Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.110003
Revised: June 15, 2025
Accepted: September 4, 2025
Published online: December 9, 2025
Processing time: 157 Days and 7.5 Hours
Mucopolysaccharidosis type VI (MPS VI) is a chronic, progressive, inherited disease with multiorgan involvement and a restricted life expectancy.
To investigate the epidemiological, clinical, and genetic characteristics of patients with mucopolysaccharidosis type 6 and their outcomes using the Russian Fed
In a retrospective cohort study, clinical, laboratory data, molecular genetic analysis results, and enzyme replacement therapy (ERT) data were extracted and analyzed from the Russian MPS VI registry for 53 patients, comprising 26 males (49.1%) and 27 females (50.9%).
The median age of first symptoms was 2 years, ranging from the first months of life to 20 years. A positive family history of MPS VI was reported in 19/53 (35.8%) patients, a negative family history in 24 (45.3%), and missing information in 10 (18.9%). The main features of the disease were hepatomegaly (n = 23; 60.5%), splenomegaly (n = 15, 39.5%), involvement of otolaryngological organs (n = 24/33; 72.7%), umbilical and inguinal hernia (n = 19/36; 52.8%), heart involvement (n = 26/32; 81.3%) with valve involvement (n = 25/26; 96.2%) and linear growth delay (n = 30/39, 76.9%). Two patients (3.8%) died. The most common variants identified in the ARSB gene were c.454C>T and c.194C>T. At the time of data collection, ERT had ever received 48/53 (90.5%) patients.
No correlation was observed between the age of onset of the first symptoms, the severity of clinical manifestations, enzyme activity, or nucleotide variants in the ARSB gene.
Core Tip: The prevalence of mucopolysaccharidosis type VI (MPS VI) in Russia is close to that in the United States and Japan. The main clinical symptoms included the Hurler phenotype, heart involvement, delayed linear growth, otolaryngological symptoms, a short neck, hepatomegaly, and inguinal hernias. All patients exhibited orthopedic manifestations, including chest deformity, spine stiffness, and joint stiffness. The pathogenic variant c.454C>T in the ARSB gene is the most common in patients with MPS VI in Russia, followed by pathogenic variant c.194C>T (p.Ser65Phe). There was no corre
- Citation: Vechkasova AO, Zakharova EY, Buchinskaya NV, Vashakmadze ND, Namazova-Baranova LS, Ivanov DO, Kutsev SI, Kostik MM. Clinical and genetic characteristics of mucopolysaccharidosis type VI according to the Russian registry. World J Clin Pediatr 2025; 14(4): 110003
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/110003.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.110003
Mucopolysaccharidosis type VI (MPS VI) or Maroteaux-Lamy syndrome is a rare autosomal recessive disorder associated with lysosomal glycosaminoglycans (GAG) storage. It has a characteristic progressive course and multisystem, multiorgan involvement[1]. The enzyme ARSB breaks GAG: Dermatan and chondroitin sulfate. Genetic variants in the ARSB gene result in reduced ARSB activity, leading to MPS VI[2]. The ongoing international registry of patients with lysosomal storage diseases, established in 2022, includes data on patients with MPS VI[3], and no separate registry exclusively for patients with MPS VI exists.
The disease is characterized by a broad spectrum of symptoms from slowly to rapidly progressive forms. The manifestation of the rapidly progressive form of MPS VI occurs during the first 2-3 years of life. However, slowly progressive forms have later and milder clinical manifestations compared to rapidly progressive forms. The average age of disease manifestation in slowly progressive forms is 10 years, with an average diagnosis age of 23.5 years, ranging from 9 to 42 years[1]. Some experts suggest splitting the slowly progressive form into osteoarticular and cardiac types based on the primary clinical manifestation[4].
The rate of progression depends on the degree of dermatan sulfate deposition in the tissues. High levels of GAG in urine (> 200 μg/mmol creatinine) are characteristic of patients with severe clinical manifestations and rapidly progressive forms who have not received enzyme replacement therapy (ERT). However, a GAG level in urine < 100 μg/mmol creatinine is characteristic of slowly progressive forms[5].
MPS VI-characteristic phenotypic features include coarse facial features, prominent frontal tubercles, hypertelorism of the eyes, flattened nasal bridge, low-set ears, full lips, gingival hyperplasia, macroglossia, short neck, and thick and coarse hair (Hurler phenotype). Clinical manifestations of MPS VI include damage to the musculoskeletal system in the form of scoliosis and/or kyphosis, lordosis, dysostosis multiplex, joint stiffness with subsequent development of contractures and the formation of a characteristic sign of mucopolysaccharidosis (MPS)-claw-shaped deformity of the hand. Short stature is also a typical hallmark of the disease[6].
The most common first sign in patients with slowly progressive forms is a decreased range of motion of the large joints of the upper and lower extremities, primarily the hip joint[4].
