Published online Mar 9, 2025. doi: 10.5409/wjcp.v14.i1.99177
Revised: October 8, 2024
Accepted: November 12, 2024
Published online: March 9, 2025
Processing time: 157 Days and 13.3 Hours
2D-echocardiography (2DE) has been the primary imaging modality in children with Kawasaki disease (KD) to assess coronary arteries.
To report the presence and implications of incidental congenital coronary artery anomalies that had been misinterpreted as coronary artery abnormalities (CAAs) on 2DE.
Records of children diagnosed with KD, who underwent computed tomography coronary angiography (CTCA) at our center between 2013-2023 were reviewed. We identified 3 children with congenital coronary artery anomalies in this cohort on CTCA. Findings of CTCA and 2DE were compared in these 3 children.
Of the 241 patients with KD who underwent CTCA, 3 (1.24%) had congenital coronary artery anomalies on CTCA detected incidentally. In all 3 patients, ba
CTCA is essential for detailed assessment of coronary arteries in children with KD especially in cases where there is suspicion of congenital coronary artery anomalies. Relying solely on 2DE may not be sufficient in such cases, and findings from CTCA can significantly impact therapeutic decision-making.
Core Tip: Congenital coronary artery anomalies are liable to be misinterpreted as coronary artery abnormalities (CAAs) on 2D-echocardiography in children with Kawasaki disease. Computed tomography coronary angiography is essential for the detailed evaluation of coronary arteries.
- Citation: Pilania RK, Nadig PL, Basu S, Tyagi R, Thangaraj A, Aggarwal R, Arora M, Sharma A, Singh S, Singhal M. Congenital anomalies of coronary artery misdiagnosed as coronary dilatations in Kawasaki disease: A clinical predicament. World J Clin Pediatr 2025; 14(1): 99177
- URL: https://www.wjgnet.com/2219-2808/full/v14/i1/99177.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i1.99177
Kawasaki disease (KD) is the most common childhood vasculitis and has a special predilection for coronary arteries[1]. 2D-echocardiography (2DE) is a convenient, noninvasive, and inexpensive bedside imaging modality to assess coronary arteries and cardiac function in children with KD[1,2]. It can be repeated as often as required. However, the findings on 2DE are operator-dependent and may have poor reproducibility. Limitations of 2DE in assessing coronaries in children with KD include difficulties in (1) Assessment of left circumflex (LCx) coronary artery; (2) Assessment of mid and distal segments of coronary arteries, and (3) In detecting coronary thrombus and stenosis. Further, a poor acoustic window in adolescent children (because of thick chest wall) creates difficulties for the operator[2-4]. In addition, certain anatomical variations in coronary arteries can be misinterpreted as dilated coronaries, resulting in diagnostic difficulties[5].
Computed tomography coronary angiography (CTCA) has emerged as a new modality for assessing coronary arteries along their entire course[3,4,6,7]. It has a high reproducibility and provides explicit details of anatomy. Though high radiation exposure was a concern in the initial days, in the current era, with advancements in dual-source CT Scanners and radiation-optimized protocols, radiation exposure of < 1 millisievert can be achieved[3,4]. The present study aims to report the presence and implications of incidental congenital coronary artery anomalies that had been misinterpreted as CAAs on 2DE.
This study was conducted at a federally funded not-for-profit tertiary care teaching institute from North-West India. Our unit has over 29 years of experience managing children with KD. We follow the largest single-center cohort of KD in India.
Between January 1994-June 2023, 1150 patients were diagnosed and treated with KD at our center. Diagnosis of KD was based on the American Heart Association guidelines[8]. Initial Coronary artery assessment in children with KD was performed using 2DE in accordance with standard guidelines. The coronary artery Z-scores were calculated based on body surface area measurements using the method described by Dallaire et al[9], which is widely recognized for its accuracy in pediatric populations. We started using CTCA as a diagnostic imaging modality in KD in October 2013 on a 128-slice dual-source CT scanner and have carried out this investigation in 241 patients till October 2023. Over the last 10 years or so, CTCA has emerged as an important imaging modality in children with KD. We have recently published the suggested indications for CTCA in KD[4-6]. At our center, the indications for performing CTCA are as follows[3,4]: (1) Children with KD who present with coronary artery abnormalities (CAAs) on 2DE at presentation; (2) Children with KD and resistance to intravenous immunoglobulin (IVIg) therapy; (3) Children with KD in infancy; (4) Children with KD and either myocarditis, or KD shock syndrome; and (5) Children with KD with macrophage activation syndrome.
