INTRODUCTION
Computed tomography (CT) has become a crucial tool for diagnosing and assessing various conditions in children. Since its introduction in the 1970s, CT technology has evolved significantly. It has progressed from basic single-source systems to advanced scanners with large detector elements, faster helical acquisitions, and the ability to capture multiple energy levels. Over the last two decades, dual-source CT systems have become more common in clinical practice, especially in pediatric care. The latest advancement in CT technology is photon-counting detectors (PCDs). On September 29, 2021, the Food and Drug Administration cleared the first photon-counting CT (PCCT) (developed by Siemens Healthineers) for clinical use[1]. This was followed by other medical imaging equipment manufacturers such as GE Healthcare and Philips producing similar machines[2,3]. This new technology has mainly been utilized in models and adult patients so far, with few applications in children. This narrative article will provide an overview of PCCT by describing its technical principles, highlighting its advantages compared to conventional CT technology, and presenting its current applications in pediatric cardiovascular imaging.
DETECTOR TECHNOLOGY
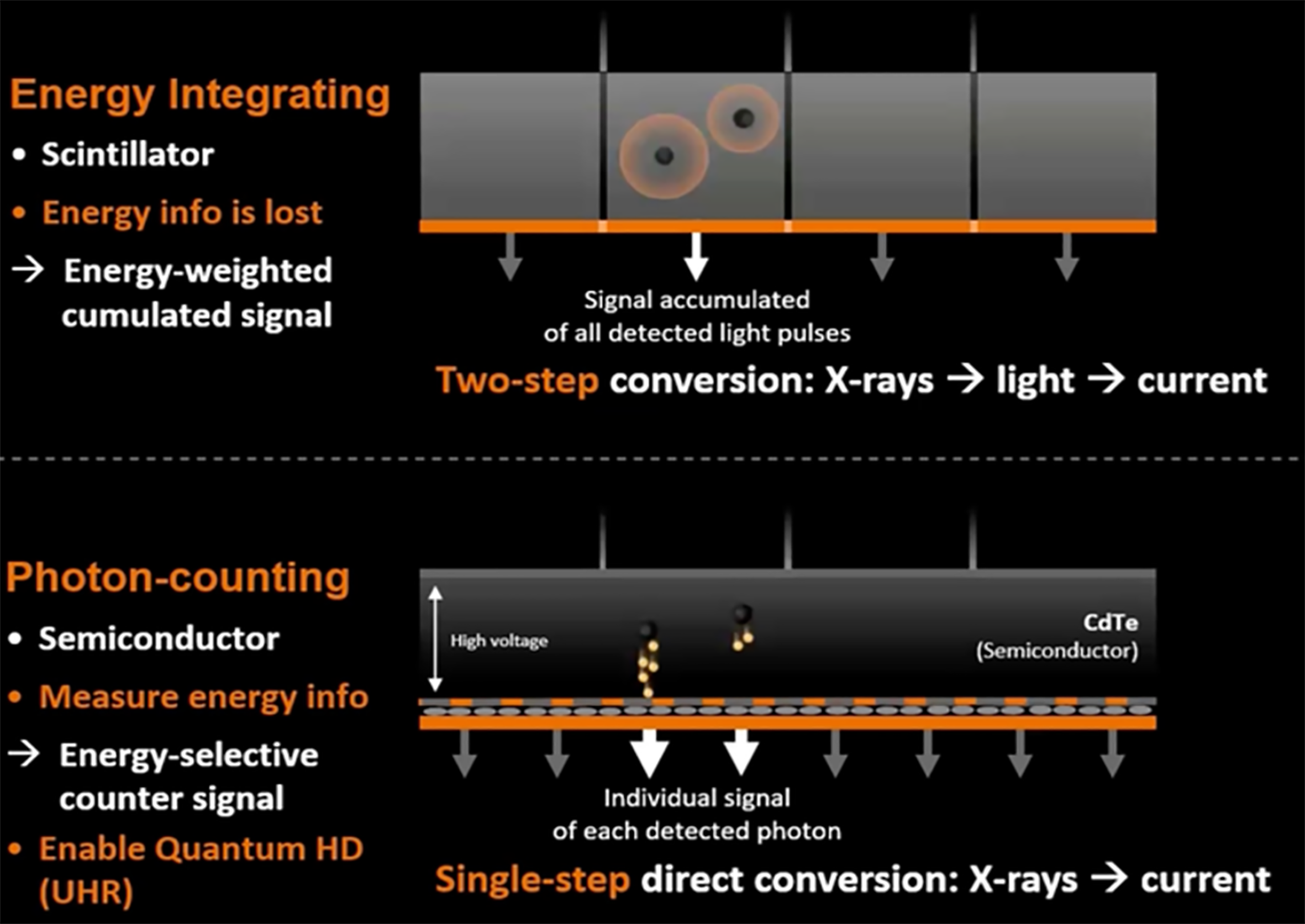
Regular CT detectors convert X-ray photons into visible light indirectly through solid-state scintillator components. A scintillator is a material that exhibits scintillation which in turn evokes the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate (i.e., re-emit the absorbed energy in the form of light). The light energy is then absorbed by specific photodiode detectors called energy-integrating detectors (EIDs). These detectors are capable of giving out an electric signal representing the total X-ray energy received by the detector during a measurement[4,5].
Conversely, PCDs consist of a single thick semiconductor with voltage applied to it, as opposed to a scintillator device. This allows the photons to be directly converted into an electric signal that matches the energy of the incoming photon (Figure 1). When photons hit the semiconductor, they create an electron cloud, which is directed by the applied voltage towards the detector anode. These detectors are linked to an electronic system application-specific integrated circuit comprising parallel channels, enabling the signal to be processed, and amplified sequentially to be categorized based on energy level. Two to eight energy levels are used to discriminate photons making photon counting analogous to color photography, where each photon’s differing energy affects the output, in comparison to charge integration, which considers only the intensity of the signal, as in black and white photography[4-6].
