Published online Mar 9, 2025. doi: 10.5409/wjcp.v14.i1.100938
Revised: November 14, 2024
Accepted: December 2, 2024
Published online: March 9, 2025
Processing time: 111 Days and 18.4 Hours
Pediatric inflammatory bowel disease (PIBD) is a chronic inflammatory disorder of the gastrointestinal tract, with rising global incidence and prevalence. Over the past two decades, biologics have added to the therapeutic armamentarium and revolutionized the approach to treatment of inflammatory bowel disease. The available biologics include monoclonal antibodies which target inflammatory cytokines (anti-tumor necrosis factor alpha, anti-interleukin 12/23) or recruitment of leucocytes to the gastrointestinal tract (anti-alpha4beta7 integrin) and small molecules (Janus kinase inhibitors, sphingosine 1-phosphate-inhibitors) which modify the proinflammatory signaling. Considering their potential disease-modifying ability, recent pediatric guidelines from the West have advocated upfront use of biologics in appropriate clinical scenarios as a top-down approach rather than the conventional step-up approach. Although real-world studies are available regarding the clinical efficacy of biologics in PIBD, there is paucity of long-term outcome and safety data in children. Also, little information is available about the best approach in the newly industrialized - developing countries where PIBD is rising but at the same time, infections are prevalent and resources are limited. In this review, we summarize the efficacy and safety profile of biologics and small molecule drugs and discuss the challenges in the management of PIBD, especially in the developing world, and future directions.
Core Tip: Biologics have revolutionized the treatment of pediatric inflammatory bowel disease. The increasing number of biologics (anti-tumor necrosis factor alpha, anti-interleukin 12/23 agents, anti-alpha4beta7 integrin agents) and the arrival of small molecules (Janus kinase inhibitors, sphingosine 1-phosphate-inhibitors), have added to our therapeutic armamentarium. The ultimate objective of therapy is to achieve clinical remission and mucosal healing by providing “personalized therapy” keeping in mind the disease particulars, and selecting the therapy based on the efficacy, cost and safety of the biologics. In this review, we have summarized the recent available literature for guiding the pediatric gastroenterologist about the practical use of biologics and small molecules for children with inflammatory bowel disease.
- Citation: Samanta A, Srivastava A. Biologics in the management of pediatric inflammatory bowel disease: When and what to choose. World J Clin Pediatr 2025; 14(1): 100938
- URL: https://www.wjgnet.com/2219-2808/full/v14/i1/100938.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i1.100938
Inflammatory bowel disease (IBD) is phenotypically classified as Crohn’s disease (CD), ulcerative colitis (UC) and IBD unclassified. Pediatric IBD (PIBD; age ≤ 17 years) constitutes up to 10%-25% of all IBD patients. Recent epidemiological studies suggest that PIBD is rising across the globe including the developing countries[1-6]. The complex patho
Biologics are traditionally defined as pharmacological agents synthesized from living organisms or their products that are tailored to specifically target an immune or genetic mediator of disease. The term “small molecules/small molecular drugs” describes a heterogenous group of drugs that have common features like oral bioavailability, ability to cross biological barriers, and modulation of different biological targets[7]. Anti-tumor necrosis factor alpha (TNFα) agents [infliximab (IFX) and adalimumab (ADA)] were the first biologics to be used in IBD. With better understanding of the underlying pathophysiology, newer classes of biologicals have been added. Currently available biologics and small molecules include the following: (1) Anti-TNFα agents (IFX, ADA, golimumab, and certolizumab); (2) Anti-alpha4beta7 (anti-α4β7) integrin antagonist [vedolizumab (VDZ)]; (3) Anti-interleukin (IL) 12/23 antagonist [Ustekinumab (UST)], anti-IL-23 (e.g., Risankizumab and mirikizumab); and (4) Oral Janus kinase (JAK) inhibitors: Pan JAK 1,2,3 (e.g., tofacitinib), selective JAK inhibitor (e.g., filgotinib and upadacitinib) as well as sphingosine1-phosphate-inhibitors (e.g., ozanimod).