Medical records of children diagnosed with KD who underwent CTCA during this period were reviewed. Patients with KD in whom incidental congenital coronary artery anomalies were identified on CTCA were analyzed, and results correlated with findings of 2DE. The manuscript was approved by the Departmental Review Board.
Of the 241 patients with KD who underwent CTCA for detailed assessment of CAAs, 3 patients were identified to have incidental congenital coronary artery anomalies (1.25%) on CTCA. Details of the three patients are as follows.
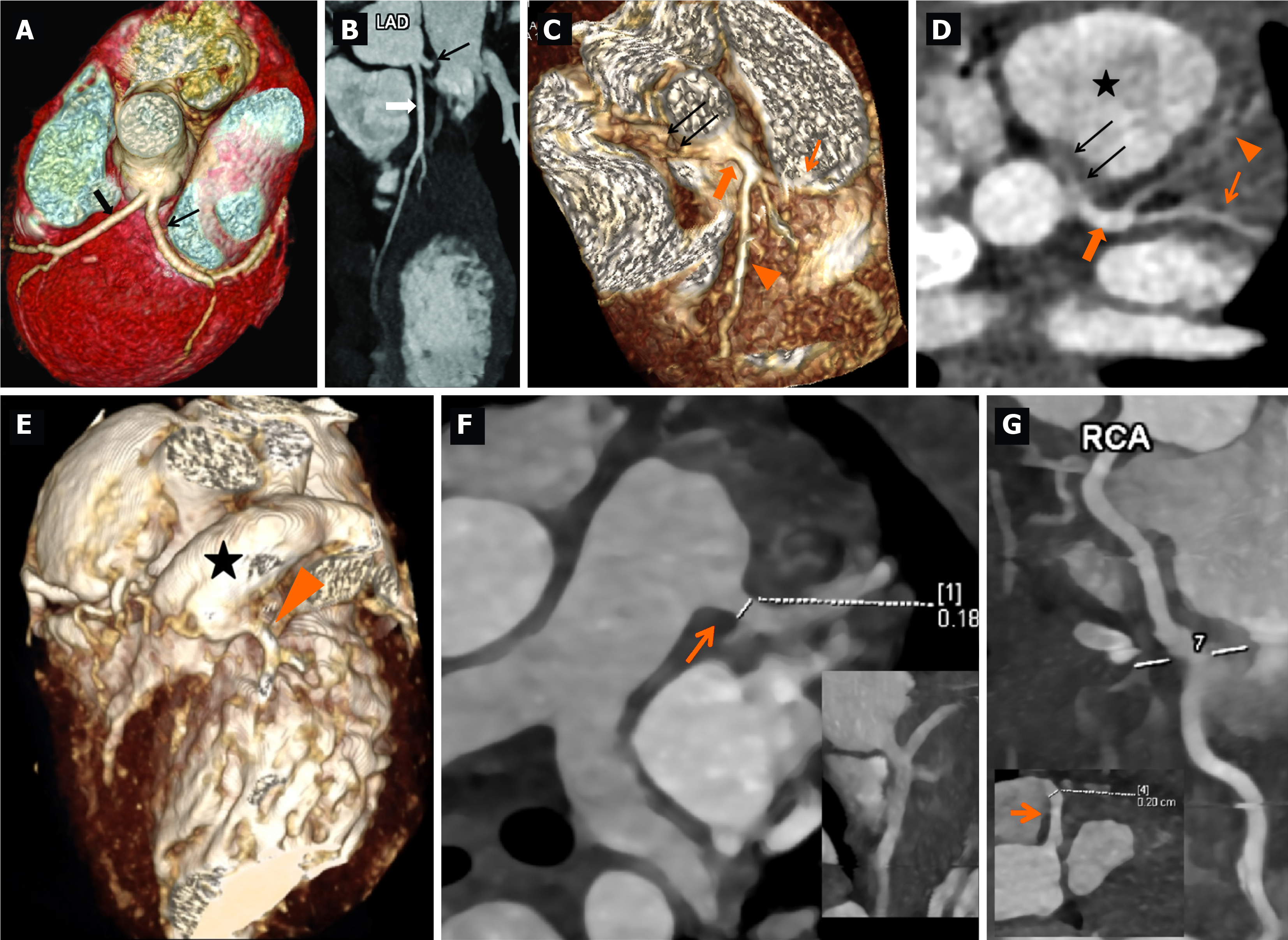
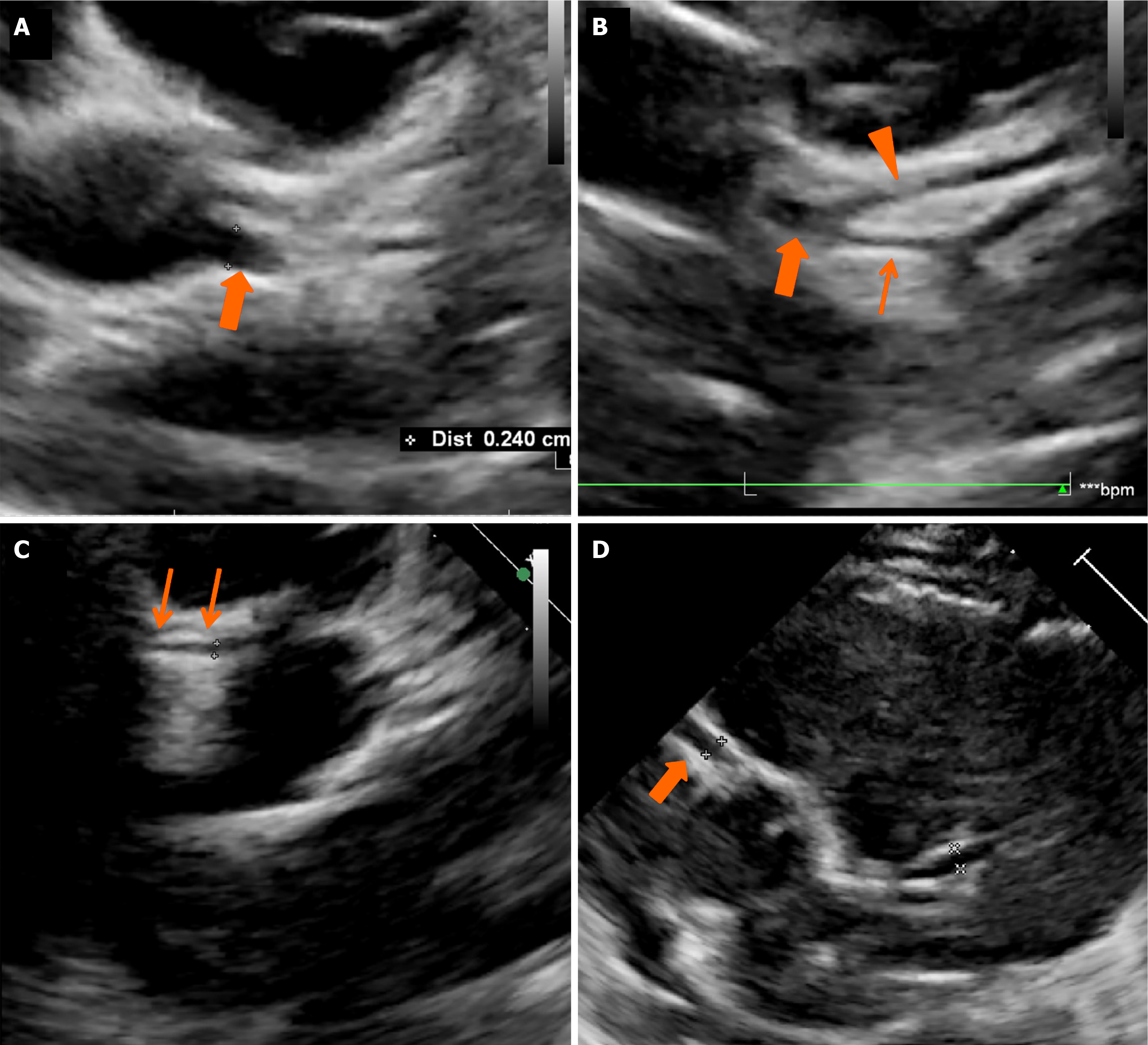
11-year-old boy who presented with fever, irritability, vomiting, loose stools, vomiting, and mucocutaneous changes in the form of red and cracked lips. Physical examination showed conjunctival injection, erythematous rash over lower limbs, and altered sensorium with poor Glasgow Coma Scale (E2V5M2). Systemic examination was unremarkable. Investigations showed anemia, thrombocytopenia, neutrophilic leucocytosis, hematuria, elevated creatinine, and liver enzymes (Table 1). Work-up for infectious causes (scrub typhus, leptospirosis, Dengue fever, and malaria) was non-contributory. During illness, on day 6 of illness, he developed periungual desquamation. 2DE for coronary artery assessment showed a left main coronary artery (LCA) aneurysm (10 mm; Z = +14.7 Z). He received IVIg 2 g/kg and hemodialysis and showed marked clinical improvement after treatment and laboratory parameters, including renal functions. He was discharged on low-dose aspirin therapy (3-5 mg/kg/day). CTCA done at 3 weeks during convalescence showed separate origins of the left anterior descending artery (LAD) and LCx from the left sinus with no LCA (Figure 1A and B). Aspirin was discontinued on follow-up.
