Published online Mar 9, 2025. doi: 10.5409/wjcp.v14.i1.91622
Revised: October 10, 2024
Accepted: November 13, 2024
Published online: March 9, 2025
Processing time: 354 Days and 10.8 Hours
The kidneys play a critical role in maintaining glucose homeostasis. Under normal renal tubular function, most of the glucose filtered from the glomeruli is re
Core Tip: Familial renal glucosuria (FRG) is the most common inherited defect in renal glucose transport. Mutations in the sodium/glucose cotransporter 5A2 gene, which encodes sodium-glucose cotransporter (SGLT) 2, lead to FRG. Although generally considered a benign condition, FRG has inspired pharmacological interest, especially as SGLT2 inhibitors have become a therapeutic target for reducing hyperglycemia in type 2 diabetes. The glucose-lowering mechanism of SGLT2 inhibitors closely resembles the pathophysiology of renal glucosuria. Detailed clinical and laboratory examinations of patients with FRG could provide further insights into the complications, significance, and long-term outcomes of SGLT2 inhibitor therapy.
- Citation: Torun Bayram M, Kavukcu S. Renal glucosuria in children. World J Clin Pediatr 2025; 14(1): 91622
- URL: https://www.wjgnet.com/2219-2808/full/v14/i1/91622.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i1.91622
Glucosuria refers to the presence of glucose in the urine. In healthy individuals, nearly all filtered glucose is reabsorbed in the proximal convoluted tubule, which helps maintain blood glucose levels within a normal range[1]. Consequently, glucose is typically absent or found only in trace amounts in urine. Glucosuria is defined as a urine glucose level that exceeds normal values, usually over 25 mg/dL in random urine samples[2]. Normally, the renal tubules reabsorb almost all glucose in the glomerular filtrate. However, glucosuria occurs when the filtered glucose load surpasses the renal tubules’ maximum reabsorptive capacity, known as the tubular maximum for glucose (TmG). This can happen in cases of elevated plasma glucose, as in diabetes mellitus, or when the tubules’ ability to reabsorb glucose is impaired, as seen in Fanconi syndrome (which also affects the absorption of phosphate and amino acids) or in isolated glucosuria, an inherited condition called familial renal glucosuria (FRG).
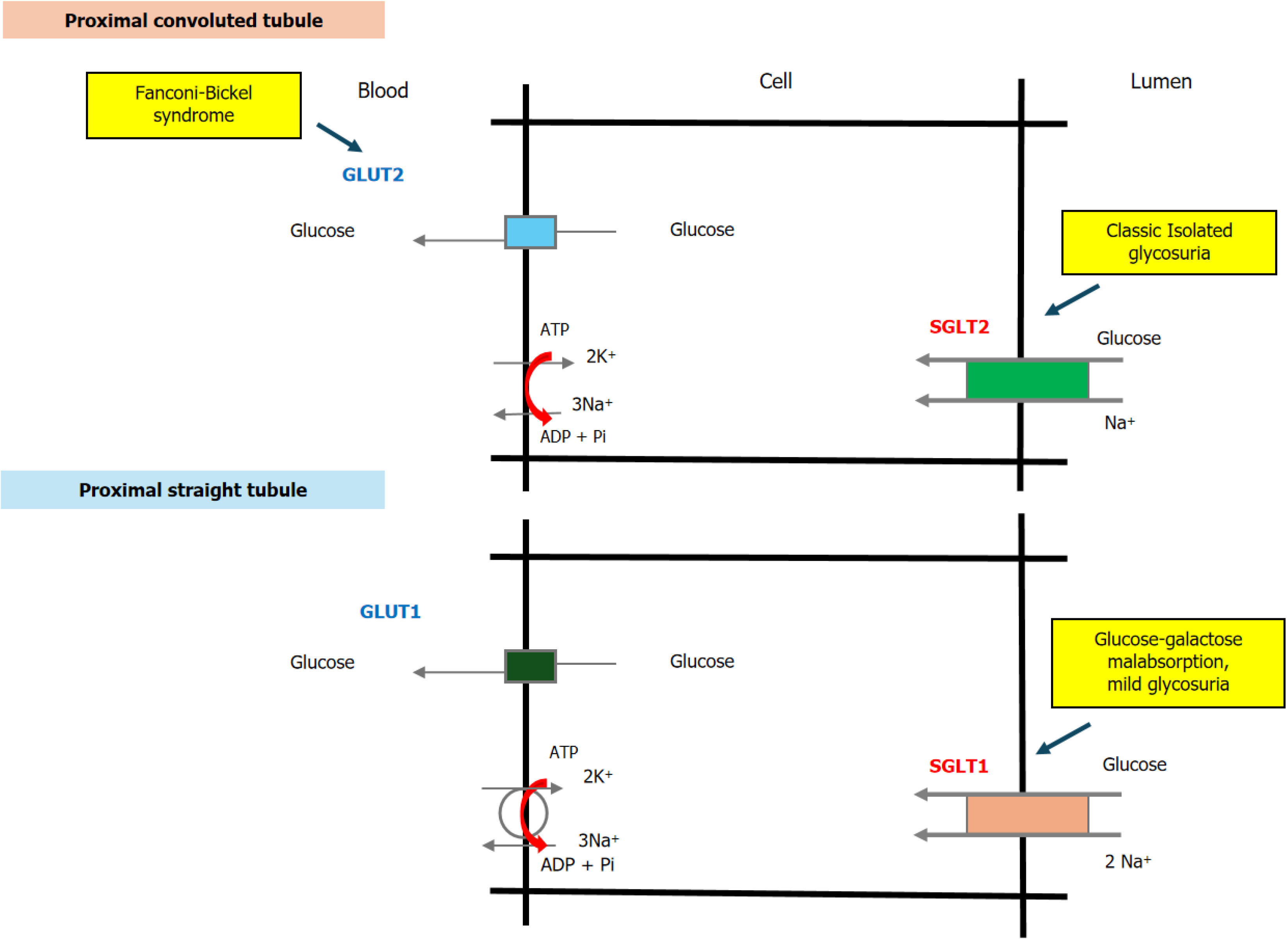
FRG is the most common inherited defect in renal glucose transport, although it remains a rare hereditary renal tubular disorder. Patients with FRG experience glucosuria owing to glucose reabsorption dysfunction in the proximal renal tubule, despite normal blood glucose levels. The sodium-glucose cotransporter (SGLT) 2 is a transmembrane protein located in the brush border of S1 segment proximal tubule cells, where it reabsorbs approximately 90% of the filtered glucose load[3,4]. Mutations in the sodium/glucose cotransporter (SLC) 5A2 gene, which encodes SGLT2, result in FRG[5]. Other hereditary causes of renal glucosuria (RG) include intestinal glucose-galactose malabsorption (GGM) and Fanconi-Bickel syndrome (FBS). Mutations in the SLC5A1 gene, which encodes SGLT1, lead to GGM. FBS is associated with mutations in SLC2A2, which encodes glucose transporter (GLUT) 2, a basolateral membrane-bound glucose transporter in the proximal convoluted tubule[6].