Typical radiographic changes include thickened, short metacarpals, irregular and hypoplastic carpal and tarsal bones, dysplastic femoral head and severe hip dysplasia, abnormal development of the vertebral bodies, broad and short ribs, thickened and shortened clavicles, hypoplastic distal ulna and radius, but these features are similar to mild forms of other types of MPS[7].
Ophthalmological manifestations include corneal opacity, refractive errors, edema and atrophy of the optic disc, and glaucoma[8]. These patients are also characterized by frequent rhinosinusitis, adenoiditis, tonsillar hypertrophy, otitis media, and hearing loss[8]. Hearing loss can be either conductive, sensorineural, or mixed, causing delayed speech development in some patients.
Involvement of otolaryngological organs in MPS is associated with GAG deposition in mucous membranes and other tissue layers, such as the tongue and tonsils, leading to nasopharyngeal and oropharyngeal airway obstruction and sleep apnea[9,10]. Patients with MPS VI may have features of obstructive and restrictive lung disease. Obstructive lung disease is associated with narrowing of the bronchial airways and tracheobronchomalacia, which can lead to acute airway obstruction or collapse. Restrictive lung disease is characterized by a small, rigid rib cage and abdominal distension combined with kyphosis, scoliosis, and lumbar hyperlordosis[10,11]. Ventriculomegaly, communicating hydrocephalus, spinal cord compression in the cervical spine with the development of myelopathy, and carpal tunnel syndrome are the most common neurological manifestations of this type of MPS. Patients with MPS VI typically experience normal intellectual development[1].
Сardiovascular damage may be observed similar to other types of MPS. The mitral, aortic, and tricuspid valves are most often involved, and surgical intervention, including valve replacement, is often required. Pulmonary hypertension, cardiomyopathy, and endomyocardial fibroelastosis are less common symptoms. Cardiac and respiratory failure is the main leading cause of death due to the progress of cardiopulmonary damage[7]. National data of MPS VI is limited to a few countries.
The registry of patients with MPS VI in the Russian Federation was launched in 2008, and the last update and expansion were made in 2022. The registry includes data on patients with MPS, confirmed by both biochemical and/or molecular genetic tests. Geneticists or other specialists familiar with MPS VI who monitor patients at their residences entered information about patients. The responsible physician enters information about patients in each subject of the Russian Federation. All patients with MPS VI are eligible for inclusion in the registry, regardless of whether they receive ERT, age, or disease severity.
The clinical diagnosis of MPS VI was confirmed by the high excretion of GAG (dermatan sulfate) in urine, enzymatic deficiency of ARSB in leukocytes, plasma, or dried blood spots, and molecular genetic testing.
This study utilized data from the "Registry of Patients with MPS VI of the Russian Federation", which was provided by the Association of Medical Geneticists and the Federal State Budgetary Scientific Institution "Research Center for Medical Genetics". The patient or his legal representative signed an informed consent to include the data. Data were collected and analyzed using the Quinta CRM platform (certificate of state registration of computer programs #2016615129 for Quinta: Universal software for remote collection, processing, and management of geographically distributed clinical and epidemiological data; copyright holder-Aston Consulting) in partnership with Aston Consulting.
The registry includes the patient's data, existing clinical manifestations, results of biochemical, instrumental, and molecular genetic studies, data on surgical interventions, the time of symptom onset, diagnosis, initiation of ERT, and outcomes.
The retrospective cohort study included data on 53 patients with MPS VI, extracted in February 2025 from the Russian national registry for the period from 2008 to 2025.
The study was approved by the Ethics Committee of St. Petersburg State Pediatric Medical University (Protocol No. 1, dated January 19, 2009). Participation in the registry was voluntary. Patients' parents signed informed consent permitting the inclusion of data in the registry and subsequent use for scientific purposes in an anonymized form.
The following information was extracted from the registry for patients.
Demographics: Age, sex, residence, data about family history of MPS VI, age of first symptom(s), age and time to diagnosis. The age of the first symptom was determined retrospectively by an experienced physician who included the patient in the registry based on their personal opinion on whether the symptom could be associated with MPS VI.
Clinical manifestations: Which organs and/or organ systems were involved in the pathogenesis of the disease in most cases, how often neurological manifestations were observed, and which ones, etc.
Laboratory data: Laboratory data include genetic analysis (Sanger sequencing), urinary GAG excretion, and ARSB activity, as available.
Treatment: Number of patients treated with ERT, time from diagnosis to start of therapy, duration of therapy.
Outcomes: Proportion of patients alive and deceased.
We utilized the STATISTICA software package, version 10.0 (StatSoft Inc., St. Tulsa, OK, United States). Numerical indicators were presented with the median and interquartile range (IQR; 25th-75th percentiles), and categorical variables were presented with absolute numbers and percentages (%). We used the Pearson χ2 criteria to compare independent categorical variables and the Mann-Whitney criteria for independent numerical variables. Differences were considered statistically significant if the P value was less than 0.05.