ADVANTAGES OF PCD SYSTEMS
Noise reduction
Noise in CT images come from two main sources: Quantum and electronic factors. Quantum noise arises due to the fluctuating number of photons reaching the detector, which is influenced by the random interactions of X-rays in the body. On the other hand, electronic noise is a product of the analogue circuits in modern CT hardware. Electronic noise becomes more apparent when there are fewer photons, which is often the case in pediatric CT scans that use lower voltages. PCD systems are designed to differentiate the energy of each photon, and sort them into specific energy levels. By setting thresholds on the lower energy levels above the system’s inherent electronic noise, PCD systems effectively remove electronic noise while keeping the contrast information found in lower-energy photons intact. In tests using phantoms, PCD systems were found to reduce noise by 58%-89% compared to traditional EIDs at the same radiation doses. Similarly, in real-life musculoskeletal exams, PCD systems showed similar noise levels but at lower radiation doses, ranging from 31%-47%[7-11].
Improved spatial resolution
The clarity of a CT image is limited by the size of the detector elements. Traditional detectors (such as EIDs) typically offer a resolution of around 0.5 mm × 0.5 mm at the center of the scan area, considering magnification effects. EID systems need reflective barriers between detector pixels to prevent light from escaping one pixel and reaching another, which reduces the effective detector area and efficiency. PCDs, however, do not require these barriers as they directly convert X-rays into an electric signal using a continuous semiconductor layer. As a result, PCDs have a larger total surface area available for detection. Different manufacturers offer PCD systems with element sizes ranging from 0.11 mm × 0.11 mm to 0.5 mm × 0.5 mm. The current PCD system from Siemens Healthineers (NAEOTOM Alpha) produces a maximum in-plane resolution of 0.11 mm, while the latest EID system (SOMATOM Force) offers a maximum in-plane resolution of 0.24 mm. Comparisons between PCD and EID systems have shown that PCDs offer far greater spatial resolution. In phantom models, the maximum in-plane resolution for PCDs is about 125 μM, while for EID systems, it’s around 278 μM. PCD CT has consistently demonstrated improved resolution in imaging small structures including coronary arteries, temporal bones, and pulmonary nodules compared to EID CT[12-16].