The incidence of RG in the general population is approximately 0.29%, though this rate varies depending on screening criteria. No specific racial or ethnic group appears to be more affected, and the condition does not show a higher prevalence in either males or females. Although some reports focus on younger individuals, RG can occur across all age groups.
Patients with FRG may experience symptoms such as polyuria, enuresis, mild growth delay, episodic dehydration, starvation ketosis, genitourinary infections, lower body weight, reduced obesity, lower systemic blood pressure, and diaper dermatitis. However, these occurrences are rare and typically mild. As a result, FRG is generally considered a benign condition with an excellent prognosis[7]. This benign nature has made SGLT2 inhibitors a pharmacological target for reducing hyperglycemia in type 2 diabetes[8]. The glucose-lowering mechanism of SGLT2 inhibitors resembles the pathophysiology of RG, suggesting that lower doses of these inhibitors could aid glycemic control in children with type 2 diabetes.
This review summarizes the general characteristics of proximal tubular glucose transport, explores glucose transport systems, and reviews the molecular pathophysiology and genetic aspects of hereditary glycosuria. It also describes the clinical features and treatment options for these tubulopathies, with a special focus on FRG, GGM, and FBS. Additionally, given that SGLT2 inhibitors exhibit effects similar to those observed in FRG, this review may offer insights into the safety of SGLT2 inhibitors and guidance for monitoring patients undergoing treatment with these drugs.
Glucose reabsorption by the renal tubule is nearly complete, with less than 0.05% of filtered glucose appearing in the urine[1]. In the proximal convoluted tubule, approximately 90% of filtered glucose is reabsorbed, with the remaining amount reclaimed in the proximal straight tubule[1,3]. This process relies on carrier-mediated, sodium (Na+)-dependent transport across the brush-border membrane of the proximal tubule[1,3]. The Na+ electrochemical gradient that drives glucose transport is maintained by Na+-K+-ATPase in the basolateral membrane. Glucose exits the cell via Na+-in
The kinetic properties of glucose reabsorption vary along the length of the nephron[9,10]. Two distinct Na+-dependent glucose transport systems exist: (1) A low-affinity/high-capacity cotransporter in the early proximal tubule; and (2) A high-affinity/low-capacity transporter in the late proximal tubule. The Na+-glucose coupling ratio is 1:1 in the convoluted portion and 2:1 in the straight portion of the proximal tubule[9,10]. The glucose transporter in the early proximal tubule is responsible for reabsorbing most of the filtered glucose from the tubular lumen, while the transporter in the late proximal tubule removes any remaining glucose[9,10].