Data about 53 patients with MPS type VI were available in the registry at the time of the study, of whom 51 (96.2%) are currently alive and two patients (3.8%) died. The cohort consisted of 26 male (49.1%) and 27 female (50.9%). The median age of first symptoms was 2 years, ranging from the first months of life to 20 years. A positive family history of MPS VI was reported in 19/53 (35.8%) patients, a negative family history in 24 (45.3%), and data were missing for 10 (18.9%) patients.
The median time from the onset of the first symptom to molecular confirmation was 4 years, with a range of 0 to 36 years. The median time from enzyme activity assessment to genetic confirmation was 0 months, with a range of 0 to 13 years.
The primary symptoms of the disease were hepatomegaly (n = 23, 60.5%) and splenomegaly (n = 15, 39.5%). Hepatosplenomegaly was observed in 13 of 38 patients (34.2%). Umbilical and inguinal hernias had 19/36 (52.8%). Delayed linear growth was seen in 30/39 (76.9%) patients. The Hurler phenotype was noted in 34 of 39 (87.2%) patients, and a short neck was diagnosed in 28 of 39 (71.8%) patients.
According to the registry data, heart involvement (n = 26/32; 81.3%) was detected in 25/32 patients (78.1%) with changes in the heart valves. Respiratory complications were reported in 9 of 26 (34.6%) patients.
Thirty-nine out of 39 patients (100%) exhibited orthopedic manifestations of the disease: Chest deformity (n = 26, 66.7%), spine involvement (n = 30, 76.9%), and joint stiffness (n = 38, 97.4%).
The otolaryngological organs involvement had (24/33; 72.7%) of patients, and hearing loss was reported in 14/32 patients (43.8%).
Hydrocephalus was diagnosed in 7/32 (21.9%) patients, and epilepsy only in 1/33 patients (3%). Delayed psychomotor development before the first year was 5/31 (16.1%), and delayed psychomotor development before 3 years was 5/30 (16.7%). Mental retardation at the age of 5-12 and over 12 years was observed with the same frequency in 16.1% (n = 5/31, 16.1%). Visual impairment was diagnosed in 31 of 35 (88.6%) individuals.
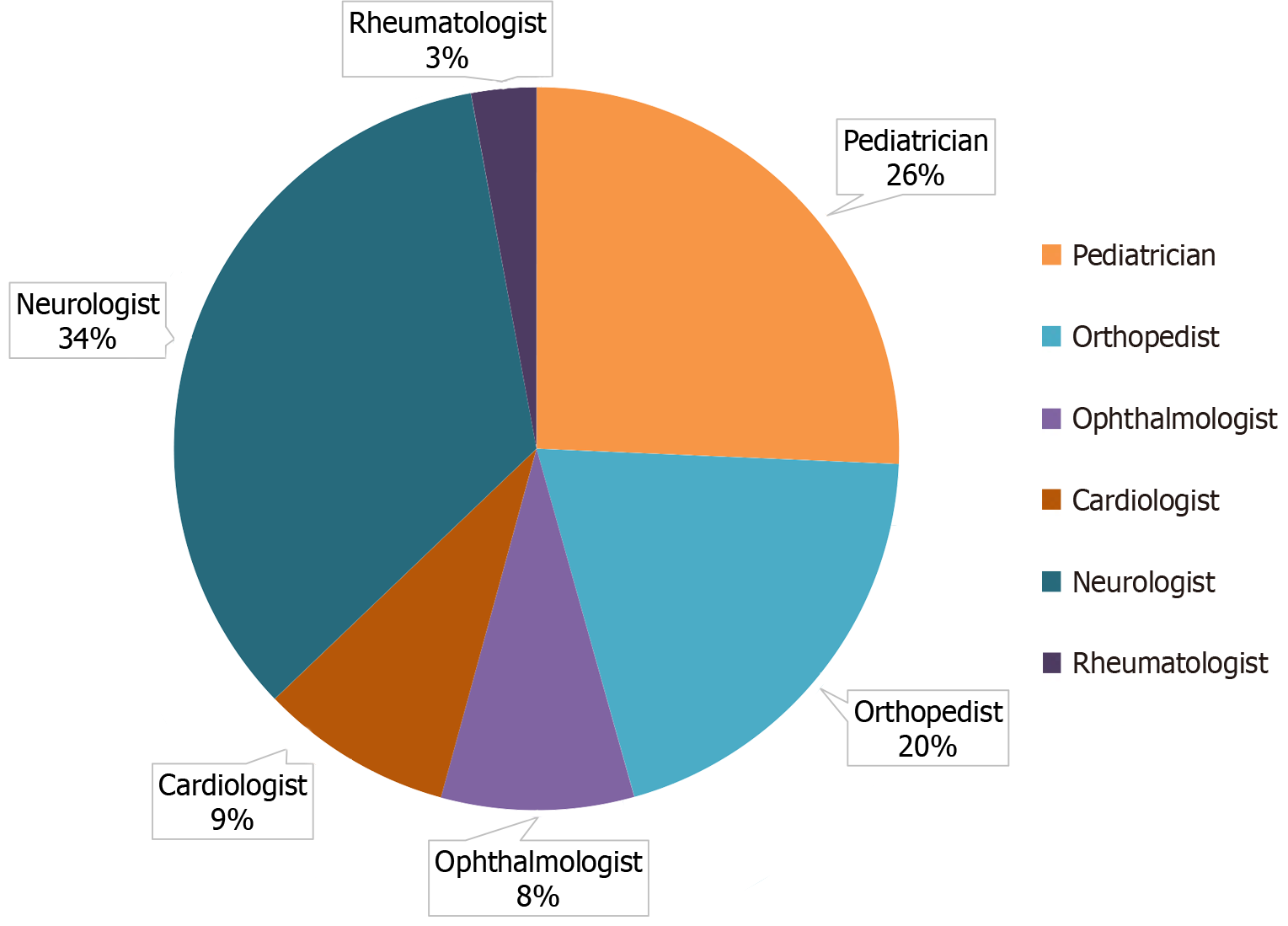
The leading specialists (data provided for 35 patients) who referred patients to a geneticist were neurologists (n = 12, 34.3%), pediatricians (n = 9, 25.7%), and orthopedists (n = 7, 20%). The complete list of specialists is presented in Figure 1.
