Published online Mar 9, 2025. doi: 10.5409/wjcp.v14.i1.99231
Revised: October 3, 2024
Accepted: October 31, 2024
Published online: March 9, 2025
Processing time: 155 Days and 19.1 Hours
Gestational diabetes mellitus (GDM) is a metabolic disorder, recognised during 24-28 weeks of pregnancy. GDM is linked with adverse newborn outcomes such as macrosomia, premature delivery, metabolic disorder, cardiovascular, and neurological disorders. Recent investigations have focused on the correlation of genetic factors such as β-cell function and insulin secretary genes (transcription factor 7 like 2, potassium voltage-gated channel subfamily q member 1, adipo
Core Tip: Higher morbidity and mortality rates were reported in neonates born to diabetic mothers. Gestational diabetes mellitus (GDM) is linked to both genetic and epigenetic alterations. Therefore, it would be beneficial to implement a strategy to find molecular biomarkers in GDM, such as genetic and epigenetic variations in genes associated with β-cell function and insulin signaling pathways. Implementing this strategy would result in GDM risk prediction, and improved maternal and newborn pregnancy outcomes while contributing to their future well-being.
- Citation: Shamsad A, Gautam T, Singh R, Banerjee M. Genetic and epigenetic alterations associated with gestational diabetes mellitus and adverse neonatal outcomes. World J Clin Pediatr 2025; 14(1): 99231
- URL: https://www.wjgnet.com/2219-2808/full/v14/i1/99231.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i1.99231
Gestational diabetes mellitus (GDM) is a pregnancy-related metabolic complication characterized by the development of persistent hyperglycemia in women who do not have a history of diabetes, arising during their gestational period. The chronic metabolic condition normally resolves after pregnancy. GDM can impact the development of several organ systems, with cardiovascular and neural tube defects being common anomalies. Other problems may include fetal growth abnormalities, pre-eclampsia, premature birth, and perinatal mortality. Neuro-developmental research on offspring born to mothers with diabetes has shown an increased prevalence of attention deficit hyperactivity disorder, learning im
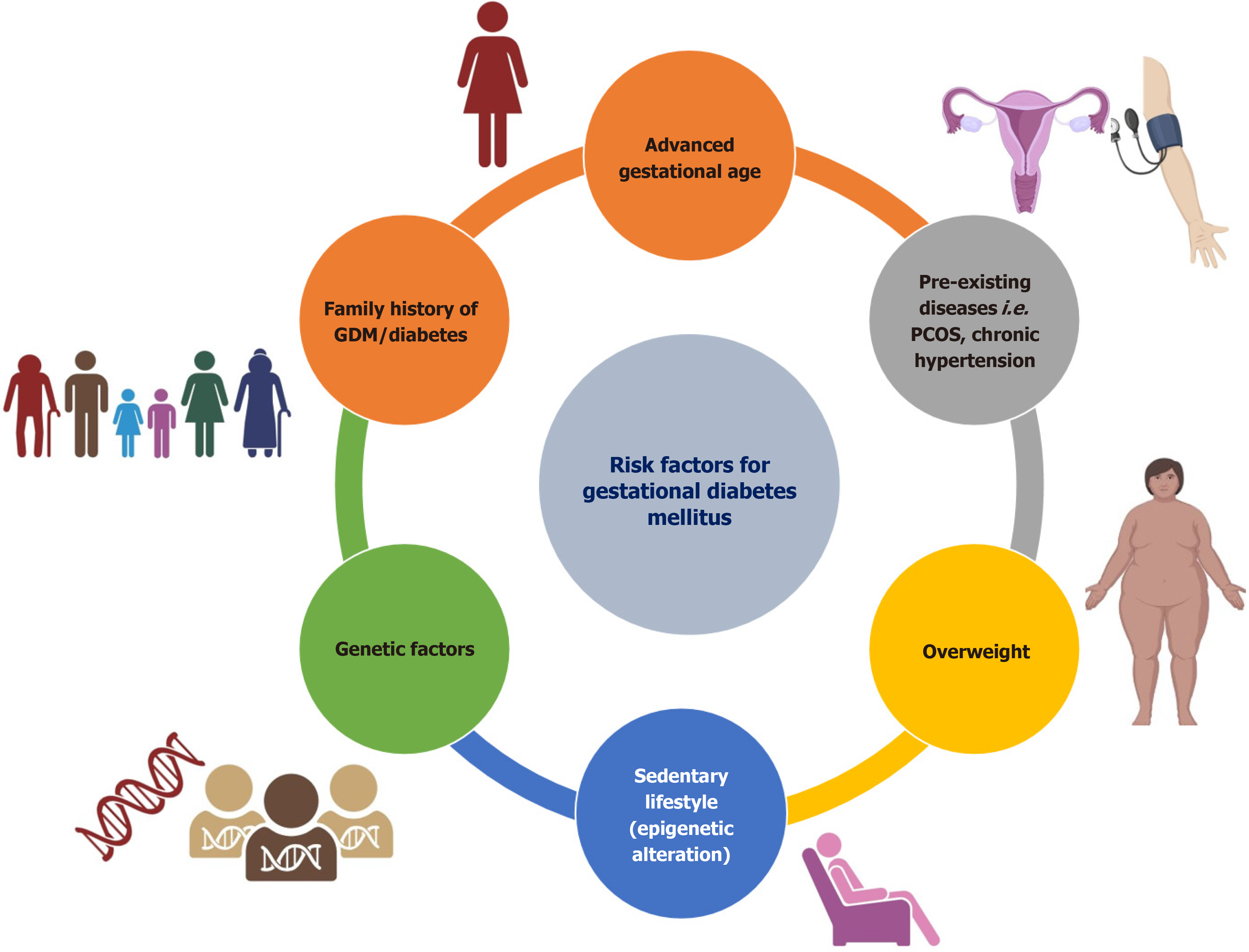
Annually, 5 million women in India, accounting for 16.55% of the female population, are diagnosed with GDM. According to a Report,1 in 6 pregnant women have GDM, worldwide 21 million newborns are affected by diabetes. It is noteworthy that 83% of these cases are caused by GDM[2,6]. However, there is a significant variation in the frequency of GDM, which can be attributed to disparities in diagnostic criteria, screening methods, and the research environment, such as urban vs rural or hospital vs community settings[7]. A 2-hour 75-gram oral glucose tolerance test is often used for diagnosing GDM between 24-28 weeks of pregnancy[8]. GDM is associated with many risk factors such as high parity, overweight or obese, family history of hyperglycemia, and advanced maternal age (Figure 1). The exact underlying etiology of GDM has not yet been uncovered. During pregnancy, the maternal pancreas fails to adjust the increased insulin requirements during gestation[9]. Hyperglycemia is often a result of impaired glucose tolerance, which is caused by malfunction of the pancreatic β-cells in instances of chronic insulin resistance conditions during gestation. GDM is characterized by a wide range of short- and long-term effects that impact both the mother and the fetal health. However, there is currently no widely recognized treatment or preventative method for GDM, except for lifestyle modifications such as exercise and dietary adjustments[2,10]. Insulin treatment may be used in certain instances; nevertheless, its ef
