001a | 0.88:1.84 | < 0.001a |
| Modified shock index on admission | 4.5 | 0.73:8.4 | 0.02a | -3.9:3.2 | 0.859 |
| Age in months | -0.18 | -0.096:0.06 | 0.652 | ||
| Sex2 | 0.71 | -1.07:2.5 | 0.43 | ||
| Na on admission | 0.032 | -0.4:0.4 | 0.88 | ||
| K on admission | 0.947 | -0.28:2.1 | 0.131 | -0.09:1.2 | 0.786 |
| Weight Z scores | -0.73 | -1.4:-0.07 | 0.03 | -5.4:1.9 | 0.356 |
| Height Z scores | -1.06 | -2:-0.04 | 0.04 | -1.87:2.1 | 0.88 |
| BMI Z scores | -0.4 | -1:0.08 | 0.096 | -1.43:3.6 | 0.387 |
For more clarity, we compared Na levels as hyponatremia, normal, or hypernatremia on admission, day 2, day 4, and at discharge, as shown in Table 9. There were more children in the control group with hyponatremia at discharge (P value = 0.02).
| Interventional (n = 70) | Control (n = 70) | P value | |
| On day 2 | |||
| Hyponatremia on day 2 | 38 (54.3) | 34 (48.5) | 0.491 |
| Normal natremia on day 2 | 32 (45.7) | 36 (51.4) | |
| On day 4 | |||
| Hyponatremia on day 4 | 37 (52.8) | 35 (50) | 0.731 |
| Normal natremia on day 4 | 33 (47.2) | 35 (50) | |
| On discharge | |||
| Hyponatremia on discharge | 21 (30) | 39 (55.7) | 0.021,a |
| Normal natremia on discharge | 49 (70) | 31 (44.3) | |
The ORS was well tolerated, with good adherence observed among participants throughout the study period. No adverse events related to ORS supplementation were reported.
Pneumonia is a leading cause of pediatric death and morbidity worldwide. Accurate diagnosis and identification of the causes of pneumonia are critical for evaluating the burden of illness, implementing appropriate prevention or treatment methods, and developing more effective therapies[23]. Electrolyte disturbances have been linked to a wide range of acute infections, including pneumonia, predominantly hyponatremia, and are present in the vast majority of community-acquired pneumonia cases. Hyper and hypokalemia are less common electrolyte disturbances[3].
Electrolyte disturbances, notably hyponatremia, are prevalent in children hospitalized with lower respiratory tract infections, particularly pneumonia, with estimated rates reaching 40% in some cohorts. Recent studies have shown that reduced serum sodium levels are independently associated with greater disease severity, longer hospital stays, increased requirements for mechanical ventilation or intensive care, hemodynamic instability, and higher fatality rates[24-26]. These findings emphasize the importance of electrolyte monitoring in pediatric patients with pneumonia and underscore the need for additional research evaluating preventive or prophylactic strategies to increase sodium and potassium levels and thus improve clinical outcomes. Therefore, we were encouraged to study the impact of prophylactic ORS supplementation on clinical outcomes and length of hospital stay in pediatric pneumonia.
Hyponatremia is common in infants and children hospitalized with pneumonia and is linked to increased disease severity. The underlying process is incompletely understood, yet the syndrome of inappropriate antidiuretic hormone secretion is thought to play an important role. Traditional choices for managing hyponatremia in such children are challenging[27].
Hyponatremia is a common finding in children with lower respiratory tract infections and seems to be linked to disease severity and hospitalization duration[28]. This may have been the case in our study, as the interventional group experienced lower respiratory scores and shorter hospital stays. Hyponatremia was associated with an increased length of hospital stay in another study[29].
Low sodium levels can affect gastrointestinal tract functional capacity[30]. This was found in our study, in which the fasting time was significantly shorter in the intervention group (P value = 0.012). This may add to the possible value of supplementation with ORS in hospitalized patients.
ORS are formulated to have a greater effect on sodium levels than on potassium levels[31]. This may be the basis for our results, as potassium levels at discharge did not differ significantly between the interventional and control groups. This may underscore the fact that hyponatremia is more prevalent than hypokalemia in children hospitalized with pneumonia[32]. However, compared with those in the interventional group, potassium levels in the control group shifted to lower levels. This finding may further reveal the importance of ORS supplementation in vulnerable children.