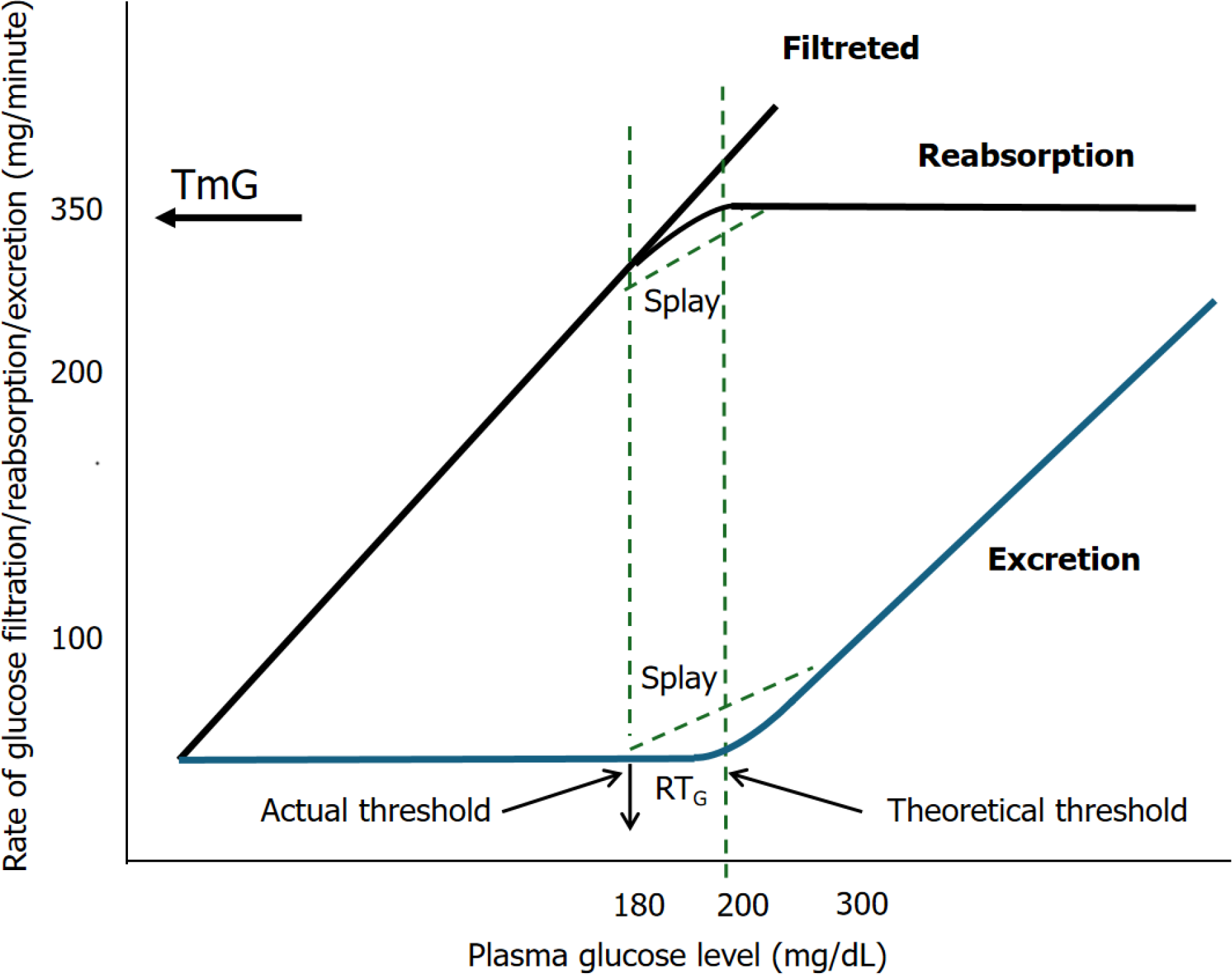
The capacity of the kidney to reabsorb glucose is limited[11]. As plasma glucose concentration increases, the amount of filtered glucose rises linearly. Up to a plasma glucose concentration of 200 mg/dL, all filtered glucose is reabsorbed because the reabsorptive capacity of SGLTs has not yet reached saturation. However, the reabsorption curve becomes nonlinear at plasma glucose concentrations between 200 mg/dL and 250 mg/dL as the cotransporters approach sa
The reported TmG values in children range between 260 mg/min/1.73 m² and 350 mg/min/1.73 m², with lower values observed in infants[6,12,13]. When the load of filtered glucose exceeds TmG, the glucose excretion rate increases linearly, paralleling the filtered load curve. In contrast, both the reabsorption and excretion curves exhibit non-linear shapes. This deviation from linearity indicates a threshold that occurs at a plasma glucose concentration lower than TmG, a phe
SGLTs are membrane proteins that facilitate glucose reabsorption in the proximal tubule. SGLT1 and SGLT2 are located in the apical membrane of proximal tubular cells, while GLUT2, a uniporter, is present in the basolateral membrane (Figure 1). The SGLT proteins belong to the SLC5 family of Na+-glucose transporters, which includes 12 human proteins, 6 of which are expressed in the kidney and other organs[15,16]. SGLT1, the prototype of the SGLTs, is encoded by the SLC5A1 gene located on chromosome 22q11.2[15,17]. SGLT2, classified as a low-affinity Na+-glucose cotransporter, is encoded by the SLC5A2 gene on chromosome 16p11.2[11,18]. The amino acid sequences of SGLT2 and SGLT1 are 59% identical. While SGLT1, a high-affinity/low-capacity transporter, is predominantly found in the straight proximal tubule (S3 segment), SGLT2 is highly expressed in the early segments of the proximal tubule (S1 and S2) (Figure 1). The Na+-to-glucose coupling ratios for SGLT1 and SGLT2 are 2:1 and 1:1, respectively[19-21]. Variants in the SLC5A1 and SLC5A2 genes are responsible for GGM and FRG, respectively.