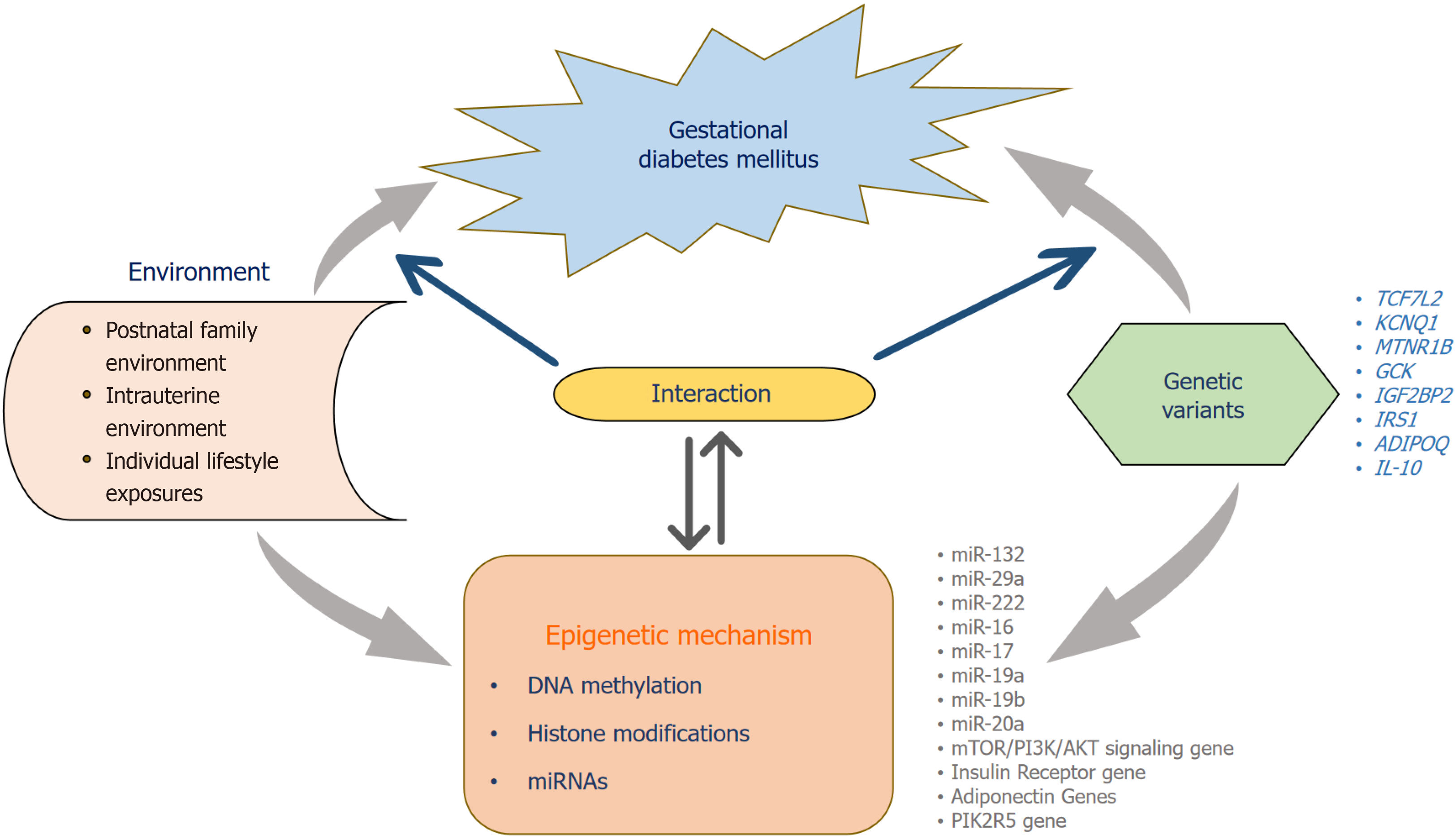
During pregnancy, the accumulation of genetic and epigenetic changes in genes is linked to insulin secretion[12]. Earlier research indicated that genetic variations in genes involved in essential metabolic pathways such as insulin secretion, lipids, and glucose metabolism are linked to the onset of GDM[13,14]. In addition to genetic factors, epigenetic modifications have been identified as linked to GDM[15,16]. Epigenetics refers to the mechanisms that regulate gene expression without altering the underlying DNA sequence[17]. Epigenetic modifications are crucial in guiding the processes of development and differentiation throughout embryonic stages[18]. They play a crucial role in modifying gene expression that impacts pregnant women and newborns, consequently heightening the likelihood of developing metabolic syndrome and diabetes in the future[19]. Previous studied reported altered genetic variants of several genes that regulate β-cell function and insulin secretion such as transcription factor 7 like 2 (TCF7 L2), potassium voltage-gated channel subfamily q member 1 (KCNQ1), melatonin receptor 1b gene (MTNR1B), insulin-like growth factor 2 messenger RNA-binding protein 2 (IGF2BP2), insulin receptor substrate - 1 (IRS1), adiponectin (ADIPOQ), interleukin-10 (IL-10) genes may increase susceptibility to GDM[2,15]. Epigenetic mechanisms, such as DNA methylation, histone modification, and miRNA expression regulate gene expression and are interconnected[20,21]. Recent studies indicate that DNA methylation patterns in the placenta and cord blood of women experiencing GDM differ from those that were found in normoglycemic pregnancies[22]. Exposure of the fetus to the intrauterine environment in mothers with GDM leads to long-term consequences and increases the likelihood of developing metabolic diseases in the future[23]. Genetic al
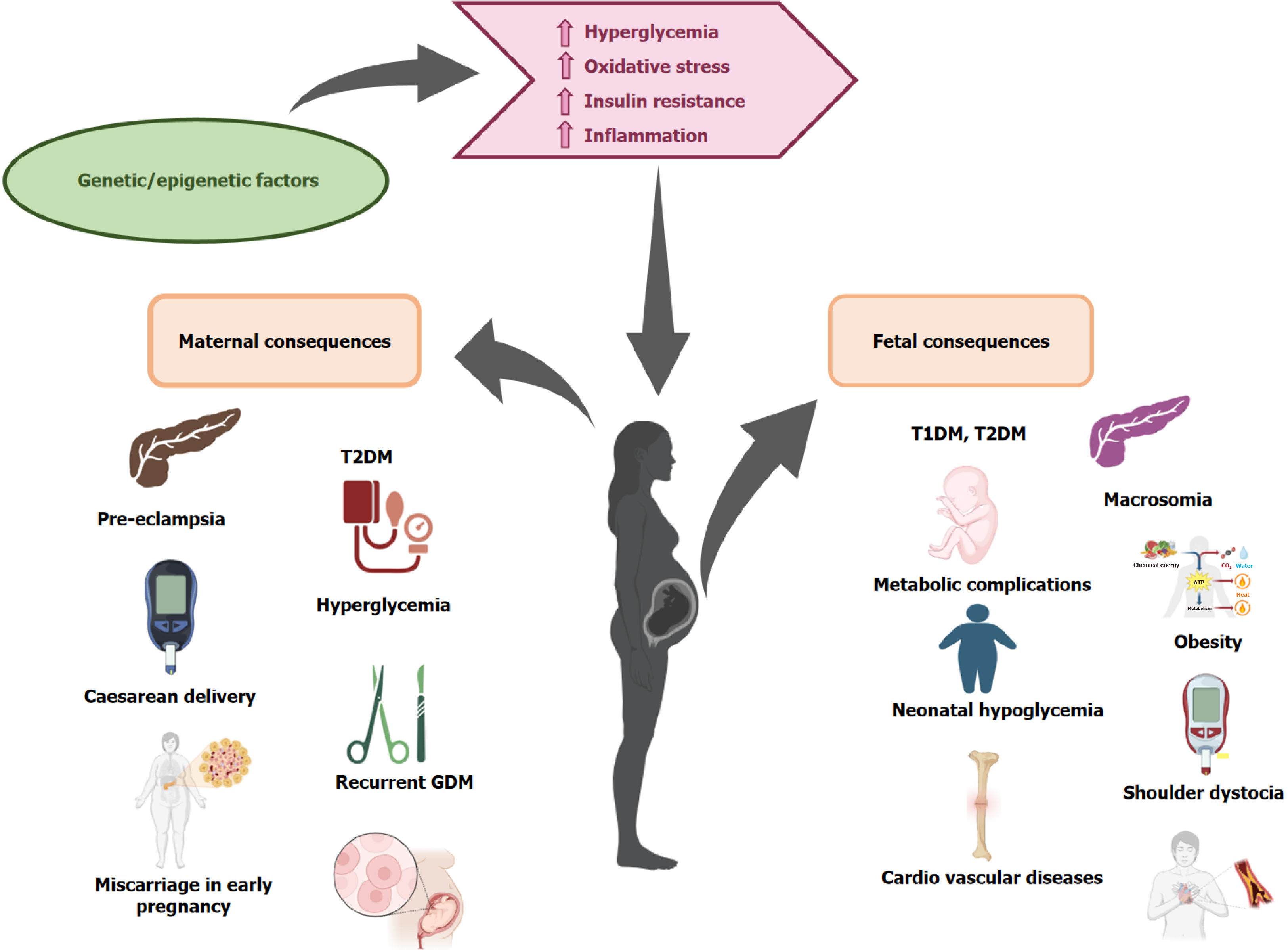
The Pedersen theory explains how GDM affects the fetus due to insulin resistance, inducing fetal hyperglycemia and fetal macrosomia by increased transfer of glucose over the placenta to the fetus. Macrosomia is a birth weight exceeding 4000 or 4500 g, distinguished by a rise in subcutaneous fat, muscular mass, and a larger head circumference. It can result from shoulder dystocia and shoulder injuries, leading to birth complications such as fractures of the humerus and clavicle bones and injury to the brachial plexus (Erb's palsy). Abnormal fetal development is due to the transfer of additional growth substances (amino acids and lipids) through the placenta[27,28]. GDM poses risks to the fetus and newborn, including macrosomia, elevated fetal glucose levels, and increased insulin production. This leads to chronic fetal hypoxia, increasing the risk of intrauterine fetal mortality[29] (Figure 2). Fetal hypoxia leads to polycythemia, an excessive increase in red blood cell volume in venous blood, characterized by a venous hematocrit level of more than 65% and increased blood viscosity, can cause bluish discoloration (cyanosis), hypoglycemia, decreased muscle tone (hypotonia), challenges with eating, difficulty in breathing, skin redness (plethora), and hyperbilirubinemia[30,31]. Hypoglycemia in neonates born to mothers with GDM is caused by high insulin production, hindering the normal responses that counteract the placental insufficiency leads to reduced glucose supply, such as lipolysis, glycogenolysis, β-oxidation of fatty acids, and gluconeogenesis. Additionally, it increases glucose utilization by peripheral tissues. The Hyperglycemia and Adverse Pregnancy Outcome research shows a direct correlation between preeclampsia and glucose tolerance tests, a risk factor for premature delivery, and Neonatal Intensive Care Unit admission of neonates for respiratory support[32,33].