The following biochemical studies were used to diagnose the disease: (1) Total urine GAG [elevated GAG levels were detected in 17/20 (85%) patients]; (2) Assessment of fractional GAG excretion [elevated dermatan sulfate levels were detected in 17/18 (94.4%) patients]; and (3) Assessment of enzyme activity in a dry blood spot [a decrease in ARSB activity was detected in 34/34 (100%) patients].
The majority of patients (n = 48/53; 90.5%) had ever received ERT. Of these, 46/48 (95.8%) were alive, 2/48 (4.2%) had died from various causes at the time of data collection, and three patients had not received ERT.
Multiple organ failure at age 25 was indicated as the underlying cause of death in one of the two patients. In the second patient, the cause of death and age at death were not specified. Detailed demographic characteristics of MPS VI patients from the registry analysis as of February 2025 are presented in Table 1.
| Parameter | n | Results (n = 53), n (%) or median (Q1; Q3)/ minimum and maximum |
| Sex, female/male, n (%) | 27/26 | 27/53 (50.9)/27/53 (49.1) |
| Current age of the patient, years | 53 | 19.0 (12.0; 30.0)/1.0-52.0 |
| Age of first symptoms, years | 38 | 2 (1.0; 4.0)/0.0-20.0 |
| Age of molecular genetic diagnostics, years | 40 | 11 (5/0; 19.0)/0.0-45 |
| Age at diagnosis, years | 44 | 7 (3.0; 14.0)/0.0-41 |
| Time since first symptoms to genetic confirmation, years | 34 | 7 (2.0; 15.0)/0.0-36 |
| Ever received ERT, n (%) | 48 | 48/51 (94.1) |
| Time since first symptoms of ERT, years | 25 | 7 (4.0; 14.0)/0.0-36 |
| Time since diagnosis to ERT, years | 27 | 2 (0.0; 10.0)/0.0-21 |
| Alive/died, n (%) | 53 | 51 (96.2)/2 (3.8) |
| Age of death, years | 1 | 25 |
Nineteen (36.5%) patients had relatives suffering from a similar disease, mainly siblings (n = 16).
Patients with MPS VI are distributed unevenly in Russia: The majority of patients live in the Ural and Central Federal Districts-10 (prevalence 0.01 per 100000 residents), nine (0.009 per 100000 residents) in the North-Caucasian Federal District, and seven (prevalence 0.007 per 100000 residents) in the Northwestern Federal District. The exact number of patients is registered in the Privolzhsky and Siberian Federal Districts-6 (prevalence 0.006 per 100000 residents), and the smallest number of patients is registered in the Southern Federal District-3 (prevalence 0.003 per 100000 residents) and Far Eastern Federal District-2 (prevalence 0.002 per 100000 residents).