Glucose that accumulates inside tubular epithelial cells is transported to the interstitium through Na+-independent facilitative glucose transporters, primarily GLUT2, which operate according to concentration gradients[1,22]. These basolateral glucose transporters are part of the SLC2 family of facilitative hexose transporters, which comprises 14 glucose transporters (GLUT1–14)[22-24]. GLUT1 (SLC2A1), the first facilitative glucose transporter to be sequenced, is the most widely distributed transporter in human tissues[22,23]. It is a high-affinity transporter located in the basolateral membrane of S3 segment cells in the proximal straight tubule of the kidney (Figure 1)[25,26]. In contrast, GLUT2 (SLC2A2) has a low affinity for glucose and is the predominant glucose transporter in hepatocytes. It is also present in the basolateral membranes of intestinal and renal tubular cells[24-27]. In the kidney, GLUT2 is strongly expressed in the proximal convoluted tubule (S1/S2 segments) and, to a lesser extent, in the proximal straight tubule (S3 segment) (Figure 1)[24,27]. FBS is associated with mutations in the SLC2A2 gene, while mutations in SLC2A1 primarily present with neurological symptoms and do not exhibit a renal phenotype. Several other members of the SLC2 family have been identified in the kidney and implicated in glucose transport, but their functional roles are not well understood[22,24]. For example, GLUT4 has been detected in the thick ascending limb of the nephron and may play a role in local metabolic regulation[28]. GLUT5 primarily functions as a fructose transporter in the apical membrane of the rat proximal straight tubule[28,29], and GLUT12 is found in the apical membrane of distal tubules and the collecting duct, although its function remains unclear[30].
In summary, the transepithelial organization of renal glucose transport in the proximal tubule involves both apical Na+-dependent and basolateral Na+-independent glucose transporters[1,22]. The first step in glucose transport occurs at the apical membrane, where SGLTs bind Na+. This interaction generates an electrochemical Na+ gradient through the action of Na+/K+-ATPase, which serves as the driving force for symporter activity, resulting in glucose accumulation within the epithelial cells. In the second step, the glucose concentration gradient between the cell and the plasma facilitates the passive exit of glucose across the basolateral membrane via GLUT2. In the early proximal tubule, SGLT2 (a low-affinity/high-capacity glucose transporter) and GLUT2 (a Na+-independent transporter) account for most of the glucose re
FRG is characterized by the excretion of variable amounts of glucose in the urine, without any abnormal fasting blood glucose levels, impaired glucose tolerance, or signs of tubular dysfunction[7]. The prevalence of this condition in children is not well established, as routine urine analyses are not commonly performed in most medical centers. However, Urakami et al[32] conducted an annual urine glucose screening program in schools in Tokyo, which found a positive glucosuria rate of approximately 0.1% in the first test and about 0.05% in the second test. Notably, nearly 70% of these schoolchildren were subsequently diagnosed with RG following detailed examinations, including an oral glucose tolerance test and HbA1c measurements[32]. In the general Caucasian population, the prevalence of RG has been reported to be 0.29%[33].
Initially, the inheritance pattern of FRG was thought to be either autosomal dominant or recessive. However, cohort studies have indicated that the inheritance is co-dominant with incomplete penetrance[34]. In the early 2000s, it was established that RG was associated with a defect in SGLT2, although the human gene for this transporter had not yet been characterized[35]. In 2002, the SGLT2 gene, designated SLC5A2, was first identified as the mutated gene associated with FRG[5]. Located on chromosome 16p11.2, SLC5A2 consists of 2019 base pairs, with 14 exons encoding 672 amino acids that form 14 transmembrane helices[36,37]. To date, 123 variants of the SLC5A2 gene have been identified, with the most common being missense, nonsense, frameshift, and splicing mutations[38-42]. In patients with FRG without an identified SLC5A2 variant, mutations in the PDZK1IP1 gene have also been observed. This gene encodes MAP17, a membrane protein that enhances the transport activity of SGLT2[38,43].
FRG is a heterogeneous condition classified into three types (A, B, and O) based on the analysis of renal titration curves for glucose reabsorption[21,44,45]. Patients with type A FRG, also known as classic RG, exhibit a minimal FminG (the minimum threshold), defined as the filtered glucose load at which 1 mg of glucose per min appears in the urine, along with reduced TmG. In contrast, type B FRG is characterized by a low FminG threshold but a normal TmG with increased splay. It is suggested that type A FRG arises from a reduced capacity of the glucose transport system due to a uniform defect across all nephrons, while type B FRG indicates a decrease in the affinity of the transport system due to nephron heterogeneity[44]. The third type, type O glycosuria, is characterized by virtually absent tubular reabsorption of glucose, resulting in severe glucosuria (≥ 10 g/1.73 m²/24 h)[21]. Type O RG is a rare subtype, with cases involving homozygous mutations classified as type O with severe glycosuria, while heterozygous cases exhibiting milder glucosuria are categorized as type A or B[46,47].
Most patients with FRG do not exhibit significant symptoms or serious effects associated with excessive urinary glucose excretion, such as polyuria. However, there have been reports of polyuria, enuresis, mild growth delays, episodic dehydration, starvation ketosis, and genitourinary infections in patients with type O FRG[7,29,32,46]. A study involving approximately 2.5 million adolescents indicated that FRG is associated with lower body weight, reduced obesity, and lower systemic blood pressure[48]. Wang et al[7] also noted low body weight in one of their cases. Dorum et al[46] observed low body weights in their patients, with one patient’s height falling below the third percentile; however, systemic blood pressure measurements remained within normal limits for all cases. Additionally, these authors reported dermatitis in the diaper area of infants, suggesting that glucosuria might predispose them to fungal infections. Activation of the renin-angiotensin-aldosterone system may occur owing to natriuresis and potential extracellular volume depletion, which has been reported in some patients with FRG[49]. However, Lee et al[50] demonstrated that the renin-angiotensin-aldosterone system remained unaffected in cases of FRG. Furthermore, Xu et al[38] found no evidence of a correlation between Na+ excretion and glucose excretion.