PIBD is usually more severe than adult IBD, and associated with unique complications like growth failure[8,9]. The conventional treatment strategy for PIBD is a “step-up” approach with biologics reserved for those not responding to therapy with steroids, 5-ASA, IM (AZA, MTX) and exclusive enteral nutrition[10]. However, emerging evidence suggests that early biologic use might enhance the control of the inflammatory gut milieu and thereby alter the disease course and its complications[11]. In an elegantly done prospective study in 191 propensity-matched pairs of newly diagnosed pediatric CD patients, Kugathasan et al[11] showed that children receiving early (within 12 weeks of diagnosis) anti-TNFα therapy were less likely to have penetrating complications [hazard ratio = 0.30, 95% confidence interval (CI): 0.10-0.89; P = 0.029] but not stricturing complications (1.13, 95%CI: 0.51-2.51; P = 0.76) as compared to those who did not receive early anti-TNFα therapy. Similarly, a meta-analysis of 13 studies [2 randomized controlled trials (RCT) and 11 cohort studies), involving 861 children and young adults, showed that early (within 12 weeks of diagnosis) biologic use was associated with a significantly higher rate of clinical remission [risk ratio (RR) = 1.30, 95%CI: 1.10-1.54], lower relapse rates (RR = 0.33, 95%CI: 0.21-0.53), and improved mucosal healing (RR = 1.47, 95%CI: 1.10-1.97) compared to late biologics/conventional therapy[12]. It was also associated with better normalization of linear growth.
Observing this potential benefit of early biologic therapy, the European Crohn’s and Colitis Organization (ECCO)-European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) guidelines of 2020[13] advocated upfront biologic therapy at diagnosis in children with CD and extensive disease, deep colonic ulcers, perianal disease, stricturing/penetrating phenotype or growth failure (Table 1). In children with UC, the indications for biologics include chronic active UC, not responding to conventional therapy, steroid-dependent UC and patients with acute severe UC (ASUC), not responding to intravenous corticosteroids[14,15]. Table 1 summarizes the recommendations of guidelines for use of biologics in PIBD[6,13-16].
| Characteristics | ECCO-ESPGHAN guidelines CD, 2020[13], UC, 2018[14,15] | Asian Pan-Pacific Society for Pediatric Gastroenterology, Hepatology, and Nutrition PIBD Working Group, 2022[6] | Canadian association of Gastroenterology Guidelines, 2019[16] |
| Indications CD | Upfront biologics for the following: (1) Extensive disease and deep colonic ulcers; (2) Perianal disease; (3) Stricturing or penetrating disease; and (4) Growth failure | Upfront biologics if severe luminal or perianal disease | Upfront biologics if severe luminal or perianal disease |
| As step-up therapy: Moderate to severe active CD not responding to conventional therapy | Step-up approach: Moderate to severe active CD not responding to conventional therapy | Step-up approach: Moderate to severe active CD not responding to conventional therapy | |