Improved contrast resolution
Soft-tissue contrast resolution is notably greater at lower energies because materials with low absorption transmit more low-energy photons. Traditional EID systems sum up the total energy of all detected photons over a period, meaning low-energy photons contribute less to the final image. Instead, PCD systems function by identifying the energy of each photon and accounting for them all equally, giving more weight to low-energy photons and enhancing overall image contrast. Furthermore, PCD systems allow signal binning based on energy levels, which can generate virtual monoenergetic images (VMIs)[17,18]. These low-energy VMIs not only enhance soft-tissue contrast but also improve the resolution of iodinated contrast material around its K-edge. Additionally, analyzing multiple energy spectra simultaneously enables the separation of different materials like calcium and water. PCD CT can also distinguish between multiple contrast agents. One study involving pigs demonstrated PCD CT’s material separation capabilities by creating dual-phase liver images from a single scan after the injection of two different contrast agents. This allowed the hepatic arteries, enhanced by iodinated contrast material, and the hepatic veins, enhanced by gadolinium-based contrast material, to be visualized separately[19-23].
Improved temporal resolution
Pediatric cardiovascular PCCT relies heavily on technical advancements for efficient and accurate diagnosis. Siemens Healthineers[6] has utilized dual X-ray sources rotating 90 degrees to offer a remarkable temporal resolution of 66 ms, which is approximately twice as fast as single-source CT. This enhancement facilitates high-pitch spiral imaging at 737 mm/second, enabling single-beat cardiac acquisition, even in challenging scenarios like dealing with agitated or rolling patients or managing chest pain[6,24].
Reducing artifacts
Artifacts are a common occurrence in clinical CT imaging and can imitate or hide genuine pathology. In particular, beam-hardening artifacts can cause distortion of CT images due to X-ray beams interacting with high-density materials more than low-density ones (like soft tissues). The distortion can be in the form of streaks or shading. In PCDs, constant weighting is used to normalize attenuation measurements across different energy levels, reducing beam-hardening artifacts. Additionally, high-energy thresholds act as a filter, which is beneficial in this regard. Calcium-blooming and metal artifacts commonly arise from volume averaging, motion, and beam hardening. PCDs significantly alleviate these artifacts due to enhanced spatial resolution and improved material decomposition, enabling precise separation of high-density materials (such as metals) from surrounding soft tissues. Moreover, PCCT’s faster acquisition times shorten scan durations and minimizes motion artifacts due to patient movement, which is highly beneficial in the context of pediatric imaging where there can be challenges in ensuring patients remain still during the scanning phase[25-28].
PEDIATRIC CARDIOVASCULAR IMAGING APPLICATIONS
PCD CT has been used in adults to assess the degree of coronary artery stenosis and calcifications. However, in the pediatric population, cardiac imaging is more challenging due to the smaller heart size, faster heart rates, and complex congenital or post-surgical anatomies, which can affect when intravenous contrast media should be administered. These challenges are especially pronounced when very young children require imaging for pre- and postoperative assessment of congenital heart conditions[29].