The rising global rates of obesity and GDM have significant public health implications, particularly for future generations. Several risk factors like parity, advanced maternal age, and gestational weight gain during pregnancy are significantly related to GDM and cause long-term consequences on newborn’s health[34]. Neonates born to mothers with GDM are susceptible to developing obesity, insulin resistance, Type 2 Diabetes Mellitus, cardiovascular disorders, and other metabolic illnesses[35,36] (Figure 2). Mothers with high glucose levels during pregnancy have a two-fold higher risk of their children being overweight or obese during childhood. This correlation persisted even after considering the mother's pre-pregnancy body mass index and the neonate's birth weight[37]. Maternal obesity substantially increases the probability of developing GDM and is the most effective predictor of obesity in children throughout the perinatal period[38]. Elevated systolic blood pressure in children increases the risk of hypertension in adulthood, a leading cause of cardiovascular disease[39]. The role of the maternal surrounding environment affects the intrauterine programming to determine the offspring obesity is still unknown. Insulin resistance during pregnancy is linked with GDM which in
Pregnancy is an intricate physiological phenomenon that is controlled by various hormones and signaling molecules, to facilitate embryonic growth and development, which involves significant changes in the circulatory, renal, hematologic, respiratory, and metabolic systems[43-45]. Throughout the pregnancy period, the placenta serves as an endocrine organ by releasing numerous hormones that are crucial for regulating maternal physiology. Moreover, it facilitates the delivery of nutrients and gasses to the growing embryo, thereby encouraging fetal development[46]. The trophoblast of the growing placenta releases hormones [leptin, insulin, glucocorticoid, insulin growth factor (IGF), prostaglandin, gonadotrophin, etc.] in a tightly regulated way to optimize the absorption of these hormones by the endometrium, thus promo
Maternal hormones such as IGF1, insulin, and leptin promote the activation of mTOR signaling, while hypoxia and adiponectin inhibit or reduce its activity. Restricting protein intake during pregnancy has an impact on the metabolism of glucose and insulin in the muscular tissue of the fetus[55]. Obesity and exposure to EDC can cause pregnancy complications, affecting the mTOR signaling pathway and placental nutrient transporter efficiency, and adversely affecting fetal development[49,56]. Placental hormones increase during pregnancy, leading to increased insulin resistance, since these hormones antagonize the effects of insulin. The main aim of the mechanism is to provide an adequate level of glucose to the fetus. Elevated maternal glucose levels cause a rise in insulin production, allowing the mother to maintain normal glucose levels[57]. GDM occurs when insulin production is insufficient to compensate for increased resistance or beta cell dysfunction during pregnancy. β-cell dysfunction is the loss of β-cells' ability to accurately measure glucose levels in blood and secrete enough insulin in response[58]. During gestation, insulin sensitivity changes due to pregnancy de
These hormones function together to facilitate optimal fetal growth. Nevertheless, the increased level of placental hormones can also induce inflammation inside the body, resulting in oxidative stress generation and damage to DNA and cells. Progesterone levels rise during pregnancy specifically from the first to the third trimester, affecting the phosphoinositide 3-kinase (PI3K) pathway by reducing IRS-1 expression and preventing glucose transport and absorption through GLUT4. During pregnancy, estrogen levels rise and reduce insulin action, while human placental lactogen (hPL) functions similarly to insulin or anti-insulin medications[2,60]. Increased levels of hPL during pregnancy facilitate the absorption of glucose and the synthesis of glycogen in the mother's body. However, pituitary growth hormone and human placental growth hormone have diabetogenic effects, causing hyperinsulinemia, reduced glucose uptake, impaired formation of glycogen, and decreased insulin production[61]. During the third trimester of pregnancy, in
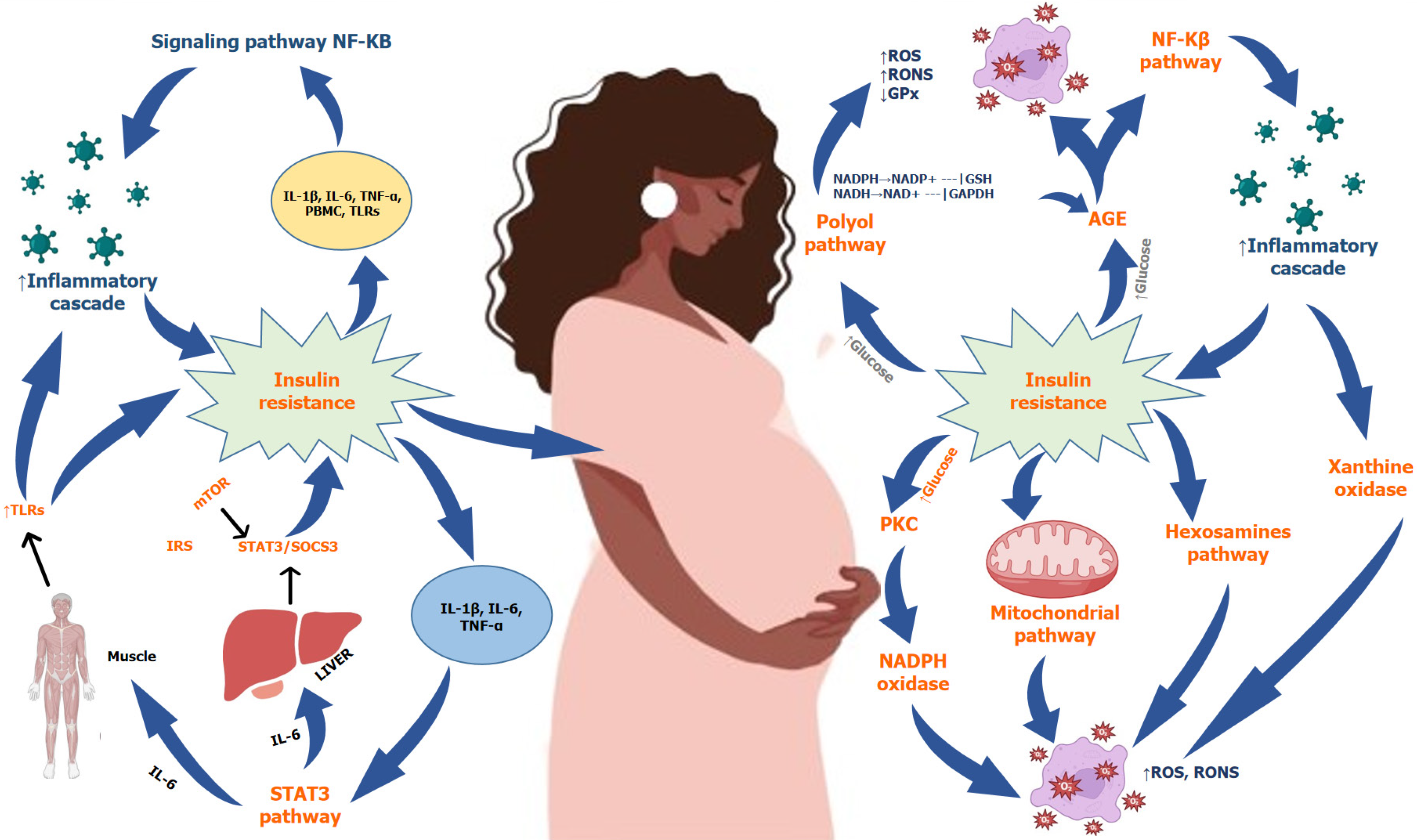
Genetic variations are increasingly being linked with insulin resistance during pregnancy causing GDM. Research findings indicate that the alterations in genes that are crucial for metabolic processes during gestation elevate the probability of developing GDM[20]. The genes most prone to be linked with GDM are mostly those involved in β-cell function, such as regulating the insulin signal transduction, as well as several inflammatory and antioxidant genes[63,64]. The impact of these genes on maternal physiology during gestation has been examined by two different methods including the study of different genetic variants of regulatory genes in pregnant women to investigate the connection between the genes and the risk of GDM development. However, this method has shown little efficacy in identifying the particular genes implicated in the pathogenesis of GDM, mostly due to the limited sample numbers and the scarcity of genetic variations in the genomic region. Another method is Genome-wide association studies that found genetic polymorphism from many loci is linked with the risk of GDM. However, type 2 diabetes mellitus (T2DM) is distinguished by the presence of insulin resistance and shares a common genetic architecture with GDM, various genes that are linked with an increased risk of T2DM have also been examined in individuals with GDM[65]. Research on key genes involved in insulin signaling homeostasis has shown a correlation between single nucleotide polymorphisms (SNPs) and the likelihood of developing GDM[66] (Figure 4).
TCF7 L2 gene is situated at the long arm of the 10th chromosome (10q25.3) and encodes a transcription factor that contains a high mobility group box. This transcription factor is involved in the regulation of blood glucose homeostasis[67,68]. A study on the Chinese women population reported that AT genotypes of SNP rs4506565 and TC genotype rs7901695 are at a considerably higher risk for GDM[69]. Previous research on the populations of Saudi Arabia, Egypt, and Bangladesh has shown that individuals with the CT genotype of the SNP rs7903146 (C>T) had 2-3 times increased risk of developing GDM[70-72].
KCNQ1 gene is located on the short arm of 11th chromosome (11p15). The voltage-gated channel is present in pancreatic β-cells and has the ability to control insulin secretion by regulating the electrical potential of the cell membrane and the activity of ion channels. This eventually affects the synthesis of insulin[73]. Two separate study of rs2237892 and rs2237895in Korean population by Shin et al[74], and Kwak et al[75], demonstrated that the C alleles of both SNPs found to be linked with the risk of GDM. These SNPs are also significantly associated with insulin sensitivity[74,75]. Another study conducted on Chinese populations revealed that the presence of the C allele of rs2237892 had an elevated risk of GDM[76]. A study on a group of 960 Caucasian and African American women also confirmed that the SNP rs2237895 was found to be directly linked with an increase in gestational weight gain during pregnancy in women with GDM[77]. Similarly, another research found that the C allele of SNP rs2237895 is associated with a higher risk of GDM in Pakistani women[78].
The gene encodes a receptor that specifically binds to the melatonin hormone. melatonin has a role in the regulation of the circadian cycle, insulin signaling pathway, and metabolism of glucose, among other physiological processes[79]. The genetic variant rs10830963/G of the MTNR1B gene has a notable influence on the progression of GDM and glycemic traits, such as insulin response and glucose absorption, and has been significantly associated with the risk of GDM in many populations[80,81]. Other studies found that T allele of SNP rs1387153 has a strong correlation with an increased risk of developing GDM[82,83]. A study discovered that the SNP rs4753426 of the MTNR1B gene may enhance the susceptibility to the risk of developing GDM. The study stated that the presence of the C allele is associated with elevated fasting plasma glucose levels and reduced pancreatic β-cell function[84].