| Characteristics | Case 1 | Case 2 | Case 3 |
| Age of presentation/sex | 11 years/male | 3.3 years/male | 4 months/female |
| Clinical presentation | Complete KD | Incomplete KD | Incomplete KD |
| Haemoglobin (g/L) | 105 | 97 | 102 |
| Total leukocyte counts (× 109/L) | 22.4 | 6 | 7.6 |
| Differential leukocyte counts | N62L24M08E06 | N67L20M7E06 | N51L36M10E3 |
| Platelet counts at presentation (× 109/L) | 0.49 | 4.46 | 6.1 |
| ESR (mm/hour) | 54 | 29 | |
| CRP (mg/L) (n < 6 mg/L) | 229 | 45.63 | 107 |
| NT-proBNP (n < 125 pg/mL) | 39000 | 1318 | 2649 |
| Serum albumin (g/dL) | 1.7 | 4.1 | 3.6 |
| ALT (IU/L) | 21 | 17 | 951 |
| AST (U/L) | 39 | 37 | 534 |
| Blood urea (mg/dL) | 219 | 32 | |
| Serum creatinine (mg/dL) | 5.5 | 0.11 | |
| Urine microscopy | 10-12 RBCs/hpf, 5-6 WBCs/hpf | ||
| Others | Skin biopsy- features of small vessel vasculitis. Cerebrospinal fluid analysis: Cells- nil, Sugar-88 mg/dL, Protein- 31 mg/dL, Gram stain and Indian ink negative, culture sterile | ||
| 2DE findings | LCA: 10 mm (+ 14.7 z) | LCA: 3.34 mm (+ 3.44 Z); LAD: 1.5 mm (- 0.17 Z); LCx: 1.53 mm (0.09 Z) RCA: 0.99 mm (-2.06 Z) | LCA: Not visible. Proximal LAD: 1.48 mm (+ 1.59 Z). Mid LAD: 2.01 mm (+ 3.42 Z). RCA: 2.15 mm (+ 4.03Z) |
| Treatment received | IVIg (2 g/kg); aspirin (3-5 mg/kg/day) | IVIg (2 g/kg); aspirin (3-5 mg/kg/day); infliximab (5mg/kg) | IVIg (2 g/kg); aspirin (3-5 mg/kg/day) |
| CTCA findings | Separate origins of the LAD and LCx from left sinus with no LCA | Single coronary artery arising from left sinus with left dominant circulation. Anomalous origin of RCA from common trunk left sinus with interarterial course. No evidence of any stenosis/ectasia/aneurysm was seen | Anomalous origin of LCA from MPA c/w ALCAPA with dilated LAD and LCX. Diffusely dilated RCA (likely due to ALCAPA). No aneurysm was seen in the coronary arteries |
A 3.3-year-old boy presented with prolonged fever, maculopapular rash, perianal redness, and dorsal edema. Upon history review, the parents noticed periungual peeling on day 6 of the illness. A clinical possibility of incomplete KD was considered. He had anemia, thrombocytosis, and elevated acute phase reactants (Table 1). He had been administered IVIg (2 g/kg) and low-dose aspirin (3-5 mg/kg/day) at a hospital elsewhere and referred to our institute for further management. Coronary artery assessment by 2DE showed LCA aneurysms [LCA: 3.34 mm; +3.44 Z, LAD: 1.5 mm; -0.17 Z, LCx: 1.53 mm; 0.09 Z, and right coronary artery (RCA): 0.99 mm; -2.06 Z] (Figure 2A-C). He also received infliximab (5mg/kg). CTCA revealed a single coronary artery from the left sinus with the left dominant circulation with anomalous origin of RCA from the common trunk left sinus with the inter-arterial course (Figure 1C and D). Given the morphologically normal coronary arteries, oral aspirin was discontinued.