To the best of our knowledge, our study is the first to outline the possible effect of ORS as an adjuvant therapy in infants and children hospitalized with pneumonia and its effect on hospital length of stay.
The observed reduction in hospital stay is encouraging but should be considered in light of our single-center design and relatively small sample size. Larger, multicenter trials are needed before generalizations can be made or routine preventive ORS usage in all pediatric pneumonia cases can be suggested. Furthermore, studies with longer follow-up periods are needed to outline the long-term safety of ORS in such vulnerable age groups.
Recommendation: Further studies are needed to clarify the possible use of ORS as a routine supplement in children with pneumonia.
This study may increase the awareness of health care providers regarding the possible use of ORS as an adjuvant prophylactic therapy in infants and children admitted with pneumonia. In this single-center trial, ORS supplementation was associated with shorter hospitalization. Larger trials are needed to confirm these findings.
| 1. | Alzomor O, Alhajjar S, Aljobair F, Alenizi A, Alodyani A, Alzahrani M, Aljubab A, Al Banyan E, Alshehri M, Alfwaz T, Alghoshimi M, Alhammadi M, Almazer Y, Elsidig N, Alghamdi F, Alsubaie S, Alshahrani D. Management of community-acquired pneumonia in infants and children: Clinical practice guidelines endorsed by the Saudi Pediatric Infectious Diseases Society. Int J Pediatr Adolesc Med. 2017;4:153-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Sakellaropoulou A, Hatzistilianou M, Eboriadou M, Athanasiadou-Piperopoulou F. Hyponatraemia in cases of children with pneumonia. Arch Med Sci. 2010;6:578-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Pande V, Jadhav R, Ilyaz M, Mane S, Dua J. Dyselectrolytemia in Children With Severe Pneumonia: A Prospective Study. Cureus. 2024;16:e53940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Matani A, Sharon N, Reiss N, Yana M, Cleper R, Amir AZ. Hyponatremia in Pediatric Community-acquired Pneumonia is Associated with Bacterial Etiology and Severity Markers. Isr Med Assoc J. 2025;27:231-237. [PubMed] |
| 5. | Hassb El-Naby MM, Mohammed Aly HA, Ahmed MESM, Bayoumy IM. Assessment of Serum Electrolytes in Hospitalized children with Pneumonia. Al-Azhar J Pediatr. 2025;28:4485-4496. [DOI] [Full Text] |
| 6. | Impact of hyponatremia on outcome of children with community acquired pneumonia. Al-Azhar J Pediatr. 2023;26:3274-3285. [DOI] [Full Text] |
| 7. | Aghsaeifard Z, Heidari G, Alizadeh R. Understanding the use of oral rehydration therapy: A narrative review from clinical practice to main recommendations. Health Sci Rep. 2022;5:e827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Freige C, Spry C. Oral Rehydration Solutions versus Drink of Choice in Children with Dehydration: A Review of Clinical Effectiveness [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, 2020. [PubMed] |
| 9. | Powers KS. Dehydration: Isonatremic, Hyponatremic, and Hypernatremic Recognition and Management. Pediatr Rev. 2015;36:274-83; quiz 284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Ibrahim HAA, Sobhi R, Khaled Farouk N, Mohamed AbdelAziz F. Omega and heart rate variability in overweight and obese schoolchildren. Pediatr Res. 2025;98:957-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | USAID Global Health Supply Chain Program. Manual for Procurement & Supply of Quality-Assured MNCH Commodities. 2023. |
| 12. | Dietary Reference Intakes for Sodium and Potassium. Washington (DC): National Academies Press (US); 2019-Mar-5. [PubMed] |
| 13. | Crame E, Shields MD, Mccrossan P. Paediatric pneumonia: a guide to diagnosis, investigation and treatment. Paediatr Child Health. 2021;31:250-257. [DOI] [Full Text] |