The glucokinase (GCK) gene is situated on the short arm of the 7th chromosome (7p13). GCK gene encodes a protein, that is a part of the hexokinase protein family and serves as a vital regulating enzyme in the pancreaticβ-cells. Its main function is to catalyze the phosphorylation of glucose and control the secretion of insulin[85]. Research conducted on Brazilian women revealed that those who had the C allele of rs780094 had a 1.41 times higher risk of developing GDM[86]. Another case-control study on the Chinese population found that SNP rs1799884 was linked with the risk for GDM[87]. Additional meta-analyses have shown an association between rs1799884 polymorphisms and GDM in both Caucasian and Asian populations[88]. Another meta-analysis in the Caucasian population reported SNP rs780094 of GCKR was discovered as a contributing factor for GDM[89]. The presence of the C allele of SNP rs780094 in the Asian and Malaysian populations was shown to dramatically enhance the risk of GDM development[90].
The gene-coded IGF2BP2 protein, that binds to the messenger RNA (mRNA) of insulin-like growth factor 2. IGF2BP2 is mostly expressed in pancreatic β cells and plays a vital role in embryonic growth and development, cellular differentiation, cellular metabolism, and insulin control[91]. The expression of the IGFBP2 gene is controlled by the interaction of many transcription factors with the regulatory domain located at the promoter site[92]. The SNP rs4402960 has been shown to exhibit a robust association with the susceptibility to developing GDM and weight gain during gestation in Korean and Chinese populations[93,94]. A meta-analysis was performed to identify the major risk alleles that elevate the probability of developing GDM in Asian populations and found that the individuals with the genetic variant rs4402960 had a considerably increased risk of developing GDM[95]. Similarly, another research found that individuals with TT genotypes of SNP rs4402960 and AA genotypes of rs11705701 had a strong association with advanced maternal age and adverse effects on newborns[96].
IRS1 gene coded the insulin receptor substrate 1 is a crucial adaptor protein essential for insulin signal transduction by transferring the signals between insulin and insulin-like growth factor-1 receptor to regulate the insulin response[97]. The presence of IRS gene SNP rs1801278 (C>T) variant was found to be associated with GDM women in the Chinese population[98]. Another study in Saudi Arabia found that T allele of rs1801278 was shown to be correlated with a high risk of developing GDM[99]. Similar research was found in the Pakistani population[100]. Another research in the Chinese population found that the T allele of rs1801278 was more prevalent in the GDM groups (8.7%) compared to the control groups (5.1%)[101].
The gene is situated in the long arm of the 3rd chromosome (3q27.3) and is predominantly expressed in adipose tissue. ADIPOQ gene encodes a structural protein that has a resemblance to collagens X and VIII, as well as complement factor C1q. these components are mostly active in the bloodstream and has a role in metabolic and hormonal functions. mutations in this gene cause Adiponectin deficiency[102]. Adiponectin is a hormone that regulates insulin sensitivity by controlling fatty acid oxidation and glucose signaling[103]. During pregnancy, adiponectin is also secreted by the pla
IL-10 gene is present on the long arm of 1st chromosome (1q32.1). It serves as an anti-inflammatory cytokine that is synthesized by activated T cells, monocytes, and B cells[109]. Multiple studies have shown that alteration in the gene
During the crucial period of early development, the control of gene expression is regulated by environmental and lifestyle factors via epigenetic processes. This is facilitated by a collection of genomic imprinters that are specifically programmed to perform their functions. The period of fetoplacental development is flexible and may be modified and influenced by changes in the maternal environment, whether they are induced by internal or external factors. These changes can significantly impact embryonic programming[114]. Epigenetic modifications include a variety of processes, including DNA methylation, microRNAs (miRNAs), and histone changes (Figure 4). The placenta has a crucial function in fetal development. It not only controls the interchange of substances between the mother and fetus, which allows for em
DNA methylation is a well-researched and well-understood epigenetic process. The process comprises the incorporation of a methyl group (CH3) to the 5th Carbon inside the cytosine-phosphate-guanine dinucleotides (CpG island)[116]. The process is catalyzed by the DNA methyltransferase enzyme. The S-adenosyl-methionine acts as the methyl donor in the transfer process. Methylation of genes located in CpG-dinucleotide-rich promoter regions is frequently associated with transcriptional repression. The hypermethylation of the promoter region causes alteration in the DNA sequence where the transcription factors bind, which results in altered gene expression[117]. DNA methylation is a process that may be reversed. During pregnancy DNA methylation plays a crucial role in the regulating genome to transcribe genetic in
Multiple studies have reported alteration in DNA methylation of certain placental regulatory genes, such as leptin, ADIPOQ, peroxisome PGC-1α, ABCA1, LPL, and important inflammatory cytokines genes. Overall, previous data indicate a robust correlation between altered DNA methylation of several regulatory genes with metabolic diseases such as GDM[120-122]. Pregnancy is a state of various physiological modifications in the DNA methylation pattern of blood plasma. This has led to a growing interest in using maternal blood to test for the identification of significant molecular biomarkers of GDM. Research investigating the DNA methylation patterns throughout the whole genome in fetal cord blood and maternal placenta from GDM pregnancies has shown inconsistent results[123,124]. An analysis of 1030 placenta samples found that women with GDM had a substantial increase in overall placental DNA methylation. Furthermore, there was a significant association reported between overall placental DNA methylation and the prevalence of GDM (P = 0.0009)[125]. A research found that an increase in promoter methylation in the areas of IGFBP-1 and IGFBP-2 is associated with reduced mRNA expression in the placenta of GDM women. These genes are responsible for encoding IGF-binding proteins[126]. Another research found the association between DNA hypermethylation and decreased expression of the insulin receptor and adiponectin gene in GDM mothers and their offspring[127]. A study conducted by Kang et al[128] for genome-wide DNA methylation examination in Chinese women with GDM used the Illumina Infinium Human Methylation EPIC Bead Chip array, the results of this study revealed that the 200 loci with differential methylation were associated with 151 genes[128].
Another study found that offspring of GDM mothers exhibited elevated DNA methylation of the adiponectin gene region at the promoter reduced the expression of the ADIPOQ in subcutaneous fat. Nevertheless, there was no significant modification seen in the methylation level of the adiponectin gene in the blood plasma of the GDM mother[129]. A study reported hypermethylation of the PIK3R5 gene in the blood of pregnant women with GDM during the early stages of pregnancy. This altered hypermethylation causes the alteration of PI3K/AKT/mTOR signal transduction in pregnant women[130]. The constraints posed by sample selection and the presence of methylated cell types have hindered a comprehensive investigation of the causes of GDM. In the future, investigating the differential methylation patterns of genes that regulate insulin will enhance our comprehension of how alterations in DNA methylation in maternal and placental cells contribute to the development of insulin resistance in women with GDM.
miRNAs are small around 19-23 nucleotides highly conserved non coding RNA molecules. miRNAs control the ex
MiRNAs play a vital role in regulating metabolism and embryonic growth during pregnancy. The differential expression of miRNAs is influenced by the surrounding environment of pregnant women, so the altered epigenetic modification of these miRNAs is associated with the progression of GDM. Genome-wide research conducted in 2013 revealed the expression of over 600 miRNAs in the placenta and their association with maternal physiology[136]. In a recent study, Poirier et al[137] examined placental miRNAs that experience abnormal regulation throughout pregnancy in women with GDM[137]. The placenta plays a crucial role in regulating the mother's metabolism throughout pregnancy, and it is believed that the different levels and expression of certain placental miRNAs contribute to these physiological modifications. Placental miRNAs are circulated into the mother's bloodstream. Consequently, these miRNAs have the potential to serve as indicators of placental malfunction causing GDM[138,139]. In 2011, Zhao et al[140] conducted the first analysis of serum miRNA expression of GDM women, by using Taqman low-density arrays and then confirming the results by applying qRT-PCR technique and identifying three specific miRNAs, namely miR-132, miR-29a, and miR-222, that exhibited substantial downregulation in Chinese GDM women (n = 24) as compared to a control group (n = 24)[140].
The differential expressions of these miRNAs (miR-132, miR-29a, and miR-222) were confirmed by various studies. These miRNAs are believed to have a role in maintaining glucose levels, enhancing insulin responsiveness, and regulating the activity of β-cells[141]. Various research conducted on different populations has reproduced similar trials, with contradictory findings. A recent study by Pheiffer et al[142] found that the levels of miR-132, miR-29a, and miR-222 were lower in the blood plasma of South African women with GDM (n = 28) compared to a control group (n = 53). This difference was statistically significant[142]. These data indicate that the expression of these serum miRNAs is common across both South African and Chinese populations. Unlike the findings of Zhao et al[140], another study by Tagoma et al[143] in the Estonian women population demonstrated that the expression of miR-222 was elevated in the plasma sample of GDM women (n = 13) in comparison to the control group (n = 9)[143]. Wander et al[144] found that there were no discernible variations The study examined the levels of miR-222 and miR-29a in the plasma sample of 36 GDM women of American Caucasian ethnicity and compared with the control group of 80 individuals[144]. Zhu et al[145] used high-throughput DNA sequencing and qRT-PCR techniques to examine miRNAs in plasma samples obtained from Chinese women with GDM (n = 10) and control (n = 10) during the 16th and 19th weeks of pregnancy. Another study on in
The research emphasizes several miRNAs as biomarkers for early detection of GDM. However, the findings often demonstrate variability, perhaps because of variations in sample kinds and sizes, women’s gestational age, and the analytical techniques used. These disparities might arise from variances in the biological material used (serum or plasma), at the stage of pregnancy, or other unidentified variables that have not been taken into consideration. Currently, there is no agreement on the most effective strategy for quantifying circulating miRNAs during profiling. Various quantification techniques are recognized to differ in terms of sensitivity and specificity. This might potentially affect the precision and understanding of the data. Furthermore, the study of circulating miRNA profiling is significantly challenged by the process of data standardization. In order to improve the precision of molecular biomarkers in predicting the likelihood of GDM, future research should focus on incorporating a combination of these indicators into risk stratification models.