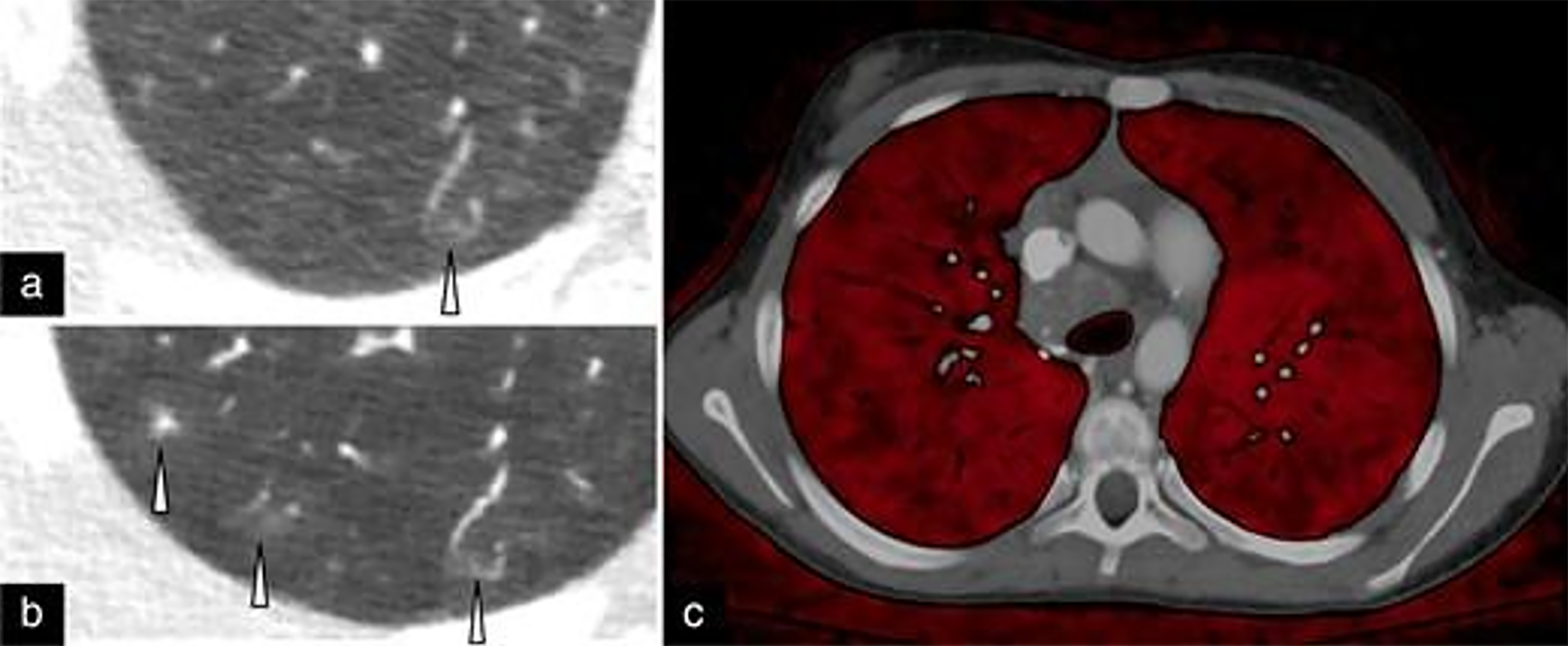
In a study involving 113 children with a median age of 66 days, Dirrichs et al[30] demonstrated that PCCT provided superior cardiovascular imaging quality compared to dual-source CT at an equitable radiation dose in children with suspicion of congenital cardiac defects. However, additional research is required to determine if ultra-high spatial resolution, iodine mapping, tissue characterization, or artifact-reducing algorithms could further enhance image quality. Figure 2 highlights the increased spatial resolution of PCD compared to EID-CT to identify small peripheral structures in the lung of a pediatric patient with hereditary hemorrhagic telangiectasia syndrome[29].