A 4-month-old girl presented with acute febrile illness, erythematous rash, respiratory distress, and hypotensive shock. She required mechanical ventilation and inotropic support. Laboratory investigations showed thrombocytosis, elevated transaminases, and raised inflammatory markers (Table 1). During the course of illness, she developed periungual desquamation. A clinical possibility of KD with myocarditis was considered. 2DE showed left ventricular dysfunction with left ventricular ejection fraction of 15%, severe mitral regurgitation, mild pericardial effusion, dilatation of RCA (2.15 mm; +4.03Z), and a small aneurysm in LAD coronary artery (2.01 mm; +3.42Z) (Figure 2D). She was treated with IVIg (2 g/kg) along with low-dose aspirin (3-5 mg/kg/day). CTCA showed the anomalous left coronary artery from the pulmonary artery (ALCAPA) with predominant right circulation (Figure 1E-G). She underwent corrective surgery for ALCAPA and remains well on follow-up.
KD is an acute self-limiting medium vessel vasculitis of childhood with a predilection for the coronary arteries. The most important complication of KD, which also results in significant morbidity is the development of CAAs[8]. Delays in diagnosis and treatment of KD can result in the development of CAAs in 15-25% of patients[10]. 2DE is the established modality for the assessment of CAAs of KD. However, 2DE has several limitations. It is operator-dependent and has lower sensitivity for detection of thrombus and stenosis. The distal segments of coronary arteries, the LCx coronary artery, and congenital coronary artery anomalies are difficult to evaluate on 2DE[3-6,11]. Because of these limitations, CTCA is now being increasingly used for the assessment of coronary arteries in children with KD[3-6].
We identified the presence of incidental congenital coronary artery anomalies in 3/241 (1.24%) children. One of these had ALCAPA (patient 3), which is considered to pose a risk of cardiac dysfunction due to chronic ischemia and thus necessitated immediate surgical correction. Because of the right dominant circulation, the RCA appears dilated in 2DE. The other two had minor coronary artery anomalies: One with separate origins of LAD and LCx from the left sinus with an absent left main trunk (patient 1), while the other had a single coronary artery (patient 2). In both these patients, the congenital anomaly had been misinterpreted as CAAs.
About 66 different varieties of coronary artery anomalies are described and classified in the literature[12]. Of these, only a few pose a risk of myocardial injury. Prevalence of congenital coronary artery anomalies has been variably reported in the literature, from 0.17% in autopsy studies[13] to 1.3% in adults evaluated by digital subtraction angio
2DE is standard of care imaging in the assessment of coronary arteries and cardiac functions in children with KD as it is a readily available bedside modality but has many inherent limitations. In our cohort of patients with KD, 2DE was performed at admission and then daily during admission. All 3 patients were found to have CAAs on 2DE by different operators. CTCA was performed in these 3 patients after initial stabilization as per our protocol. At our center, we offer radiation-optimized CTCA in the assessment of CAAs of KD. This is a non-invasive day-care examination with minimal radiation exposure (usually < 1 millisievert)[3,4]. The interval between baseline 2DE and CTCA in these 3 patients ranged from 2 days-3 weeks (patient 1:3 weeks; patient 2:2 days; patient 3:3 days).
Magnetic resonance coronary angiography (MRCA) has also been used in the assessment of CAAs. However, it has several drawbacks when compared to CTCA. One of the primary limitations of MRCA is its lower spatial and temporal resolution – this can impact the accuracy of imaging. Additionally, MRCA typically takes much longer (usually 45-60 minutes), and necessitates general anesthesia in infants and younger children to prevent motion artifacts. MRCA is usually a modality of choice for follow-up of thrombotic CAAs[16,17].
CTCA is a safer and non-invasive technique that can demonstrate coronary arteries along their entire course. Though radiation exposure and motion artifacts were shortcomings, recent advances in CTCA imaging have enabled a high resolution with motion-free cardiac images at any heart rate with optimal radiation exposure. These images with high spatial and temporal resolution can delineate coronary artery anatomy with exquisite details of all coronary artery segments, depict changes in luminal caliber, and characterize plaque and other intramural changes. CTCA is rapidly emerging as a non-invasive imaging modality for evaluating CAAs in KD[3,4,17]. Timely diagnosis and findings of CTCA in the 3 patients being reported herein have significantly impacted therapeutic decision-making.
We have recently published the suggested indications for CTCA in KD[3,4]. Based on our experience over the last 10 years we suggest that CTCA should be carried out in the following situations during the acute phase: (1) Children with KD who present with CAAs on 2DE at presentation; (2) Children with KD and resistance to intravenous immunoglobulin therapy; (4) Children with KD in infancy; (5) Children with KD and either myocarditis or KD shock syndrome; and (6) Children with KD with macrophage activation syndrome.