Pregnancy is characterized by a period of increased metabolic activity, during which it is crucial to maintain glucose homeostasis. Despite ongoing debate, there is still disagreement on the criteria used for diagnosis. Hence, it is essential to establish a reliable diagnostic criterion for GDM, and all endeavours should be focused on researching effective and secure therapies that can manage maternal insulin homeostasis during pregnancy. Since the genetic and epigenetic factors play a role in the onset of GDM, the current research is to identify novel biomarkers to support insulin balance throughout pregnancy. The regulation of insulin homeostasis genes through genetic variations and epigenetic factors, such as the differential expression of miRNA and promoter methylation, could serve as distinctive methods for forecasting the risk of GDM and its associated outcomes. While these molecular biomarkers hold significant promise, there are several limitations that need to be addressed prior to their application in therapeutic settings. However, rapid improvements in technology could potentially address these challenges and pave the way for a fast, affordable test capable of readily identifying GDM women and neonates at elevated risk in the early stages of pregnancy. Consequently, comprehensive research must be undertaken across various populations, and the establishment of a global organization is essential to oversee the analytical standards for molecular biomarkers in intricate diseases.
| 1. | Márquez-Valadez B, Valle-Bautista R, García-López G, Díaz NF, Molina-Hernández A. Maternal Diabetes and Fetal Programming Toward Neurological Diseases: Beyond Neural Tube Defects. Front Endocrinol (Lausanne). 2018;9:664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Shamsad A, Kushwah AS, Singh R, Banerjee M. Pharmaco-epi-genetic and patho-physiology of gestational diabetes mellitus (GDM): An overview. Health Sci Rev. 2023;7:100086. [DOI] [Full Text] |
| 3. | Vounzoulaki E, Miksza JK, Zaccardi F, Tan BK, Davies MJ, Khunti K, Gillies CL. Association of ethnicity and socioeconomic status with health outcomes in women with gestational diabetes: Clinical practice research datalink cohort study. Diabetes Metab Syndr. 2024;18:103010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Li W, Wang L, Guo J, Dong W, Zhang S, Li W, Leng J. Seasonal variation and its interaction with pre-pregnancy BMI for GDM: a large population-based study in Tianjin, China. Sci Rep. 2023;13:22837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Li Y, Ren X, He L, Li J, Zhang S, Chen W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 6. | Shah CS, Vaishnav SB, Mankad SP, Sharma TS, Sapre SA, Raithatha NS, Patel MR, Mannari JG. Silent upsurge of gestational diabetes: Are we aware? A rural tertiary care experience of Central Gujarat. J Family Med Prim Care. 2022;11:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Bhattacharya S, Nagendra L, Krishnamurthy A, Lakhani OJ, Kapoor N, Kalra B, Kalra S. Early Gestational Diabetes Mellitus: Diagnostic Strategies and Clinical Implications. Med Sci (Basel). 2021;9:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Kotzaeridi G, Blätter J, Eppel D, Rosicky I, Linder T, Geissler F, Huhn EA, Hösli I, Tura A, Göbl CS. Characteristics of gestational diabetes subtypes classified by oral glucose tolerance test values. Eur J Clin Invest. 2021;51:e13628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Sferruzzi-Perri AN, Lopez-Tello J, Napso T, Yong HEJ. Exploring the causes and consequences of maternal metabolic maladaptations during pregnancy: Lessons from animal models. Placenta. 2020;98:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Mathiesen L, Buerki-Thurnherr T, Pastuschek J, Aengenheister L, Knudsen LE. Fetal exposure to environmental chemicals; insights from placental perfusion studies. Placenta. 2021;106:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Nakshine VS, Jogdand SD. A Comprehensive Review of Gestational Diabetes Mellitus: Impacts on Maternal Health, Fetal Development, Childhood Outcomes, and Long-Term Treatment Strategies. Cureus. 2023;15:e47500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 12. | Lecoutre S, Maqdasy S, Breton C. Maternal obesity as a risk factor for developing diabetes in offspring: An epigenetic point of view. World J Diabetes. 2021;12:366-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Ortega-Contreras B, Armella A, Appel J, Mennickent D, Araya J, González M, Castro E, Obregón AM, Lamperti L, Gutiérrez J, Guzmán-Gutiérrez E. Pathophysiological Role of Genetic Factors Associated With Gestational Diabetes Mellitus. Front Physiol. 2022;13:769924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 14. | Brito Nunes C, Borges MC, Freathy RM, Lawlor DA, Qvigstad E, Evans DM, Moen GH. Understanding the Genetic Landscape of Gestational Diabetes: Insights into the Causes and Consequences of Elevated Glucose Levels in Pregnancy. Metabolites. 2024;14:508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (7)] |
| 15. | Yahaya TO, Salisu T, Abdulrahman YB, Umar AK. Update on the genetic and epigenetic etiology of gestational diabetes mellitus: a review. Egypt J Med Hum Genet. 2020;21:13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Ustianowski Ł, Udzik J, Szostak J, Gorący A, Ustianowska K, Pawlik A. Genetic and Epigenetic Factors in Gestational Diabetes Mellitus Pathology. Int J Mol Sci. 2023;24:16619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Clark J, Rager JE. Epigenetics: An overview of CpG methylation, chromatin remodeling, and regulatory/noncoding RNAs. In: Fry RC, editor. Environmental Epigenetics in Toxicology and Public Health. Amsterdam: Academic Press, 2020. [DOI] [Full Text] |
| 18. | Safi-Stibler S, Gabory A. Epigenetics and the Developmental Origins of Health and Disease: Parental environment signalling to the epigenome, critical time windows and sculpting the adult phenotype. Semin Cell Dev Biol. 2020;97:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 19. | Giglio RV, Stoian AP, Patti AM, Rizvi AA, Sukhorukov V, Ciaccio M, Orekhov A, Rizzo M. Genetic and Epigenetic Biomarkers for Diagnosis, Prognosis and Treatment of Metabolic Syndrome. Curr Pharm Des. 2021;27:3729-3740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Rosik J, Szostak B, Machaj F, Pawlik A. The role of genetics and epigenetics in the pathogenesis of gestational diabetes mellitus. Ann Hum Genet. 2020;84:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Lizárraga D, García-Gasca A. The Placenta as a Target of Epigenetic Alterations in Women with Gestational Diabetes Mellitus and Potential Implications for the Offspring. Epigenomes. 2021;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Chen F, Fei X, Zhu W, Zhang Z, Shen Y, Mao Y, Zhu Q, Xu J, Zhou W, Li M, Du J. Placental DNA methylation changes in gestational diabetes mellitus. Epigenetics. 2022;17:2109-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Ornoy A, Becker M, Weinstein-Fudim L, Ergaz Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int J Mol Sci. 2021;22:2965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 24. | Yi Y, Wang T, Xu W, Zhang SH. Epigenetic modifications of placenta in women with gestational diabetes mellitus and their offspring. World J Diabetes. 2024;15:378-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 25. | Grilo LF, Tocantins C, Diniz MS, Gomes RM, Oliveira PJ, Matafome P, Pereira SP. Metabolic Disease Programming: From Mitochondria to Epigenetics, Glucocorticoid Signalling and Beyond. Eur J Clin Invest. 2021;51:e13625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Valent AM, Choi H, Kolahi KS, Thornburg KL. Hyperglycemia and gestational diabetes suppress placental glycolysis and mitochondrial function and alter lipid processing. FASEB J. 2021;35:e21423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Reim PK, Engelbrechtsen L, Gybel-Brask D, Schnurr TM, Kelstrup L, Høgdall EV, Hansen T. The influence of insulin-related genetic variants on fetal growth, fetal blood flow, and placental weight in a prospective pregnancy cohort. Sci Rep. 2023;13:19638. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Malhotra A, Stewart A. Gestational diabetes and the neonate: challenges and solutions. RRN. 2015;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Desoye G, Carter AM. Fetoplacental oxygen homeostasis in pregnancies with maternal diabetes mellitus and obesity. Nat Rev Endocrinol. 2022;18:593-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 30. | Hutter D, Kingdom J, Jaeggi E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a review. Int J Pediatr. 2010;2010:401323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 31. | Zinjani S. Common Medical Conditions in the Neonates. In: Saha U, editor. Clinical Anesthesia for the Newborn and the Neonate. Springer: Singapore, 2023. [DOI] [Full Text] |
| 32. | Moyce Gruber BL, Cole LK, Xiang B, Fonseca MA, Klein J, Hatch GM, Doucette CA, Dolinsky VW. Adiponectin deficiency induces hepatic steatosis during pregnancy and gestational diabetes in mice. Diabetologia. 2022;65:733-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Kumar P, Sajan S. An Update on Neonatal Hypoglycemia. In: Rigobelo EC, editor. Hypoglycemia - Causes and Occurrences. Licensee IntechOpen, 2011. [DOI] [Full Text] |