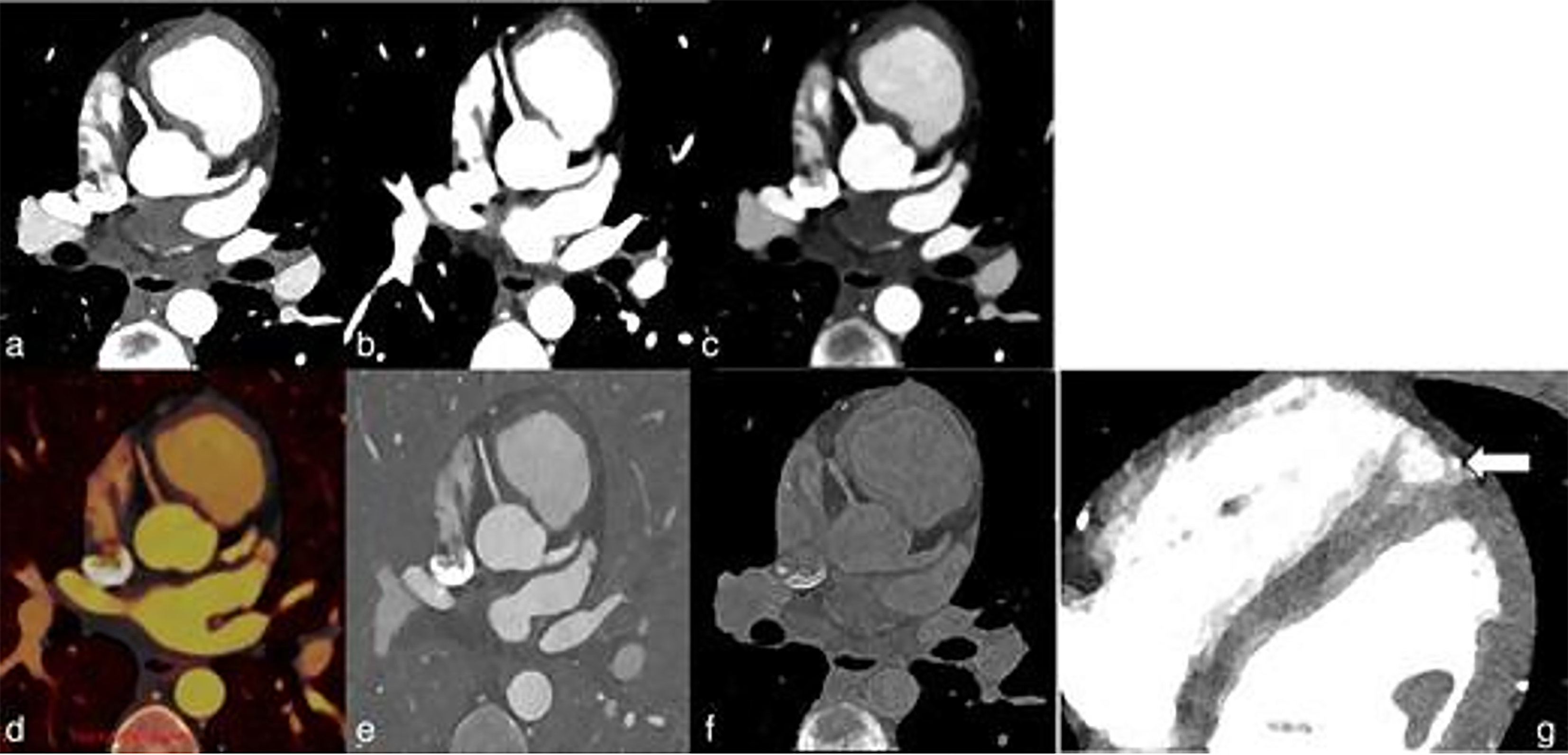
Multi-energy capabilities can be combined with high-pitch (pitch = 3.2) cardiac (66 ms temporal resolution) scanning to simultaneously reduce motion artifact and radiation dose. One application is to improve the visualization of cardiac anomalies in pediatric patients (Figure 3). This enhanced spatial resolution obtained using PCDs could potentially aid in creating three-dimensional models to guide surgical procedures. One of the trademark features of PCD CT is the extraordinary improvement in pediatric coronary imaging.
When utilizing imaging modalities in on children, it’s crucial to consider radiation exposure carefully, as children are more sensitive to radiation and have an increased risk of developing cancer after exposure in childhood. CT is particularly important in this context, as it contributes the most in radiation exposure in medicine. However, the role of CT cannot be understated and is often the gold-standard technique for planning interventions and influence decision-making in many pediatric diseases. The main advantage of PCD CT in pediatric radiology is its ability to reduce radiation exposure significantly. “Low dose” PCD CT offers far lower radiation doses while maintaining the accuracy of important anatomical structures and reducing image noise. This dose reduction effect is particularly accentuated disease that require serial scanning[30].