Strengths of this study is that our unit has over 29 years of experience managing children with KD. We follow the largest single-center cohort of KD in India. We have experience with CTCA in 241 patients with KD-this is arguably one of the largest experiences on CTCA in KD. There are certain limitations inherent to the retrospective study design from a single center.
To conclude, this study underscores the misinterpretation risk of congenital coronary anomalies as CAAs in KD using 2DE. The incidental discovery of such anomalies in 1.24% of CTCA-scanned KD patients highlights the need for advanced imaging. CTCA remains crucial for coronary artery assessment in children with KD. One patient necessitated immediate intervention due to an anomalous left coronary artery, initially misdiagnosed as a CAA. These findings emphasize CTCA's pivotal role in precise diagnosis and treatment planning in KD, particularly for coronary artery anomalies. Further research is needed to validate these results and enhance clinical guidelines.
The authors gratefully acknowledge the role of all faculty members, clinical fellows, nursing officers, research, and technical staff who have been involved in the care of these patients.
| 1. | McCrindle BW, Cifra B. The role of echocardiography in Kawasaki disease. Int J Rheum Dis. 2018;21:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Singhal M, Pilania RK, Jindal AK, Gupta A, Sharma A, Guleria S, Johnson N, Maralakunte M, Vignesh P, Suri D, Sandhu MS, Singh S. Distal coronary artery abnormalities in Kawasaki disease: experience on CT coronary angiography in 176 children. Rheumatology (Oxford). 2023;62:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Singhal M, Pilania RK, Thangaraj A, Chaudhary H, Gummadi A, Soundararajan R, Loganathan S, Sharma A, Gupta A, Bhattad S, Jindal AK, Vignesh P, Suri D, Sandhu MS, Singh S. The value of CT coronary angiography for a comprehensive assessment of left circumflex artery in Kawasaki disease: 9 years of experience from a tertiary center. Lancet Reg Health Southeast Asia. 2024;29:100471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Tsuda K, Kishimoto S, Kagiyama Y, Koteda Y, Suda K. Pitfall in Acute Care of Kawasaki Disease: Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery—Secondary Publication. J Pediatr Cardiol Card Surg. 2023;7:36-40. [DOI] [Full Text] |
| 6. | van Stijn D, Planken RN, Groenink M, Streekstra GJ, Kuijpers TW, Kuipers IM. Coronary artery assessment in Kawasaki disease with dual-source CT angiography to uncover vascular pathology. Eur Radiol. 2020;30:432-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Thangathurai J, Kalashnikova M, Takahashi M, Shinbane JS. Coronary Artery Aneurysm in Kawasaki Disease: Coronary CT Angiography through the Lens of Pathophysiology and Differential Diagnosis. Radiol Cardiothorac Imaging. 2021;3:e200550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017;135:e927-e999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 2610] [Article Influence: 290.0] [Reference Citation Analysis (1)] |
| 9. | Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr. 2011;24:60-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode MP. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 820] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 11. | Singhal M, Pilania R, Singh S. Comment on the Article “Pitfall in Acute Care of Kawasaki Disease: Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery—Secondary Publication” by Tsuda K et al (2023). J Pediatr Cardiol Card Surg. 2023;7:69-70. [DOI] [Full Text] |
| 12. | Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation. 2007;115:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 543] [Article Influence: 28.6] [Reference Citation Analysis (2)] |
| 13. | Alexander RW, Griffith GC. Anomalies of the coronary arteries and their clinical significance. Circulation. 1956;14:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 260] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Yildiz A, Okcun B, Peker T, Arslan C, Olcay A, Bulent Vatan M. Prevalence of coronary artery anomalies in 12,457 adult patients who underwent coronary angiography. Clin Cardiol. 2010;33:E60-E64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Davis JA, Cecchin F, Jones TK, Portman MA. Major coronary artery anomalies in a pediatric population: incidence and clinical importance. J Am Coll Cardiol. 2001;37:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 255] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Dietz SM, Tacke CE, Kuipers IM, Wiegman A, de Winter RJ, Burns JC, Gordon JB, Groenink M, Kuijpers TW. Cardiovascular imaging in children and adults following Kawasaki disease. Insights Imaging. 2015;6:697-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | van Stijn D, Planken N, Kuipers I, Kuijpers T. CT Angiography or Cardiac MRI for Detection of Coronary Artery Aneurysms in Kawasaki Disease. Front Pediatr. 2021;9:630462. [PubMed] [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/