The average prevalence of MPS VI in the Russian Federation is 3.9 per 100000 newborns or 0.036 per 100000 popu
| Federal district | Number of patients with MPS VI, n (%) | Number of residents in the federal district | Prevalence of MPS VI in the federal district per 100000 live births |
| Central | 10 (18.9) | 40198659 | 0.03 |
| Southern | 3 (5.7) | 16624081 | 0.02 |
| Northwestern | 7 (13.2) | 13840352 | 0.05 |
| Privolzhsky | 6 (11.3) | 28540832 | 0.02 |
| Far-Eastern | 2 (3.8) | 7866344 | 0.03 |
| North-Caucasian | 9 (16.9) | 10251083 | 0.09 |
| Ural | 10 (18.9) | 12262295 | 0.08 |
| Siberian | 6 (11.3) | 16567143 | 0.04 |
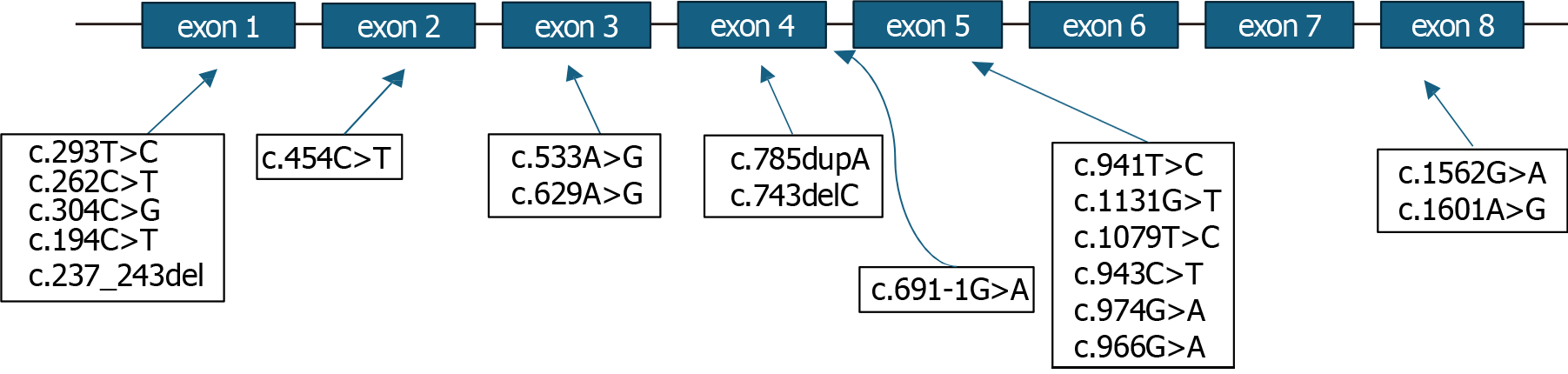
Molecular genetic testing was available for analysis in 51 of 53 (96.2%) patients, and in two patients (3.8%), the information on genetic testing was missing. Single nucleotide variants in the ARSB gene were detected in 47 of 53 (88.7%) patients, and gross rearrangements of the ARSB gene were not identified (Table 3; Figure 2).
| Single nucleotide variant | Number of patients with a specific variant, n = 51 |
| с.1601А>G (p.Ter534Trp) | 2 (3.9) |
| c.691-1G>A | 1 (2.0) |
| c.454C>T (p.Arg152Trp) | 22 (43.1) |
| c.966G>A (p.Trp322*) | 5 (9.8) |
| c.194C>T (p.Ser65Phe) | 10 (19.6) |
| c.304C>G (p.Arg102Gly) | 1 (2.0) |
| c.797A>C (p.Tyr266Ser) | 2 (3.9) |
| c.974G>A (p.Gly325Asp) | 1 (2.0) |
| c.943C>T (p.Arg315*) | 5 (9.8) |
| c.237_243delGGTGCTC (p.Val80Trpfs*32) | 1 (2.0) |
| c.629A>G (p.Tyr210Cys) | 4 (7.8) |
| c.1562G>A (p.Cys521Tyr) | 2 (3.9) |
| c.533A>G (p.His178Arg) | 2 (3.9) |
| c.262C>T (p.Gln88*) | 3 (5.9) |
| c.1079T>C (p.Leu360Pro) | 1 (2.0) |
| c.743delC (p.Pro248fs) | 1 (2.0) |
| c.785dupA (p.Asn262fs) | 1 (2.0) |
| c.293T>C (p.Leu98Pro) | 2 (3.9) |
| c.1131G>T (p.Trp377Cys) | 1 (2.0) |
| c.941T>C (р.Leu314Pro) | 3 (5.9) |
| ex5: Del2635kb | 1 (2.0) |
| с.990_1003del14 | 1 (2.0) |
The following types of single nucleotide variants were identified (number of variants, n = 17): Pathogenic (n = 10/17; 58.8%), likely pathogenic (n = 2/17; 11.8%), variants with unknown clinical significance (n = 5/17; 29.4%).
Missense variants (14 different missense variants in total) were detected in 43/51 (84.3%) patients, nonsense variants (3 different nonsense variants in total) in 12/51 (23.5%) patients, and frameshift (n = 3/51; 5.9%), splice site variants (n = 1/51; 2.0%), and intronic variants (n = 1/51; 2.0%) were the minors.
One patient had a previously undescribed 14-nucleotide deletion (c.990_1003del14), which is not mentioned in the Franklin database (https://franklin.genoox.com/clinical-db/home). The next patient had a 2635 kb deletion in exon 5 of the ARSB gene, which had not been described previously (Supplementary Table 1).
The most common variants identified in the ARSB gene were c.454C>T, detected in 22 patients from different regions of Russia, and variant c.194C>T, detected in 10 patients from different regions of Russia.
We provide the clinical and molecular epidemiology data of patients with MPS VI based on the Russian national MPS registry.
No convincing correlation has been established between the age of onset of the first symptoms, the severity of clinical manifestations, enzyme activity, and nucleotide variants in the ARSB gene, according to our data. According to studies conducted by Tomanin et al[2] and Saito et al[12], no genotype-phenotype correlation was observed. However, according to the literature, specific variants (e.g., large deletions, nonsense mutations, frameshifts, and certain missense mutations) may be associated with the classic, rapidly progressive phenotype[13].