| 34. | Dai RX, He XJ, Hu CL. The Association between Advanced Maternal Age and Macrosomia: A Meta-Analysis. Child Obes. 2019;15:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Nijs H, Benhalima K. Gestational Diabetes Mellitus and the Long-Term Risk for Glucose Intolerance and Overweight in the Offspring: A Narrative Review. J Clin Med. 2020;9:599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 36. | Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287-2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 468] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 37. | Lowe WL Jr, Lowe LP, Kuang A, Catalano PM, Nodzenski M, Talbot O, Tam WH, Sacks DA, McCance D, Linder B, Lebenthal Y, Lawrence JM, Lashley M, Josefson JL, Hamilton J, Deerochanawong C, Clayton P, Brickman WJ, Dyer AR, Scholtens DM, Metzger BE; HAPO Follow-up Study Cooperative Research Group. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia. 2019;62:598-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 38. | Andersson-Hall UK, Järvinen EAJ, Bosaeus MH, Gustavsson CE, Hårsmar EJ, Niklasson CA, Albertsson-Wikland KG, Holmäng AB. Maternal obesity and gestational diabetes mellitus affect body composition through infancy: the PONCH study. Pediatr Res. 2019;85:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Lin L, Lin J. Interactive effects and relative contribution of prepregnancy overweight and obesity, excessive gestational weight gain and gestational diabetes mellitus to macrosomia: A retrospective cohort in Fujian, China. Eur J Obstet Gynecol Reprod Biol. 2024;296:354-359. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 40. | Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290-e296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1590] [Cited by in RCA: 1615] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 41. | Kelstrup L, Bytoft B, Hjort L, Houshmand-oeregaard A, Mathiesen E, Damm P, Clausen T. Diabetes in Pregnancy: Long-Term Complications of Offsprings. In: Lapolla A; Metzger BE; Lapolla A; Metzger BE, editors. Frontiers in Diabetes. S Karger AG, 2020. [DOI] [Full Text] |
| 42. | Tam WH, Ma RC, Yang X, Li AM, Ko GT, Kong AP, Lao TT, Chan MH, Lam CW, Chan JC. Glucose intolerance and cardiometabolic risk in adolescents exposed to maternal gestational diabetes: a 15-year follow-up study. Diabetes Care. 2010;33:1382-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Salazar-Petres ER, Sferruzzi-Perri AN. Pregnancy-induced changes in β-cell function: what are the key players? J Physiol. 2022;600:1089-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 44. | Abruzzese GA, Arbocco FCV, Ferrer MJ, Silva AF, Motta AB. Role of Hormones During Gestation and Early Development: Pathways Involved in Developmental Programming. Adv Exp Med Biol. 2023;1428:31-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 45. | Ng SW, Norwitz GA, Pavlicev M, Tilburgs T, Simón C, Norwitz ER. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 46. | Cindrova-Davies T, Sferruzzi-Perri AN. Human placental development and function. Semin Cell Dev Biol. 2022;131:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 47. | Gauster M, Moser G, Wernitznig S, Kupper N, Huppertz B. Early human trophoblast development: from morphology to function. Cell Mol Life Sci. 2022;79:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 48. | Zhou H, Zhao C, Wang P, Yang W, Zhu H, Zhang S. Regulators involved in trophoblast syncytialization in the placenta of intrauterine growth restriction. Front Endocrinol (Lausanne). 2023;14:1107182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 49. | Basak S, Varma S, Duttaroy AK. Modulation of fetoplacental growth, development and reproductive function by endocrine disrupters. Front Endocrinol (Lausanne). 2023;14:1215353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 50. | Pandey Y, Pooja AR, Devi HL, Jalmeria NS, Punetha M, Kumar S, Paul A, Kumar K, Sonawane A, Samad HA, Singh G, Bag S, Sarkar M, Chouhan VS. Expression and functional role of IGFs during early pregnancy in placenta of water buffalo. Theriogenology. 2021;161:313-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Wilson RL, Troja W, Sumser EK, Maupin A, Lampe K, Jones HN. Insulin-like growth factor 1 signaling in the placenta requires endothelial nitric oxide synthase to support trophoblast function and normal fetal growth. Am J Physiol Regul Integr Comp Physiol. 2021;320:R653-R662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Bowman CJ, Streck RD, Chapin RE. Maternal-placental insulin-like growth factor (IGF) signaling and its importance to normal embryo-fetal development. Birth Defects Res B Dev Reprod Toxicol. 2010;89:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Rosario FJ, Kelly AC, Gupta MB, Powell TL, Cox L, Jansson T. Mechanistic Target of Rapamycin Complex 2 Regulation of the Primary Human Trophoblast Cell Transcriptome. Front Cell Dev Biol. 2021;9:670980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Shao X, Cao G, Chen D, Liu J, Yu B, Liu M, Li YX, Cao B, Sadovsky Y, Wang YL. Placental trophoblast syncytialization potentiates macropinocytosis via mTOR signaling to adapt to reduced amino acid supply. Proc Natl Acad Sci U S A. 2021;118:e2017092118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 55. | Kelly AC, Powell TL, Jansson T. Placental function in maternal obesity. Clin Sci (Lond). 2020;134:961-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 56. | Sandoval C, Wu G, Smith SB, Dunlap KA, Satterfield MC. Maternal Nutrient Restriction and Skeletal Muscle Development: Consequences for Postnatal Health. Adv Exp Med Biol. 2020;1265:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Olmos-Ortiz A, Flores-Espinosa P, Díaz L, Velázquez P, Ramírez-Isarraraz C, Zaga-Clavellina V. Immunoendocrine Dysregulation during Gestational Diabetes Mellitus: The Central Role of the Placenta. Int J Mol Sci. 2021;22:8087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 58. | Choudhury AA, Devi Rajeswari V. Gestational diabetes mellitus - A metabolic and reproductive disorder. Biomed Pharmacother. 2021;143:112183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 59. | Lee JH, Lee J. Endoplasmic Reticulum (ER) Stress and Its Role in Pancreatic β-Cell Dysfunction and Senescence in Type 2 Diabetes. Int J Mol Sci. 2022;23:4843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 60. | Kintiraki E, Mintziori G, Goulis DG. Pathogenesis of Gestational Diabetes Mellitus. In: Rodriguez-Saldana J, editor. The Diabetes Textbook. Cham: Springer, 2023. [DOI] [Full Text] |
| 61. | Calvo MJ, Parra H, Santeliz R, Bautista J, Luzardo E, Villasmil N, Martínez MS, Chacín M, Cano C, Checa-Ros A, D'Marco L, Bermúdez V, De Sanctis JB. The Placental Role in Gestational Diabetes Mellitus: A Molecular Perspective. touchREV Endocrinol. 2024;20:10-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 62. | Wicklow B, Retnakaran R. Gestational Diabetes Mellitus and Its Implications across the Life Span. Diabetes Metab J. 2023;47:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (1)] |
| 63. | Saucedo R, Ortega-Camarillo C, Ferreira-Hermosillo A, Díaz-Velázquez MF, Meixueiro-Calderón C, Valencia-Ortega J. Role of Oxidative Stress and Inflammation in Gestational Diabetes Mellitus. Antioxidants (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 105] [Reference Citation Analysis (0)] |
| 64. | Shamsad A, Gautam T, Singh R, Banerjee M. Association of mRNA expression and polymorphism of antioxidant glutathione-S-transferase (GSTM1 and GSTT1) genes with the risk of Gestational Diabetes Mellitus (GDM). Gene. 2024;928:148746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Popova PV, Klyushina AA, Vasilyeva LB, Tkachuk AS, Vasukova EA, Anopova AD, Pustozerov EA, Gorelova IV, Kravchuk EN, Li O, Pervunina TM, Kostareva AA, Grineva EN. Association of Common Genetic Risk Variants With Gestational Diabetes Mellitus and Their Role in GDM Prediction. Front Endocrinol (Lausanne). 2021;12:628582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 66. | Shukla AK, Shamsad A, Kushwah AS, Singh S, Usman K, Banerjee M. CD36 gene variant rs1761667(G/A) as a biomarker in obese type 2 diabetes mellitus cases. Egypt J Med Hum Genet. 2024;25:9. [DOI] [Full Text] |
| 67. | Ali Y, Muntaha S, Akter M, Gani KMA, Chowdhury SR, Sharmen F. Investigation of the association between the TCF7L2 rs12255372 (G/T) gene polymorphism and Gestational Diabetes Mellitus (GDM) in the population of Chattogram, Bangladesh. Endocr Metab Sci. 2023;13:100149. [DOI] [Full Text] |
| 68. | Zhang P, Deng M, Li W, Dai Q, He H, Zheng W, She L, Xiang B, Zeng J, Zhou F, Guo Y, Yang M. The correlation between transcription factor 7-like 2 gene polymorphisms and susceptibility of gestational diabetes mellitus in the population of central China: A case-control study. Front Endocrinol (Lausanne). 2022;13:916590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Alshammary AF, Al-Hakeem MM, Ali Khan I. Saudi Community-Based Screening Study on Genetic Variants in β-Cell Dysfunction and Its Role in Women with Gestational Diabetes Mellitus. Genes (Basel). 2023;14:924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Ma H. TCF7L2 Gene Rs7903146 Polymorphism May Confer Expression of Gestational Diabetes Mellitus in Relatively Young and Lean Mothers. Diabetes Obes Int J. 2016;1. [DOI] [Full Text] |