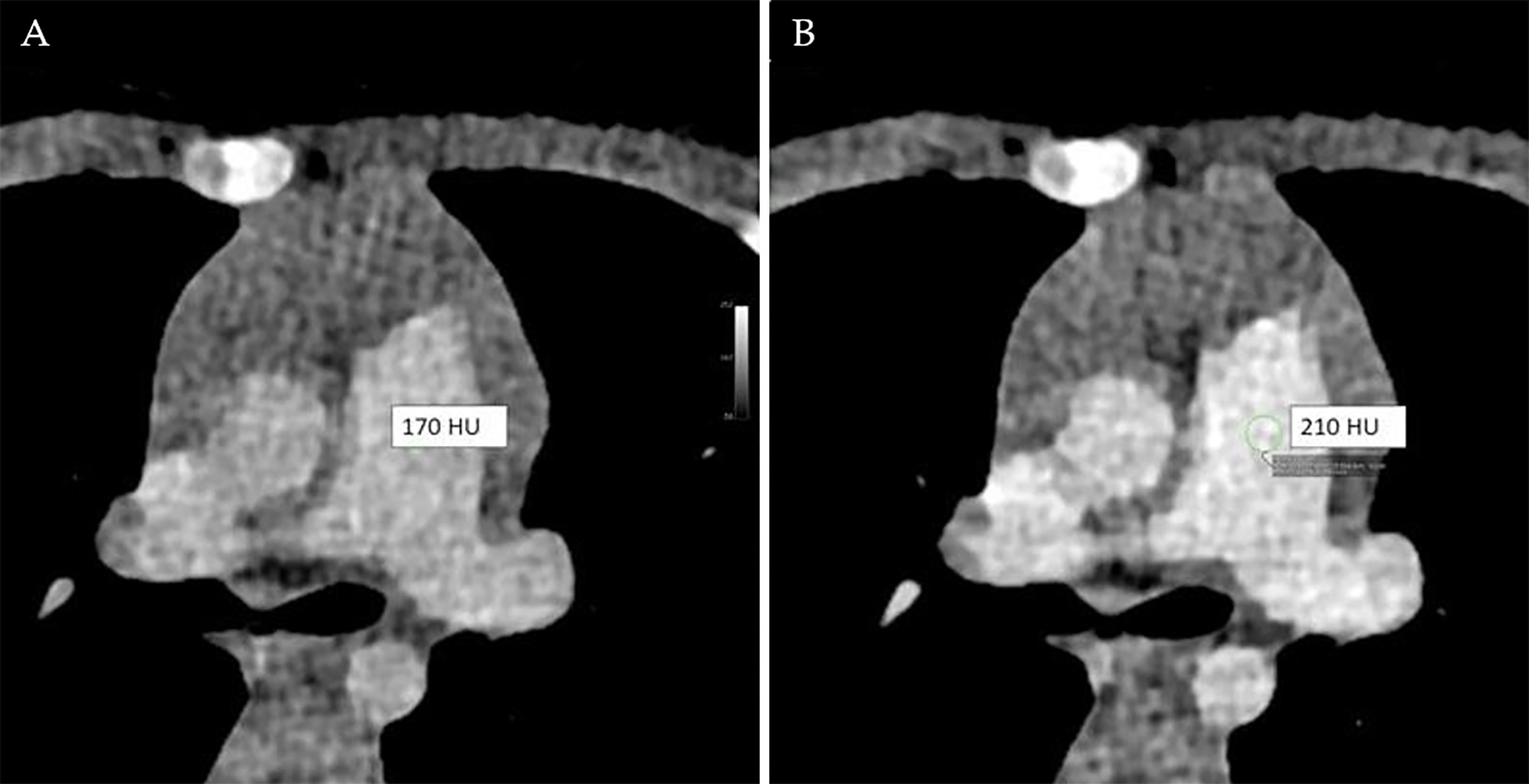
Another advantage of PCD CT is the ability to extract spectral information. Normally, a tube voltage of 120 kV or 140 kV is required for full spectral evaluation, but these levels are seldom used in pediatric patients due to the higher radiation dose involved. Consequently, tube voltage in pediatric CT is often lowered to 70 to 90 kV, which can result in reduced image quality due to increased noise. However, PCD CT’s spectral data allows for monoenergetic reconstruction, which can enhance contrast and potentially reduce the amount of contrast media needed (Figure 4)[31].