The age of manifestation and diagnosis varies greatly. The average age of the first symptoms was 2 years (0.0-20.0), and the age of diagnosis was 7 years (0.0-40.0). Despite specialists' awareness of the primary clinical manifestations of MPS VI, the correct diagnosis is often delayed by approximately 4 years. Thus, early screening programs based on the main symptoms are needed. The disease is characterized by a broad spectrum of symptoms, from slowly to rapidly progressive forms.
MPS VI occurs with equal frequency in males (26, 49.1% of 53) and females (27, 50.9%). A positive family history was found in 35.8% of patients, highlighting implications for genetic counseling in high-risk families.
According to our study, the main symptoms include musculoskeletal system symptoms (joint stiffness, chest, and spine deformity), growth retardation, hepatosplenomegaly, the presence of inguinal and/or umbilical hernias, as well as involvement of the ENT and cardiopulmonary systems. The same data were obtained from a study by foreign colleagues. Clinical manifestations of MPS VI included musculoskeletal system involvement, presenting with scoliosis and/or kyphosis, lordosis, multiple bone dysostosis, joint stiffness, and subsequent development of contractures, as well as the formation of a characteristic sign of MPS: A claw-shaped hand deformity. Short stature is also described in patients with this type of MPS, but in our cohort, 9/39 (n = 5; 23.1%) had normal linear growth[6]. Patients with this type of MPS often experience ENT involvement, including frequent rhinosinusitis, adenoiditis, tonsil hypertrophy, otitis, and hearing loss[8]. Mitral, aortic, and tricuspid valves are frequently affected. Less common symptoms include pulmonary hypertension, cardiomyopathy, and fibroelastosis[7]. The analysis of the frequency of heart (cardiomyopathy) and valve involvement in our patients was similar to that in previously published studies. According to the registry, cardiovascular disease (n = 26/32; 81.3%) was detected, with changes in the heart valves observed in 25/32 patients (78.1%) and cardiomyopathy in 11/32 (34.4%).
The feature of MPS VI neurological symptoms was low epilepsy rates (3%) while hydrocephalus (21.9%). According to literature data, hydrocephalus is one of the main symptoms of CNS damage but does not lead to cognitive deficits[14]. Seizures are a rare symptom, not typically associated with MPS VI, and occur in isolated cases[15].
An analysis of mortality in our cohort of MPS VI patients could not be performed since, at the time of registry data analysis, the majority of patients (n = 51/53; 96.2%) were alive, death occurred in 2/53 (3.8%), and the cause of death-multiple organ failure – was indicated in only one patient.
More than 220 variants with varying clinical significance have been previously identified in the ARSB gene: Most of them were missense variants (59.5%), followed by small deletions (13.5%), nonsense variants (12.0%), splice (5%) or intronic variants (5.0%), small duplications (3.0%) and large deletions (3.0%). Variants c.454C>T (p.Arg152Trp) and c.962T>C (p.Leu321Pro) are the most common variants in Russia and Turkey, respectively[2]. Two novel variants (c.990_1003del14, ex5: Del2635kb)-stress importance for genetic databases.
According to the previously published study of Voskoboeva et al[16], 28 pathogenic variants were identified in 68 patients (57 families) with MPS VI in Russia. According to the results of their study, the most common nucleotide changes were NM_000046.5: C.194C>T and NM_000046.5: C.454C>T. Five new pathogenic variants were identified; these alleles have not been described previously (NM_000046.5: C.304C>G, NM_000046.5: C.533A>G, NM_000046.5: C.941T>C, NM_000046.5: C.447_456del10 and NM_000046.5: C.990_10003del14). The nucleotide variant NM_000045.6: C.454C>T was the predominant allele among Slavic Russian patients. The nucleotide variant NM_000045.6: C.194C>T was detected only in families with MPS VI from the Republic of Dagestan. The authors also identified ethnic characteristics of the distribution of some mutations. Variants c.454C>T and c.943C>T were most frequently encountered among Russian patients of Slavic origin in the central, southeastern, and eastern regions of the Russian Federation. The nucleotide substitution c.194C>T is most common in the Republic of Dagestan, in the Republic of North Ossetia-Alania -c.691-1G>A[16]. These data are consistent with the results we obtained in our study. The most common variant identified in patients with MPS in Russia was c.454C>T, which is similar to the variant found in the Eastern European population[17]. The nonsense variant -c.979C>T (p.Arg327*) was another most common variant, according to the study by Tomanin et al[2]. It was registered 16 times (1.8%), but this variant was not detected in any patient with MPS VI in our cohort.