| 71. | Shalabi TA, Amr KS, Shaker MM. Are single nucleotide polymorphisms rs7903146 and rs12255372 in transcription factor 7-like 2 gene associated with an increased risk for gestational diabetes mellitus in Egyptian women? J Genet Eng Biotechnol. 2021;19:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Wei W, He Y, Wang X, Tan G, Zhou F, Zheng G, Tian D, Ma X, Yu H. Gestational Diabetes Mellitus: The Genetic Susceptibility Behind the Disease. Horm Metab Res. 2021;53:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Yu XX, Liao MQ, Zeng YF, Gao XP, Liu YH, Sun W, Zhu S, Zeng FF, Ye YB. Associations of KCNQ1 Polymorphisms with the Risk of Type 2 Diabetes Mellitus: An Updated Meta-Analysis with Trial Sequential Analysis. J Diabetes Res. 2020;2020:7145139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Shin HD, Park BL, Shin HJ, Kim JY, Park S, Kim B, Kim SH. Association of KCNQ1 polymorphisms with the gestational diabetes mellitus in Korean women. J Clin Endocrinol Metab. 2010;95:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Kwak SH, Kim TH, Cho YM, Choi SH, Jang HC, Park KS. Polymorphisms in KCNQ1 are associated with gestational diabetes in a Korean population. Horm Res Paediatr. 2010;74:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Ao D, Wang HJ, Wang LF, Song JY, Yang HX, Wang Y. The rs2237892 Polymorphism in KCNQ1 Influences Gestational Diabetes Mellitus and Glucose Levels: A Case-Control Study and Meta-Analysis. PLoS One. 2015;10:e0128901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Stuebe AM, Lyon H, Herring AH, Ghosh J, Wise A, North KE, Siega-Riz AM. Obesity and diabetes genetic variants associated with gestational weight gain. Am J Obstet Gynecol. 2010;203:283.e1-283.17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 78. | Fatima SS, Chaudhry B, Khan TA, Farooq S. KCNQ1 rs2237895 polymorphism is associated with Gestational Diabetes in Pakistani Women. Pak J Med Sci. 2016;32:1380-1385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Sun H, Wang X, Chen J, Gusdon AM, Song K, Li L, Qu S. Melatonin Treatment Improves Insulin Resistance and Pigmentation in Obese Patients with Acanthosis Nigricans. Int J Endocrinol. 2018;2018:2304746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Rosta K, Al-Aissa Z, Hadarits O, Harreiter J, Nádasdi Á, Kelemen F, Bancher-Todesca D, Komlósi Z, Németh L, Rigó J Jr, Sziller I, Somogyi A, Kautzky-Willer A, Firneisz G. Association Study with 77 SNPs Confirms the Robust Role for the rs10830963/G of MTNR1B Variant and Identifies Two Novel Associations in Gestational Diabetes Mellitus Development. PLoS One. 2017;12:e0169781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 81. | Ren J, Xiang AH, Trigo E, Takayanagi M, Beale E, Lawrence JM, Hartiala J, Richey JM, Allayee H, Buchanan TA, Watanabe RM. Genetic variation in MTNR1B is associated with gestational diabetes mellitus and contributes only to the absolute level of beta cell compensation in Mexican Americans. Diabetologia. 2014;57:1391-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Liu Q, Huang Z, Li H, Bai J, Liu X, Ye H. Relationship between melatonin receptor 1B (rs10830963 and rs1387153) with gestational diabetes mellitus: a case-control study and meta-analysis. Arch Gynecol Obstet. 2016;294:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Jia Y, Shen Y, Shi X, Gu X, Zhang P, Liu Y, Zhu A, Jiang L. MTNR1B gene on susceptibility to gestational diabetes mellitus: a two-stage hospital-based study in Southern China. Mol Genet Genomics. 2020;295:1369-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Shen Y, Jia Y, Li Y, Gu X, Wan G, Zhang P, Zhang Y, Jiang L. Genetic determinants of gestational diabetes mellitus: a case-control study in two independent populations. Acta Diabetol. 2020;57:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Sternisha SM, Miller BG. Molecular and cellular regulation of human glucokinase. Arch Biochem Biophys. 2019;663:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 86. | Anghebem-Oliveira MI, Webber S, Alberton D, de Souza EM, Klassen G, Picheth G, Rego FG. The GCKR Gene Polymorphism rs780094 is a Risk Factor for Gestational Diabetes in a Brazilian Population. J Clin Lab Anal. 2017;31:e22035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | She L, Li W, Guo Y, Zhou J, Liu J, Zheng W, Dai A, Chen X, Wang P, He H, Zhang P, Zeng J, Xiang B, Li S, Wang L, Dai Q, Yang M. Association of glucokinase gene and glucokinase regulatory protein gene polymorphisms with gestational diabetes mellitus: A case-control study. Gene. 2022;824:146378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Han X, Cui H, Chen X, Xie W, Chang Y. Association of the glucokinase gene promoter polymorphism -30G > A (rs1799884) with gestational diabetes mellitus susceptibility: a case-control study and meta-analysis. Arch Gynecol Obstet. 2015;292:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Lin Z, Wang Y, Zhang B, Jin Z. Association of type 2 diabetes susceptible genes GCKR, SLC30A8, and FTO polymorphisms with gestational diabetes mellitus risk: a meta-analysis. Endocrine. 2018;62:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Jamalpour S, Zain SM, Mosavat M, Mohamed Z, Omar SZ. A case-control study and meta-analysis confirm glucokinase regulatory gene rs780094 is a risk factor for gestational diabetes mellitus. Gene. 2018;650:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Simon Y, Kessler SM, Bohle RM, Haybaeck J, Kiemer AK. The insulin-like growth factor 2 (IGF2) mRNA-binding protein p62/IGF2BP2-2 as a promoter of NAFLD and HCC? Gut. 2014;63:861-863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Boughanem H, Yubero-Serrano EM, López-Miranda J, Tinahones FJ, Macias-Gonzalez M. Potential Role of Insulin Growth-Factor-Binding Protein 2 as Therapeutic Target for Obesity-Related Insulin Resistance. Int J Mol Sci. 2021;22:1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 93. | Wang Y, Nie M, Li W, Ping F, Hu Y, Ma L, Gao J, Liu J. Association of six single nucleotide polymorphisms with gestational diabetes mellitus in a Chinese population. PLoS One. 2011;6:e26953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 94. | Chon SJ, Kim SY, Cho NR, Min DL, Hwang YJ, Mamura M. Association of variants in PPARγ², IGF2BP2, and KCNQ1 with a susceptibility to gestational diabetes mellitus in a Korean population. Yonsei Med J. 2013;54:352-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 95. | Syed Mohd Bahktiar SN, Jamari MH, Wan Noor NA, Ariff Fadzilah RA, Abdul Karim MZ, Abdul Halim HH, Abu NF, Lay Kek T, Salleh MZ. Meta-Analysis of the Genetic Factors that Predisposed Asian Women to Gestational Diabetes Mellitus. Malay J Pharm Sci. 2021;19:131-152. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 96. | Tarnowski M, Bujak J, Kopytko P, Majcher S, Ustianowski P, Dziedziejko V, Safranow K, Pawlik A. Effect of FTO and IGF2BP2 gene polymorphisms on duration of pregnancy and Apgar scores in women with gestational diabetes. J Obstet Gynaecol. 2019;39:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Su J, Tang L, Luo Y, Xu J, Ouyang S. Research progress on drugs for diabetes based on insulin receptor/insulin receptor substrate. Biochem Pharmacol. 2023;217:115830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 98. | Wu L, Fang C, Zhang J, Ye Y, Zhao H. The Association between Maternal/Fetal Insulin Receptor Substrate 1 Gene Polymorphism rs1801278 and Gestational Diabetes Mellitus in a Chinese Population. Gynecol Obstet Invest. 2021;86:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Alharbi KK, Khan IA, Abotalib Z, Al-Hakeem MM. Insulin receptor substrate-1 (IRS-1) Gly927Arg: correlation with gestational diabetes mellitus in Saudi women. Biomed Res Int. 2014;2014:146495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Albegali AA, Shahzad M, Mahmood S, Ullah MI. Genetic association of insulin receptor substrate-1 (IRS-1, rs1801278) gene with insulin resistant of type 2 diabetes mellitus in a Pakistani population. Mol Biol Rep. 2019;46:6065-6070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 101. | Zhang Y, Sun CM, Hu XQ, Zhao Y. Relationship between melatonin receptor 1B and insulin receptor substrate 1 polymorphisms with gestational diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2014;4:6113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 102. | Ziora-Jakutowicz KN, Zimowski J, Ziora K, Bednarska-Makaruk M, Świętochowska E, Gorczyca P, Szczepańska M, Machura E, Stojewska M, Gołąb-Jenerał K, Blaska M, Mizgała-Izworska E, Kukla M, Rybakowski F. Evaluation of the frequency of ADIPOQ c.45 T>G and ADIPOQ c.276 G>T polymorphisms in adiponectin coding gene in girls with anorexia nervosa. Endokrynol Pol. 2021;72:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 103. | de Gennaro G, Palla G, Battini L, Simoncini T, Del Prato S, Bertolotto A, Bianchi C. The role of adipokines in the pathogenesis of gestational diabetes mellitus. Gynecol Endocrinol. 2019;35:737-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 104. | Chen J, Tan B, Karteris E, Zervou S, Digby J, Hillhouse EW, Vatish M, Randeva HS. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 2006;49:1292-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 105. | Baldelli S, Aiello G, Mansilla Di Martino E, Campaci D, Muthanna FMS, Lombardo M. The Role of Adipose Tissue and Nutrition in the Regulation of Adiponectin. Nutrients. 2024;16:2436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 106. | Huang LT, Wu SL, Liao X, Ma SJ, Tan HZ. Adiponectin gene polymorphisms and risk of gestational diabetes mellitus: A meta-analysis. World J Clin Cases. 2019;7:572-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 107. | Bai Y, Tang L, Li L, Li L. The roles of ADIPOQ rs266729 and MTNR1B rs10830963 polymorphisms in patients with gestational diabetes mellitus: A meta-analysis. Gene. 2020;730:144302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 108. | Takhshid MA, Haem Z, Aboualizadeh F. The association of circulating adiponectin and + 45 T/G polymorphism of adiponectin gene with gestational diabetes mellitus in Iranian population. J Diabetes Metab Disord. 2015;14:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Ni J, Wang Y, Zhu J, Zheng T, Yu J, Xiong Y. Interleukin-10 levels and risk of gestational diabetes mellitus: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2022;35:7609-7616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 110. | Teler J, Tarnowski M, Safranow K, Maciejewska A, Sawczuk M, Dziedziejko V, Sluczanowska-Glabowska S, Pawlik A. CCL2, CCL5, IL4 and IL15 Gene Polymorphisms in Women with Gestational Diabetes Mellitus. Horm Metab Res. 2017;49:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 111. | Kang J, Liu CH, Lee CN, Li HY, Yang CW, Huang SC, Lin SY, Jou TS. Novel Interleukin-10 Gene Polymorphism Is Linked to Gestational Diabetes in Taiwanese Population. Front Genet. 2019;10:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Song L, Zhong M. Association between Interleukin-10 gene polymorphisms and risk of early-onset preeclampsia. Int J Clin Exp Pathol. 2015;8:11659-11664. [PubMed] |