RG is often suspected when glucosuria occurs without signs of generalized proximal tubular dysfunction or hyperglycemia. Established clinical criteria for diagnosing RG include the criteria described by Marble[51]: (1) Normal plasma glucose concentration during an oral glucose tolerance test; (2) Normal plasma levels of insulin and glycosylated hemoglobin; and (3) Relatively stable urinary glucose excretion in both day and night-time urine samples. Additionally, it has been proposed that normal glucose tolerance status, assessed by oral glucose tolerance test, should be considered a diagnostic criterion regardless of the presence of glycosuria in the fasting state[52].
In FRG, the renal defect is specific to glucose, with no increase in the urinary excretion of other sugars[53]. However, some mutations in the SLC5A2 gene are associated with generalized aminoaciduria[54]. RG and generalized ami
SGLT2 inhibitors are antidiabetic agents known for their cardiac and renal protective effects, and they have become widely used in the management of type 2 diabetes in recent years. FRG is generally regarded as a benign condi
GGM is a rare, autosomal-recessive disorder characterized by severe watery diarrhea with neonatal onset, resulting from defects in the active transport of glucose and galactose across the intestinal brush border[16]. While isolated RG is generally a benign condition, GGM is a life-threatening disease that can lead to death if glucose and galactose are not eliminated from the diet[16]. GGM was the first reported condition associated with a mutation in a membrane transport protein. The initial discovery of a missense mutation in the SLC5A1 gene, which causes an amino acid change at residue 28 from aspartate to asparagine in the intestinal brush-border SGLT1 Na+-glucose cotransporter, was made in two sisters with GGM in 1991[59]. Molecular studies have since identified 72 variants of SLC5A1 in the Human Gene Mutation Database, the majority of which are missense and nonsense mutations. These mutant proteins typically result in a significant reduction in Na+-glucose transport activity due to misrouting of the protein to the cell surface[16,35].
In GGM, watery osmotic diarrhea typically begins within hours to a few weeks after birth during feeding, leading to hypernatremic dehydration. The introduction of a fructose-containing formula can immediately halt the diarrhea and facilitate rapid rehydration[60]. Although severe diarrhea may improve with age, the speed and extent of recovery can vary among individuals; however, jejunal glucose transport remains absent[61]. As a result, affected individuals may gradually tolerate carbohydrates later in life, allowing for the reintroduction of a low-carbohydrate diet[62]. Renal complications, such as nephrocalcinosis, are also common in GGM. A literature review identified nephrocalcinosis or renal dysfunction in 20 of 107 patients (18.7%)[63,64]. The exact mechanism underlying these renal manifestations remains unclear, but dehydration related to chronic diarrhea may play a role. These findings underscore the importance of ensuring high fluid intake, preventing dehydration, and conducting regular ultrasound examinations for all affected individuals[65].
Diagnosing GGM is challenging owing to its rarity and the need to differentiate it from intestinal disaccharidase and transporter deficiencies. Clinical suspicion of GGM is crucial, particularly in cases of early-onset chronic diarrhea, which should alert pediatricians to the possibility of this condition. Key diagnostic steps include halting feedings and conducting molecular tests. While genetic testing is not strictly necessary for diagnosis, it is highly recommended to confirm the clinical findings[62,66]. The affected SGLT1 Na+-glucose cotransporter is present in both the intestine and the kidney, leading to a mild defect in renal tubular reabsorption of glucose, resulting in decreased FminG in patients with GGM[16,35,53]. In contrast, FRG involves an impaired glucose transporter localized solely to the kidney, meaning that patients with this condition do not exhibit any abnormalities in the intestinal absorption of D-glucose.
The treatment of GGM requires the complete elimination of glucose and galactose from the diet. A fructose-based formula that does not contain these sugars can provide rapid recovery from diarrhea, as fructose is absorbed via a different transporter, GLUT5[65,67].
FBS is an autosomal-recessive disorder caused by mutations in the gene encoding GLUT2 (SLC2A2), which encodes the facilitative glucose transporter located in the basolateral membrane of proximal convoluted tubule cells[68]. First described by Fanconi and Bickel in 1949, the syndrome arises from either compound heterozygous or homozygous mutations in the GLUT2 (SLC2A2) gene[69]. GLUT2 is primarily expressed in hepatocytes, enterocytes, proximal tubular cells in the kidney, pancreatic β-cells, and certain neuronal cells and astrocytes[69,70].
Mutations in the GLUT2 (SLC2A2) gene can lead to variable clinical manifestations and different degrees of proximal tubular dysfunction in patients with FBS[68,71,72]. Patients typically present with hepatorenal glycogen accumulation, impaired utilization of glucose and galactose, and dysglycemia, which may include fasting hypoglycemia, postprandial hyperglycemia, glucose intolerance, and, in rare instances, diabetes mellitus. Additionally, the syndrome is associated with features of Fanconi syndrome, rickets, and growth retardation. The precise mechanisms underlying dysglycemia remain unclear; however, it is believed that intracellular glucose accumulation may cause glycotoxicity, resulting in generalized proximal tubulopathy characteristic of Fanconi syndrome[68,71,72]. Treatment of FBS focuses on symptom management, including the maintenance of glucose homeostasis, treatment of rickets, replenishment of renal solute losses, and promotion of growth.