The prevalence of MPS VI ranges from 1:43261 to 1:505160 Live births[7,18-20]. The incidence of MPS type VI was 0.013:100000 live births in Poland, 0.03:100000 in Japan, 0.05:100000 in Denmark and in the Czech Republic[21], 0.04:100000 live births (United States) and up to 0.29:100000 in British Columbia[20,22]. A very high frequency of MPS VI was found among the Turkish population living in Germany, in contrast to the non-Turkish German population (1:43261 vs 1:432610, respectively)[19]. In Saudi Arabia, the frequency was 8:100000 live births, and in Brazil, it was 20:100000[23,24]. Detailed data are presented in Table 4[25-29].
| Country | Selective screening period (years) | Number of identified patients with MPS | MPS incidence per 100000 live births | Number of identified patients with MPS VI | MPS VI incidence per 100000 live births |
| Russia[16] | 31 | 467 | 3.9 | 68 | 0.036 |
| United States of America[20] | 20 | 789 | 0.98 | - | 0.04 |
| Japan[25] | 27 | 467 | 1.53 | 8 | 0.03 |
| Denmark[18] | 30 | 33 | 1.77 | 1 | 0.05 |
| Czech Republic[26] | 34 | 119 | 3.72 | 2 | 0.05 |
| Poland[21] | 40 | 392 | 1.8 | 4 | 0.013 |
| Switzerland[25] | 34 | 51 | 1.56 | 4 | 0.11 |
| Saudi Arabia[23] | 26 | 28 | 17.0 | 13.0 | 7.85 |
| Taiwan[27] | 21 | 130 | 2.04 | 9 | 0.14 |
| South Korea[28] | 19 | 147 | 1.35 | 2 | 0.019 |
| Australia[29] | 17 | 188 | 4.46 | 19 | 0.43 |
| Germany[19] | 16 | 474 | 3.51 | 33 | 0.23 |
Voskoboeva et al[16] found the frequency of the nucleotide variant c.194C>T in the Republic of Dagestan to be 0.01, corresponding to a frequency of MPS VI of approximately 1:10000, one of the highest rates reported worldwide.
Based on an analysis of global data on the epidemiology of MPS VI, the prevalence of MPS in Russia is similar to that in the United States and Japan. However, the distribution of patients with MPS across the country is uneven, primarily due to economic factors and early access to treatment in large centers.
The study's main limitations were its small sample size, retrospective design, and missing data. Sensitivity analyses may be used to address missing genetic or family history data. Some patients were excluded from the registry due to delayed diagnosis of mild cases, or they remained undiagnosed. Additionally, some diagnosed patients declined to participate in the study. The missing data about the genetic variants of the ARSB gene reduces the possibility of associating phenotype and outcomes. A small sample size increases the risk of Type II errors, where actual effects may not be detected, espe
According to the registry data, the pathogenic variant c.454C>T in the ARSB gene is the most common in patients with MPS VI in Russia. The second most common was the pathogenic variant c.194C>T (p.Ser65Phe). The correlation between the age of onset of first symptoms, the severity of clinical manifestations and enzyme activity, and nucleotide variants in the ARSB gene has not been established. The prevalence of MPS type VI in Russia is close to the prevalence of this type of MPS in the United States of America and Japan. Orphan disease registries, including those for various types of MPS, are a simple and effective tool for assessing the clinical characteristics of the disease, demographic data, and the effectiveness of various treatments.
The authors thank Aston Consulting for technical support of the study.
| 1. | D'Avanzo F, Zanetti A, De Filippis C, Tomanin R. Mucopolysaccharidosis Type VI, an Updated Overview of the Disease. Int J Mol Sci. 2021;22:13456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Tomanin R, Karageorgos L, Zanetti A, Al-Sayed M, Bailey M, Miller N, Sakuraba H, Hopwood JJ. Mucopolysaccharidosis type VI (MPS VI) and molecular analysis: Review and classification of published variants in the ARSB gene. Hum Mutat. 2018;39:1788-1802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | MacKenzie TC. Registry of Patients Diagnosed With Lysosomal Storage Diseases. [accessed 2025 Jun 14]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT05619900 ClinicalTrials.gov Identifier: NCT05619900. |
| 4. | Jurecka A, Zakharova E, Malinova V, Voskoboeva E, Tylki-Szymańska A. Attenuated osteoarticular phenotype of type VI mucopolysaccharidosis: a report of four patients and a review of the literature. Clin Rheumatol. 2014;33:725-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Swiedler SJ, Beck M, Bajbouj M, Giugliani R, Schwartz I, Harmatz P, Wraith JE, Roberts J, Ketteridge D, Hopwood JJ, Guffon N, Sá Miranda MC, Teles EL, Berger KI, Piscia-Nichols C. Threshold effect of urinary glycosaminoglycans and the walk test as indicators of disease progression in a survey of subjects with Mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). Am J Med Genet A. 2005;134A:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Nicolas-Jilwan M, AlSayed M. Mucopolysaccharidoses: overview of neuroimaging manifestations. Pediatr Radiol. 