| 113. | Wei Q, Chen X, Chen H. Association of Single Nucleotide Polymorphisms of the IL-6, IL-10, and TNF-α Genes with Susceptibility to Gestational Diabetes Mellitus. Genet Test Mol Biomarkers. 2020;24:390-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 114. | Alegría-Torres JA, Baccarelli A, Bollati V. Epigenetics and lifestyle. Epigenomics. 2011;3:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 366] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 115. | Dalfrà MG, Burlina S, Del Vescovo GG, Lapolla A. Genetics and Epigenetics: New Insight on Gestational Diabetes Mellitus. Front Endocrinol (Lausanne). 2020;11:602477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 116. | Lim DHK, Maher ER. DNA methylation: a form of epigenetic control of gene expression. Obstet Gynaecol. 2010;12:37-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 117. | Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat Rev Genet. 2018;19:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 1023] [Article Influence: 113.7] [Reference Citation Analysis (1)] |
| 118. | Zuccarello D, Sorrentino U, Brasson V, Marin L, Piccolo C, Capalbo A, Andrisani A, Cassina M. Epigenetics of pregnancy: looking beyond the DNA code. J Assist Reprod Genet. 2022;39:801-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 119. | Koukoura O, Sifakis S, Spandidos DA. DNA methylation in the human placenta and fetal growth (review). Mol Med Rep. 2012;5:883-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 120. | Zhu W, Shen Y, Liu J, Fei X, Zhang Z, Li M, Chen X, Xu J, Zhu Q, Zhou W, Zhang M, Liu S, Du J. Epigenetic alternations of microRNAs and DNA methylation contribute to gestational diabetes mellitus. J Cell Mol Med. 2020;24:13899-13912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 121. | Finer S, Mathews C, Lowe R, Smart M, Hillman S, Foo L, Sinha A, Williams D, Rakyan VK, Hitman GA. Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Hum Mol Genet. 2015;24:3021-3029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 122. | Ondičová M, Irwin RE, Thursby SJ, Hilman L, Caffrey A, Cassidy T, McLaughlin M, Lees-Murdock DJ, Ward M, Murphy M, Lamers Y, Pentieva K, McNulty H, Walsh CP. Folic acid intervention during pregnancy alters DNA methylation, affecting neural target genes through two distinct mechanisms. Clin Epigenetics. 2022;14:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 123. | Pinney SE. Metabolic Disorders and Developmental Origins of Health and Disease. In: Rosenfeld CS, editor. The Epigenome and Developmental Origins of Health and Disease. Amsterdam: Academic Press, 2016. [DOI] [Full Text] |
| 124. | El Hajj N, Pliushch G, Schneider E, Dittrich M, Müller T, Korenkov M, Aretz M, Zechner U, Lehnen H, Haaf T. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes. 2013;62:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 125. | Reichetzeder C, Dwi Putra SE, Pfab T, Slowinski T, Neuber C, Kleuser B, Hocher B. Increased global placental DNA methylation levels are associated with gestational diabetes. Clin Epigenetics. 2016;8:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 126. | Howe CG, Cox B, Fore R, Jungius J, Kvist T, Lent S, Miles HE, Salas LA, Rifas-Shiman S, Starling AP, Yousefi P, Ladd-Acosta C, Baccarelli A, Binder EB, Chatzi VL, Czamara D, Dabelea D, DeMeo DL, Ghantous A, Herceg Z, Kajantie E, Lahti JMT, Lawlor DA, Litonjua A, Nawrot TS, Nohr EA, Oken E, Pizzi C, Plusquin M, Räikkönen K, Relton CL, Sharp GC, Sørensen TIA, Sunyer J, Vrijheid M, Zhang W, Hivert MF, Breton CV. Maternal Gestational Diabetes Mellitus and Newborn DNA Methylation: Findings From the Pregnancy and Childhood Epigenetics Consortium. Diabetes Care. 2020;43:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 127. | Ott R, Melchior K, Stupin JH, Ziska T, Schellong K, Henrich W, Rancourt RC, Plagemann A. Reduced Insulin Receptor Expression and Altered DNA Methylation in Fat Tissues and Blood of Women With GDM and Offspring. J Clin Endocrinol Metab. 2019;104:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 128. | Kang J, Lee CN, Li HY, Hsu KH, Lin SY. Genome-wide DNA methylation variation in maternal and cord blood of gestational diabetes population. Diabetes Res Clin Pract. 2017;132:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 129. | Houshmand-Oeregaard A, Hansen NS, Hjort L, Kelstrup L, Broholm C, Mathiesen ER, Clausen TD, Damm P, Vaag A. Differential adipokine DNA methylation and gene expression in subcutaneous adipose tissue from adult offspring of women with diabetes in pregnancy. Clin Epigenetics. 2017;9:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 130. | Mollet IG, Malm HA, Wendt A, Orho-Melander M, Eliasson L. Integrator of Stress Responses Calmodulin Binding Transcription Activator 1 (Camta1) Regulates miR-212/miR-132 Expression and Insulin Secretion. J Biol Chem. 2016;291:18440-18452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 131. | Wang J, Chen J, Sen S. MicroRNA as Biomarkers and Diagnostics. J Cell Physiol. 2016;231:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 599] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 132. | Peterson SM, Thompson JA, Ufkin ML, Sathyanarayana P, Liaw L, Congdon CB. Common features of microRNA target prediction tools. Front Genet. 2014;5:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 325] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 133. | Sliwinska A, Kasinska MA, Drzewoski J. MicroRNAs and metabolic disorders - where are we heading? Arch Med Sci. 2017;13:885-896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 134. | Iacomino G, Siani A. Role of microRNAs in obesity and obesity-related diseases. Genes Nutr. 2017;12:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 135. | He Y, Ding Y, Liang B, Lin J, Kim TK, Yu H, Hang H, Wang K. A Systematic Study of Dysregulated MicroRNA in Type 2 Diabetes Mellitus. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 136. | Chen DB, Wang W. Human placental microRNAs and preeclampsia. Biol Reprod. 2013;88:130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 137. | Poirier C, Desgagné V, Guérin R, Bouchard L. MicroRNAs in Pregnancy and Gestational Diabetes Mellitus: Emerging Role in Maternal Metabolic Regulation. Curr Diab Rep. 2017;17:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 138. | Chim SS, Shing TK, Hung EC, Leung TY, Lau TK, Chiu RW, Lo YM. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 653] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 139. | Liu ZN, Jiang Y, Liu XQ, Yang MM, Chen C, Zhao BH, Huang HF, Luo Q. MiRNAs in Gestational Diabetes Mellitus: Potential Mechanisms and Clinical Applications. J Diabetes Res. 2021;2021:4632745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 140. | Zhao C, Dong J, Jiang T, Shi Z, Yu B, Zhu Y, Chen D, Xu J, Huo R, Dai J, Xia Y, Pan S, Hu Z, Sha J. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS One. 2011;6:e23925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 141. | Seyhan AA. microRNAs with different functions and roles in disease development and as potential biomarkers of diabetes: progress and challenges. Mol Biosyst. 2015;11:1217-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 142. | Pheiffer C, Dias S, Rheeder P, Adam S. Decreased Expression of Circulating miR-20a-5p in South African Women with Gestational Diabetes Mellitus. Mol Diagn Ther. 2018;22:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 143. | Tagoma A, Alnek K, Kirss A, Uibo R, Haller-Kikkatalo K. MicroRNA profiling of second trimester maternal plasma shows upregulation of miR-195-5p in patients with gestational diabetes. Gene. 2018;672:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 144. | Wander PL, Boyko EJ, Hevner K, Parikh VJ, Tadesse MG, Sorensen TK, Williams MA, Enquobahrie DA. Circulating early- and mid-pregnancy microRNAs and risk of gestational diabetes. Diabetes Res Clin Pract. 2017;132:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 145. | Zhu Y, Tian F, Li H, Zhou Y, Lu J, Ge Q. Profiling maternal plasma microRNA expression in early pregnancy to predict gestational diabetes mellitus. Int J Gynaecol Obstet. 2015;130:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/