This study aims to provide a comprehensive review of the causes, pathophysiology, and clinical aspects of RG, a topic that is rarely discussed in the literature. Based on our findings, we believe that the frequency, pathophysiology, and clinical significance of RG, especially in children, are not yet fully understood. The prevalence of mild RG in children remains unclear, as these patients often present with minimal symptoms, and routine urine analysis is not commonly conducted in many healthcare settings. Consequently, there is often a delay in diagnosing patients with mild FRG. Increased screening in schools or hospitals could help identify more children with FRG. Furthermore, conducting detailed clinical and laboratory evaluations of patients with FRG, along with multicenter studies, could yield valuable insights into the complications, significance, and long-term outcomes of SGLT2 inhibitor therapy.
Research in molecular biology and genetics is increasingly focusing on renal tubular diseases. Further studies on inherited tubular transport disorders that lead to glycosuria may illuminate the molecular pathophysiology of these conditions and significantly enhance our understanding of renal glucose processing in both healthy and affected children. Identifying the molecular defects associated with RG could also pave the way for developing targeted therapeutic interventions, thereby improving our management of these complex disorders.
| 1. | Hummel CS, Wright EM. Glucose Reabsorption in The Kidney. Seldin and Giebisch's The Kidney (Fifth Edition). Netherlands: Elsevier, 2013: 2393-2404. [DOI] [Full Text] |
| 2. | Gupta RC, Goyal A, Ghosh R, Punjabi M, Singh PP. Normal range for glucose in urine: age-related changes. Clin Chem. 1982;28:2335. [PubMed] |
| 3. | Sacktor B. Sodium-coupled hexose transport. Kidney Int. 1989;36:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Ghezzi C, Loo DDF, Wright EM. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61:2087-2097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 249] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 5. | van den Heuvel LP, Assink K, Willemsen M, Monnens L. Autosomal recessive renal glucosuria attributable to a mutation in the sodium glucose cotransporter (SGLT2). Hum Genet. 2002;111:544-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Zelikovic I, Servais A. Aminoaciduria and Glycosuria in Children. Pediatric Nephrology. Germany: Springer Nature Link, 2022: 929–956. [DOI] [Full Text] |
| 7. | Wang X, Yu M, Wang T, Zhang H, Ping F, Zhang Q, Xu J, Feng K, Xiao X. Genetic analysis and literature review of Chinese patients with familial renal glucosuria: Identification of a novel SLC5A2 mutation. Clin Chim Acta. 2017;469:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 410] [Article Influence: 41.0] [Reference Citation Analysis (1)] |
| 9. | Turner RJ, Moran A. Stoichiometric studies of the renal outer cortical brush border membrane D-glucose transporter. J Membr Biol. 1982;67:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Turner RJ, Moran A. Further studies of proximal tubular brush border membrane D-glucose transport heterogeneity. J Membr Biol. 1982;70:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Wells RG, Mohandas TK, Hediger MA. Localization of the Na+/glucose cotransporter gene SGLT2 to human chromosome 16 close to the centromere. Genomics. 1993;17:787-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Elsas LJ, Rosenberg LE. Familial renal glycosuria: a genetic reappraisal of hexose transport by kidney and intestine. J Clin Invest. 1969;48:1845-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Brodehl J, Franken A, Gellissen K. Maximal tubular reabsorption of glucose in infants and children. Acta Paediatr Scand. 1972;61:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 14. | Chao EC, Henry RR. SGLT2 inhibition--a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 612] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 15. | Wright EM, Loo DD, Hirayama BA, Turk E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology (Bethesda). 2004;19:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 1065] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 17. | Hediger MA, Budarf ML, Emanuel BS, Mohandas TK, Wright EM. Assignment of the human intestinal Na+/glucose cotransporter gene (SGLT1) to the q11.2----qter region of chromosome 22. Genomics. 1989;4:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994;93:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 493] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 19. | Lee WS, Kanai Y, Wells RG, Hediger MA. The high affinity Na+/glucose cotransporter. Re-evaluation of function and distribution of expression. J Biol Chem. 1994;269:12032-12039. [PubMed] |
| 20. | Swe MT, Thongnak L, Jaikumkao K, Pongchaidecha A, Chatsudthipong V, Lungkaphin A. Dapagliflozin not only improves hepatic injury and pancreatic endoplasmic reticulum stress, but also induces hepatic gluconeogenic enzymes expression in obese rats. Clin Sci (Lond). 2019;133:2415-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol. 2010;5:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 22. | Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 970] [Article Influence: 74.6] [Reference Citation Analysis (1)] |