2018;48:1503-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Valayannopoulos V, Nicely H, Harmatz P, Turbeville S. Mucopolysaccharidosis VI. Orphanet J Rare Dis. 2010;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Harmatz P, Shediac R. Mucopolysaccharidosis VI: pathophysiology, diagnosis and treatment. Front Biosci (Landmark Ed). 2017;22:385-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Gönüldaş B, Yılmaz T, Sivri HS, Güçer KŞ, Kılınç K, Genç GA, Kılıç M, Coşkun T. Mucopolysaccharidosis: Otolaryngologic findings, obstructive sleep apnea and accumulation of glucosaminoglycans in lymphatic tissue of the upper airway. Int J Pediatr Otorhinolaryngol. 2014;78:944-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Bianchi PM, Gaini R, Vitale S. ENT and mucopolysaccharidoses. Ital J Pediatr. 2018;44:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Muhlebach MS, Wooten W, Muenzer J. Respiratory manifestations in mucopolysaccharidoses. Paediatr Respir Rev. 2011;12:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Saito S, Ohno K, Sugawara K, Sakuraba H. Structural and clinical implications of amino acid substitutions in N-acetylgalactosamine-4-sulfatase: insight into mucopolysaccharidosis type VI. Mol Genet Metab. 2008;93:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Karageorgos L, Brooks DA, Pollard A, Melville EL, Hein LK, Clements PR, Ketteridge D, Swiedler SJ, Beck M, Giugliani R, Harmatz P, Wraith JE, Guffon N, Leão Teles E, Sá Miranda MC, Hopwood JJ. Mutational analysis of 105 mucopolysaccharidosis type VI patients. Hum Mutat. 2007;28:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Shapiro EG, Eisengart JB. The natural history of neurocognition in MPS disorders: A review. Mol Genet Metab. 2021;133:8-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 15. | Kaissi AA, Hofstaetter J, Weigel G, Grill F, Ganger R, Kircher SG. The constellation of skeletal deformities in a family with mixed types of mucopolysaccharidoses: Case report. Medicine (Baltimore). 2016;95:e4561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Voskoboeva E, Semyachkina A, Miklyaev O, Gamzatova A, Mikhaylova S, Vashakmadze N, Baydakova G, Omzar O, Pichkur N, Zakharova E, Kutsev S. Epidemiology and Genetics of Mucopolysaccharidosis Type VI in Russia. Front Mol Biosci. 2021;8:780184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Jurecka A, Piotrowska E, Cimbalistiene L, Gusina N, Sobczyńska A, Czartoryska B, Czerska K, Õunap K, Węgrzyn G, Tylki-Szymańska A. Molecular analysis of mucopolysaccharidosis type VI in Poland, Belarus, Lithuania and Estonia. Mol Genet Metab. 2012;105:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Malm G, Lund AM, Månsson JE, Heiberg A. Mucopolysaccharidoses in the Scandinavian countries: incidence and prevalence. Acta Paediatr. 2008;97:1577-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Baehner F, Schmiedeskamp C, Krummenauer F, Miebach E, Bajbouj M, Whybra C, Kohlschütter A, Kampmann C, Beck M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 278] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Puckett Y, Mallorga-Hernández A, Montaño AM. Epidemiology of mucopolysaccharidoses (MPS) in United States: challenges and opportunities. Orphanet J Rare Dis. 2021;16:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Jurecka A, Ługowska A, Golda A, Czartoryska B, Tylki-Szymańska A. Prevalence rates of mucopolysaccharidoses in Poland. J Appl Genet. 2015;56:205-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia, 1969-1996. Pediatrics. 2000;105:e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 279] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Moammar H, Cheriyan G, Mathew R, Al-Sannaa N. Incidence and patterns of inborn errors of metabolism in the Eastern Province of Saudi Arabia, 1983-2008. Ann Saudi Med. 2010;30:271-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Vairo F, Federhen A, Baldo G, Riegel M, Burin M, Leistner-Segal S, Giugliani R. Diagnostic and treatment strategies in mucopolysaccharidosis VI. Appl Clin Genet. 2015;8:245-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Khan SA, Peracha H, Ballhausen D, Wiesbauer A, Rohrbach M, Gautschi M, Mason RW, Giugliani R, Suzuki Y, Orii KE, Orii T, Tomatsu S. Epidemiology of mucopolysaccharidoses. Mol Genet Metab. 2017;121:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 26. | Poupetová H, Ledvinová J, Berná L, Dvoráková L, Kozich V, Elleder M. The birth prevalence of lysosomal storage disorders in the Czech Republic: comparison with data in different populations. J Inherit Metab Dis. 2010;33:387-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Lin HY, Lin SP, Chuang CK, Niu DM, Chen MR, Tsai FJ, Chao MC, Chiu PC, Lin SJ, Tsai LP, Hwu WL, Lin JL. Incidence of the mucopolysaccharidoses in Taiwan, 1984-2004. Am J Med Genet A. 2009;149A:960-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Cho SY, Sohn YB, Jin DK. An overview of Korean patients with mucopolysaccharidosis and collaboration through the Asia Pacific MPS Network. Intractable Rare Dis Res. 2014;3:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1518] [Cited by in RCA: 1529] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/