| 1st line: Infliximab, adalimumab; 2nd line: Ustekinumab | 1st line: Infliximab, adalimumab, ustekinumab, and vedolizumab - no consensus | 1st line: Iinfliximab, adalimumab; 2nd line: Ustekinumab; no consensus on vedolizumab | |
| Indications UC | Chronically active moderate to severe UC, not responding to conventional therapy; tofacitinib - no consensus | Chronically active moderate to severe UC, not responding to conventional therapy | NA |
| ASUC if not responding to 5 days of intravenous steroid | ASUC if not responding to 5 days of intravenous steroid | ||
| 1st line: Infliximab; 2nd line: Vedolizumab | 1st line: Infliximab; vedolizumab, tofacitinib - no consensus | ||
| Co-therapy with immune-modulator | Infliximab - consider combination therapy Adalimumab - no | Infliximab and adalimumab - consider combination therapy | Infliximab - NR (male), no recommendation for or against (female) |
| Adalimumab - NR (male), no recommendation for or against (female) | |||
| If considered, methotrexate for boys and thiopurine for girls | |||
| Screening before biologic | HBV/HCV/HIV | HBV/HCV/HIV | HBV/HCV/HIV |
| TB: Mantoux or IGRA (BCG-vaccinated patients), chest X-ray, sputum | TB: Mantoux and IGRA, chest X-ray, sputum | TB: Mantoux or IGRA, chest X-ray, sputum | |
| TDM | Yes, proactive preferred | Yes, reactive | Yes, reactive |
| Target trough level | Infliximab: Induction (week 6) ≥ 15 μg/mL; maintenance ≥ 5 μg/mL | Infliximab: Induction > 10-15 μg/mL; maintenance ≥ 3-7 μg/mL | NA |
| Adalimumab: Induction (week 4) and maintenance ≥ 7.5 μg/mL | Adalimumab: Maintenance ≥ 5-8 μg/mL |
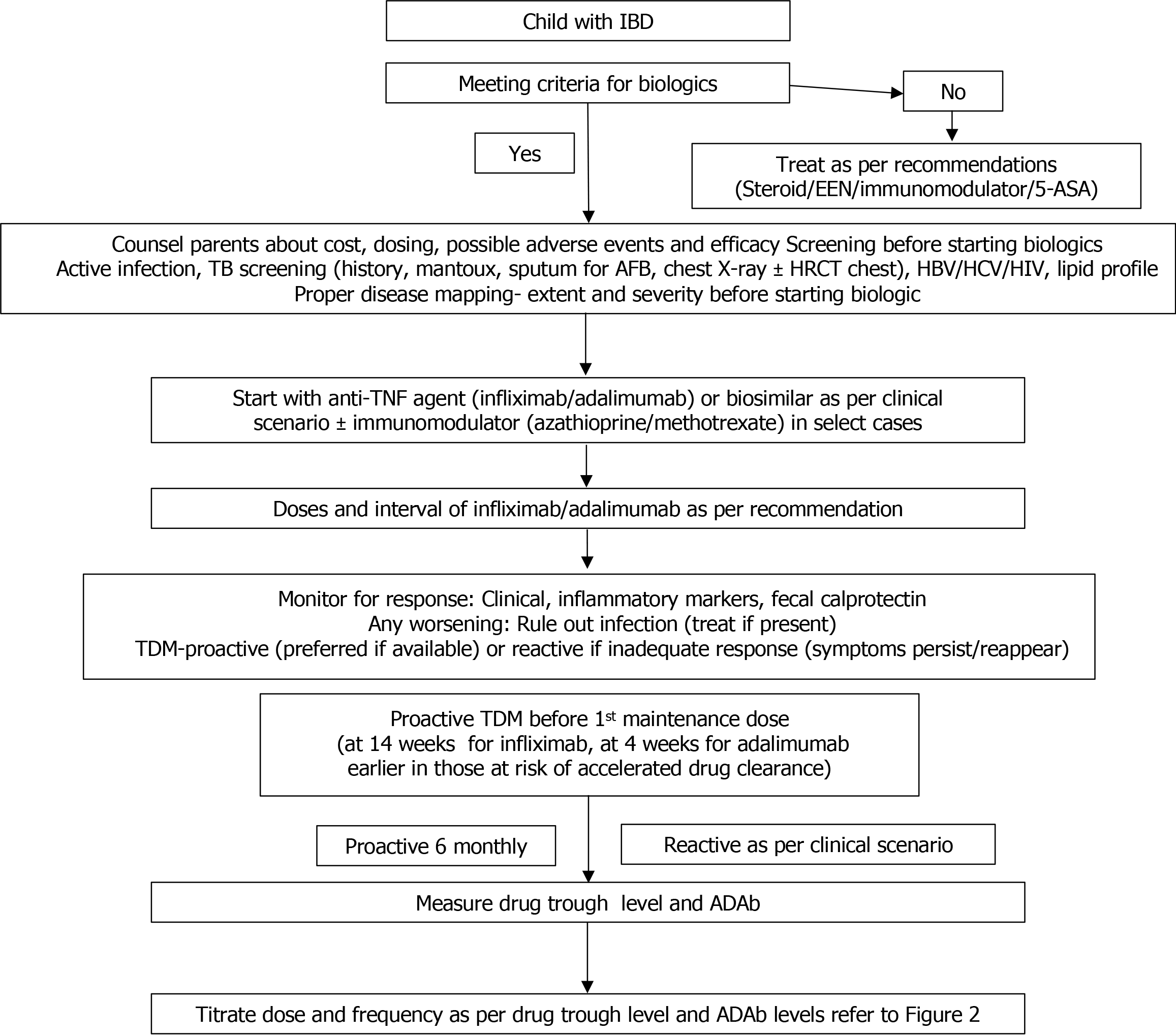
Figure 1 shows the stepwise evaluation of a patient planned for biologics and it includes the following: (1) Confirmation of diagnosis of IBD, its subtype, disease extent and indication of biologic. Previous treatment history and response to the medications should be noted; (2) A detailed discussion and proper counseling of parents regarding the medications, treatment goals, duration and cost of therapy and likely complications; (3) Detailed history of current or recent infection and any associated predisposing conditions like immunodeficiencies or comorbidities; (4) Complete clinical examination including anthropometry for growth failure and appropriate investigations to identify any focus of infection especially abscesses in patients with perianal/fistulizing CD; (5) Screening for hepatitis B, C and human immunodeficiency virus infection[17]; (6) Screening for latent tuberculosis (TB) infection (LTBI)- by a detailed history, physical examination, Mantoux test, chest X-ray and sputum examination. QuantiFERON Gold assay is a useful screening test for LTBI, especially in bacille Calmette-Guerin-vaccinated patients[18]. Evidence suggests that there is a significant risk of re-activation of TB, especially with anti-TNFa agents. A detailed discussion of LTBI and its treatment is available in excellent reviews[19]; and (7) Fasting lipid profile should be done in patients planned for tofacitinib.