Spectral or multi-energy CT utilizes two or more photon spectra to generate images. A unique aspect of PCD CT is its ability to provide spectral information in every scan using a single tube potential (120 kV), as it detects the energy of individual photons. VMIs are routinely generated at various energies depending on the diagnostic requirement. For instance, low-energy VMIs (55 keV) improve the visualization of iodinated contrast material, while higher-energy VMIs (60 and 70 keV) help reduce image noise. Diagnostic tasks often use specific energy levels: 55 keV for angiography, 60 keV for contrast-enhanced soft tissue, 65 keV for bone imaging, and 70 keV for non-contrast CT. Spectral imaging also facilitates the creation of material-specific images like iodine maps and virtual non-contrast images. Iodine maps aid in detecting contrast material in mediastinal and chest wall masses, lung nodules, and adenopathy, thereby supporting lesion characterization and evaluating treatment responses. These maps are also useful for assessing lung perfusion. Likewise, virtual non-contrast images help differentiate between iodine enhancement and other materials such as calcium or blood products in high-attenuation lesions on contrast-enhanced CT images[32].
CT assessment of metal stents can be hindered by artifacts such as blooming, beam-hardening, and motion caused by elevated heart rates, which can distort stent diameters and obscure the stent lumen. Studies in adults demonstrate that ultra-high-resolution PCD CT mitigates these artifacts through improved spatial resolution and reduced noise, enabling more precise measurements of stent diameters and stenosis, which may guide medical or surgical decision-making. In pediatric cases, stents are used to address obstructive lesions in the pulmonary artery, aorta, and veins, as well as to maintain patency in ductus-dependent circulation, collateral vessels, and surgical shunts. PCD CT enhances stent wall definition, minimizes intraluminal artifacts, and allows for better evaluation of stent diameters, luminal patency, and stenosis[32].
CONCLUSION
The limited adoption of PCCT is predominantly due to the higher associated costs as well as the complexity of implementing and maintaining this advanced technology. Additional specialized training for clinical staff would be required, as well as updates to existing protocols and workflows. Furthermore, a lack of pediatric-specific studies and evolving regulatory standards have contributed to the hesitancy in adopting PCCT widely, especially in pediatric imaging. Over time, as clinical evidence grows and costs potentially decrease, PCCT adoption is expected to increase. As more evidence becomes available, the pediatric imaging community should collaborate to establish guidelines and best practices for using PCD CT in children, including defining state-of-the-art protocols. Currently, there are discrepancies in the deployment of PCD CT among pediatric imaging practitioners, with some being new to the technology and others being experienced users at established centers. There are some important factors to consider when deciding whether to obtain a PCD CT for a given hospital, one such is the volume of pediatric cardiothoracic cases, to provide sufficient training in these machines. Furthermore, PCD CT scanners have an initial higher cost with machines costing between 3-5 times more than conventional dual source CT scanners. Moving forward we need to facilitate further clinical research evaluating the diagnostic value of PCD CT in large multi-center trials, these should ideally be focused on pediatric cardiac imaging as we believe this is where PCD CT will provide the greatest patient benefit. Overall PCD CT can lower image noise, enhance both spatial and contrast resolution, and offer multispectral capabilities, potentially accomplishing these improvements while reducing overall radiation exposure. These advancements could also allow for a reduction in the dose of contrast media and enable the capture of multiphase images in a single acquisition.