| 23. | Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141-E145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 702] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 24. | Vallon V. Glucose transporters in the kidney in health and disease. Pflugers Arch. 2020;472:1345-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 25. | Thorens B, Cheng ZQ, Brown D, Lodish HF. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990;259:C279-C285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 192] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Thorens B, Lodish HF, Brown D. Differential localization of two glucose transporter isoforms in rat kidney. Am J Physiol. 1990;259:C286-C294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 27. | Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427-3434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 605] [Article Influence: 28.8] [Reference Citation Analysis (5)] |
| 28. | Chen H, Busse LW. Novel Therapies for Acute Kidney Injury. Kidney Int Rep. 2017;2:785-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Sugawara-Yokoo M, Suzuki T, Matsuzaki T, Naruse T, Takata K. Presence of fructose transporter GLUT5 in the S3 proximal tubules in the rat kidney. Kidney Int. 1999;56:1022-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Linden KC, DeHaan CL, Zhang Y, Glowacka S, Cox AJ, Kelly DJ, Rogers S. Renal expression and localization of the facilitative glucose transporters GLUT1 and GLUT12 in animal models of hypertension and diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F205-F213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Baum M, Anslow M. Postnatal Renal Maturation. Pediatric Nephrology. Germany: Springer Nature Link, 2022. [DOI] [Full Text] |
| 32. | Urakami T, Yoda M, Yoshida K, Mine Y, Aoki M, Suzuki J. Renal glucosuria in schoolchildren: Clinical characteristics. Pediatr Int. 2018;60:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 33. | Crombie DL. Incidence of Glycosuria and Diabetes. Proceedings of the Royal Society of Medicine. 1962;55:205-211. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Li S, Yang Y, Huang L, Kong M, Yang Z. A novel compound heterozygous mutation in SLC5A2 contributes to familial renal glucosuria in a Chinese family, and a review of the relevant literature. Mol Med Rep. 2019;19:4364-4376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Brown GK. Glucose transporters: structure, function and consequences of deficiency. J Inherit Metab Dis. 2000;23:237-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 136] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 36. | Calado J, Sznajer Y, Metzger D, Rita A, Hogan MC, Kattamis A, Scharf M, Tasic V, Greil J, Brinkert F, Kemper MJ, Santer R. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23:3874-3879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Cannizzaro M, Jarošová J, De Paepe B. Relevance of solute carrier family 5 transporter defects to inherited and acquired human disease. J Appl Genet. 2019;60:305-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Xu L, Zhao R, Zhao Y, Tang X, Si N, Guo X, Yue C, Nie M, Chen L. Genetic and clinical characterization of familial renal glucosuria. Clin Kidney J. 2024;17:sfad265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 39. | Sivaji V, Raju P, Marimuthu S, Sundar S. Familial renal glycosuria identified in an Indian family. BMJ Case Rep. 2024;17:e258408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Hatano M, Udagawa T, Kanamori T, Sutani A, Mori T, Sohara E, Uchida S, Morio T, Nishioka M. A novel SLC5A2 heterozygous variant in a family with familial renal glucosuria. Hum Genome Var. 2022;9:42. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 41. | Yu L, Wu M, Hou P, Zhang H. SLC5A2 mutations, including two novel mutations, responsible for renal glucosuria in Chinese families. BMC Nephrol. 2020;21:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Barata R, Fila M, Dalla-Vale F, Bogarin R, Nunes P, Ramalho J, Rueff J, Calado J. Performance of the ACMG-AMP criteria in a large familial renal glucosuria cohort with identified SLC5A2 sequence variants. Clin Genet. 2023;104:582-586. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 43. | Coady MJ, El Tarazi A, Santer R, Bissonnette P, Sasseville LJ, Calado J, Lussier Y, Dumayne C, Bichet DG, Lapointe JY. MAP17 Is a Necessary Activator of Renal Na+/Glucose Cotransporter SGLT2. J Am Soc Nephrol. 2017;28:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 44. | Elsas LJ, Longo N. Glucose transporters. Annu Rev Med. 1992;43:377-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Sada K, Hidaka S, Imaishi N, Shibata K, Katashima R, Noso S, Ikegami H, Kakuma T, Shibata H. Clinical and genetic analysis in a family with familial renal glucosuria: Identification of an N101K mutation in the sodium-glucose cotransporter 2 encoded by a solute carrier family 5 member 2 gene. J Diabetes Investig. 2020;11:573-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Dorum S, Erdoğan H, Köksoy AY, Topak A, Görükmez Ö. Clinical features of pediatric renal glucosuria cases due to SLC5A2 gene variants. Pediatr Int. 2022;64:e14948. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 47. | Wang S, Zhao X, Zhang R, Wang C, Han Y, Shao L. Identification of ten novel SLC5A2 mutations and determination of the renal threshold for glucose excretion in Chinese patients with familial renal glucosuria. Clin Chim Acta. 2019;490:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Fishman B, Shlomai G, Twig G, Derazne E, Tenenbaum A, Fisman EZ, Leiba A, Grossman E. Renal glucosuria is associated with lower body weight and lower rates of elevated systolic blood pressure: results of a nationwide cross-sectional study of 2.5 million adolescents. Cardiovasc Diabetol. 2019;18:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 49. | Calado J, Loeffler J, Sakallioglu O, Gok F, Lhotta K, Barata J, Rueff J. Familial renal glucosuria: SLC5A2 mutation analysis and evidence of salt-wasting. Kidney Int. 2006;69:852-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 50. | Lee H, Han KH, Park HW, Shin JI, Kim CJ, Namgung MK, Kim KH, Koo JW, Chung WY, Lee DY, Kim SY, Cheong HI. Familial renal glucosuria: a clinicogenetic study of 23 additional cases. Pediatr Nephrol. 2012;27:1091-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 51. | Marble A. Renal Glycosuria. Am J Med Sci. 1932;183:811-826. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Lawrence RD. Symptomless glycosurias; differentiation by sugar tolerance tests. Med Clin North Am. 1947;31:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Wright EM, Martin MG, Turk E. Familial glucose-galactose malabsorption and hereditary glycosuria. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001: 4891–908. [DOI] [Full Text] |