| 14. | Nayani K, Naeem R, Munir O, Naseer N, Feroze A, Brown N, Mian AI. The clinical respiratory score predicts paediatric critical care disposition in children with respiratory distress presenting to the emergency department. BMC Pediatr. 2018;18:339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Liu YC, Liu JH, Fang ZA, Shan GL, Xu J, Qi ZW, Zhu HD, Wang Z, Yu XZ. Modified shock index and mortality rate of emergency patients. World J Emerg Med. 2012;3:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Nazar M, Kumar H, Krishnegowda M, Unki P, Veerappa N, Srinivas BK. Validation of the Shock Index, Modified Shock Index, and Shock Index-Paediatric age-Adjusted (SIPA) for predicting length of stay and outcome in children admitted to a paediatric intensive care unit. Egypt Pediatric Association Gaz. 2022;70:12. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Zhang H, Tao Y, Wang Z, Lu J. Evaluation of nutritional status and prognostic impact assessed by the prognostic nutritional index in children with chronic kidney disease. Medicine (Baltimore). 2019;98:e16713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Chan AS, Rout A. Use of Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in COVID-19. J Clin Med Res. 2020;12:448-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 19. | Taha M, Nael Maslamani A, Atef Abdelsattar Ibrahim H. The Predictive and Prognostic Value of Percentage Change in Calf Circumference in Infants and Children During the First Week of Admission in the Pediatric Intensive Care Unit: A Prospective Cohort Study. Clin Pediatr (Phila). 2024;63:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Mehta NM, Skillman HE, Irving SY, Coss-Bu JA, Vermilyea S, Farrington EA, McKeever L, Hall AM, Goday PS, Braunschweig C. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2017;41:706-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 21. | Tume LN, Valla FV, Joosten K, Jotterand Chaparro C, Latten L, Marino LV, Macleod I, Moullet C, Pathan N, Rooze S, van Rosmalen J, Verbruggen SCAT. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensive Care Med. 2020;46:411-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 22. | Atef Abdelsattar Ibrahim H, Elkhashab K, Khaled Ayada I, Magdy H, Sobhy Menshawy S. The Effect of Oral Immunotherapy on Preterm Neonates: A Promising Adjuvant Therapy in a Clinical Trial Study. Neonatology. 2025;1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Zar HJ, Andronikou S, Nicol MP. Advances in the diagnosis of pneumonia in children. BMJ. 2017;358:j2739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Natarajan T, Sahubar Sadique TN, Shanmugham K. Incidence of hyponatremia and its utility as an indicator of morbidity in children hospitalised with community acquired pneumonia. Int J Contemp Pediatr. 2020;7:616. [DOI] [Full Text] |
| 25. | Madasu T, Srikanth S, Kishan TR. A Study of Hyponatremia as a Predictor of Severity in Pediatric Community Acquired Pneumonia (Cap) In Children Aged 2 Months-5 Years Admitted To a Tertiary Care Center. Int J Pharm Res Technol. 2025;15:1396-1400. |
| 26. | Alkholy UM, Zidan NI, Awd-Allah Esawy NI, Yousif Hassan YM. Utility of Hyponatremia as Prognostic Factor of Community Acquired Pneumonia in Children Above Two Years old. Zagazig Univ Med J. 2025;. [DOI] [Full Text] |
| 27. | Edmonds ZV. Hyponatremia in pneumonia. J Hosp Med. 2012;7 Suppl 4:S11-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Ata Sobeih A, Abo Elfetoh Elfiky O, Abd Elalim MA, Mohammed Zakaria R. Role of hyponatremia in prediction of outcome in children with severe lower respiratory tract infections. Benha Med J. 2025;42:293-302. [DOI] [Full Text] |
| 29. | Al Yaqoubi IH, Al-Maqbali JS, Al Farsi AA, Al Jabri RK, Khan SA, Al Alawi AM. Prevalence of hyponatremia among medically hospitalized patients and associated outcomes: a retrospective cohort study. Ann Saudi Med. 2024;44:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Do C, Evans GJ, DeAguero J, Escobar GP, Lin HC, Wagner B. Dysnatremia in Gastrointestinal Disorders. Front Med (Lausanne). 2022;9:892265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Nalin DR, Harland E, Ramlal A, Swaby D, McDonald J, Gangarosa R, Levine M, Akierman A, Antoine M, Mackenzie K, Johnson B. Comparison of low and high sodium and potassium content in oral rehydration solutions. J Pediatr. 1980;97:848-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Ravioli S, Gygli R, Funk GC, Exadaktylos A, Lindner G. Prevalence and impact on outcome of sodium and potassium disorders in patients with community-acquired pneumonia: A retrospective analysis. Eur J Intern Med. 2021;85:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/