| 54. | Magen D, Sprecher E, Zelikovic I, Skorecki K. A novel missense mutation in SLC5A2 encoding SGLT2 underlies autosomal-recessive renal glucosuria and aminoaciduria. Kidney Int. 2005;67:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Pontoglio M, Barra J, Hadchouel M, Doyen A, Kress C, Bach JP, Babinet C, Yaniv M. Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell. 1996;84:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 434] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 56. | Fukui K, Yang Q, Cao Y, Takahashi N, Hatakeyama H, Wang H, Wada J, Zhang Y, Marselli L, Nammo T, Yoneda K, Onishi M, Higashiyama S, Matsuzawa Y, Gonzalez FJ, Weir GC, Kasai H, Shimomura I, Miyagawa J, Wollheim CB, Yamagata K. The HNF-1 target collectrin controls insulin exocytosis by SNARE complex formation. Cell Metab. 2005;2:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Van Lerberghe R, Mahieu E, Vanuytsel J, Vanhaute K, Vanfraechem C, Claeys L. Familial Renal Glucosuria Presenting as Paroxysmal Glucosuria and Hypercalciuria Due to a Novel SLC5A2 Heterozygous Variant. Eur J Case Rep Intern Med. 2023;10:004157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Ren Q, Gong S, Han X, Ji L. Hereditary renal glycosuria, diabetes and responses to SGLT2 inhibitor. J Diabetes. 2022;14:216-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 59. | Turk E, Zabel B, Mundlos S, Dyer J, Wright EM. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature. 1991;350:354-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 273] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Posovszky C. Congenital intestinal diarrhoeal diseases: A diagnostic and therapeutic challenge. Best Pract Res Clin Gastroenterol. 2016;30:187-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Xin B, Wang H. Multiple sequence variations in SLC5A1 gene are associated with glucose-galactose malabsorption in a large cohort of Old Order Amish. Clin Genet. 2011;79:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Hoşnut FÖ, Janecke AR, Şahin G, Vogel GF, Lafcı NG, Bichler P, Müller T, Huber LA, Valovka T, Aksu AÜ. SLC5A1 Variants in Turkish Patients with Congenital Glucose-Galactose Malabsorption. Genes (Basel). 2023;14:1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 63. | Wang W, Wang L, Ma M. Literature review on congenital glucose-galactose malabsorption from 2001 to 2019. J Paediatr Child Health. 2020;56:1779-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Al-Suyufi Y, ALSaleem K, Al-Mehaidib A, Banemai M, Aldekhail WM, Al-Muhandes A, Mohammed M, Allam R, Jambi A, Ramzan K, Imtiaz F. SLC5A1 Mutations in Saudi Arabian Patients With Congenital Glucose-Galactose Malabsorption. J Pediatr Gastroenterol Nutr. 2018;66:250-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Saadah OI, Alghamdi SA, Sindi HH, Alhunaitti H, Bin-Taleb YY, Alhussaini BH. Congenital glucose-galactose malabsorption: a descriptive study of clinical characteristics and outcome from Western Saudi Arabia. Arab J Gastroenterol. 2014;15:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Alamoudi LO, Alfaraidi AT, Althagafi SS, Al-Thaqafy MS, Hasosah M. Congenital Glucose-Galactose Malabsorption: A Case With a Novel SLC5A1 Mutation in a Saudi Infant. Cureus. 2021;13:e18440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 67. | Chan AP, Namjoshi SS, Jardack PM, Maloney L, Ardjmand A, Jackson NN, Martin MG. Long-Term Dietary Changes in Subjects with Glucose Galactose Malabsorption Secondary to Biallelic Mutations of SLC5A1. Dig Dis Sci. 2021;66:4414-4422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Santer R, Steinmann B, Schaub J. Fanconi-Bickel syndrome--a congenital defect of facilitative glucose transport. Curr Mol Med. 2002;2:213-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Sharari S, Abou-Alloul M, Hussain K, Ahmad Khan F. Fanconi-Bickel Syndrome: A Review of the Mechanisms That Lead to Dysglycaemia. Int J Mol Sci. 2020;21:6286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 70. | Freitas HS, Schaan BD, Seraphim PM, Nunes MT, Machado UF. Acute and short-term insulin-induced molecular adaptations of GLUT2 gene expression in the renal cortex of diabetic rats. Mol Cell Endocrinol. 2005;237:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Mannstadt M, Magen D, Segawa H, Stanley T, Sharma A, Sasaki S, Bergwitz C, Mounien L, Boepple P, Thorens B, Zelikovic I, Jüppner H. Fanconi-Bickel syndrome and autosomal recessive proximal tubulopathy with hypercalciuria (ARPTH) are allelic variants caused by GLUT2 mutations. J Clin Endocrinol Metab. 2012;97:E1978-E1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 72. | Enogieru OJ, Ung PMU, Yee SW, Schlessinger A, Giacomini KM. Functional and structural analysis of rare SLC2A2 variants associated with Fanconi-Bickel syndrome and metabolic traits. Hum Mutat. 2019;40:983-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/