Once a decision to initiate biologics is taken, the second step is to decide the choice of therapy i.e. which biologic, what dose and whether to combine with IM or not. Table 2 summarizes the biologics and small molecule drugs (SMD) used in children, their mechanism of action, route of administration, doses and adverse effects. Only IFX and ADA are Food and Drug Administration approved for children till date. It is important to understand the different grades of response from symptom relief to mucosal healing to appropriately assess the response to therapy. Although there is heterogeneity in the description of response across studies, the response in IBD is generally described as follows.
| Characteristics | Infliximab | Adalimumab | Ustekinumab | Vedolizumab | Tofacitinib |
| Mechanism of action | Anti-TNFα antibody (chimeric) | Anti-TNFα antibody (humanized) | Anti-IL-12/23 antibody (humanized) | Anti-α4β7 integrin antibody (humanized) | JAK inhibitor |
| Route | IV; SCb | SC | IV (1st dose) followed by SC | IV | Oral |
| Dosea | |||||
| Induction | 5mg/kg | 2.4 mg/kg, f/b 12 mg/kg | 6 mg/kg if < 40 kg BW, 130 mg if > 40 kg BW | 6 mg/kg < 40 kg BW, 300 mg > 40 kg BW | 10 mg twice daily |
| Maintenance | 5mg/kg | 0.6 mg/kg | 2 mg/kg if < 40 kg BW, 90 mg if > 40 kg BW | Same as induction dose | 5 mg twice daily |
| Schedulea | |||||
| Induction | 0 week, 2 week, and 6 weeks | 0 week, and 2 weeks | 0 week | 0 week, 2 weeks, and 6 weeks | Daily |
| Maintenance | 8 weekly | 2 weekly | 8 weekly | 8 weekly | Daily |
| Target drug level | Induction (week 6) ≥ 10-15 μg/mL; maintenance ≥ 5 μg/mL | Post-induction before 3rd dose ≥ 7.5 μg/mL | Target level not well defined | Target level not well defined | NR |
| In patients at risk for accelerated drug clearance, target concentration ≥ 25 μg/mL at 2nd infusion and ≥ 15 μg/mL at 3rd infusion[13] | Maintenance ≥ 7.5 μg/mL[13] | ||||
| Adverse events | Infection, reactivation of TB/ HBV; skin reaction (psoriasis) | Infection; headache, arthralgia, skin reaction | Infection; (GI most common), myalgia | Infection; (respiratory, GI); headache, myalgia | Varicella zoster infection, thrombosis, dyslipidemia, LFT, derangement |
| Use of concomitant immunomodulator (azathioprine/methotrexate) | Yes (initial 6-12 months) | If biological naive: No need | No | No | No |
| Biological exposed: Can add | |||||
Clinical Response: (1) CD: Decrease in pediatric CD activity index (PCDAI) score of ≥ 12.5 points; and (2) UC: Decrease of pediatric UC activity index (PUCAI) of ≥ 20 points.
Clinical Remission: (1) CD - decrease of PCDAI to < 10.0, UC - decrease of PUCAI to < 10.0; and (2) Biochemical remission (BR): Normalization of inflammatory parameters (C-reactive protein/erythrocyte sedimentation rate) and fecal calprotectin.
Mucosal healing: (1) CD: Absence of macroscopic inflammation or simple endoscopic score for CD of < 3; and (2) UC: Disease in remission, Mayo endoscopic scope = 0. The target of therapy as per stride II recommendation includes relief in symptoms with normalization of serum and fecal markers in the short-term and clinical remission (CR) with endoscopic healing, normal growth and quality of life, and no disability in the long-term[20].
IFX and ADA have been used by most pediatric gastroenterologists for a long time. Table 3 summarizes the important pediatric studies on the efficacy and safety of IFX and ADA in children.
| Ref. | CD | UC | ||||||
| Number of cases | Details of cases | Efficacy | Adverse event | Number of cases | Details of cases | Efficacy | Adverse event | |
| Age (years), mean ± SD/median age (years); IQR | Age (years), mean ± SD/ IQR | |||||||
| Infliximab | ||||||||
| Hyams et al[21], REACH trial | 112 | Moderate to severe | CRes and CR 89% and 59% at week 10; 63% and 56% at week 54th | 94.6% (serious 19.6%) | - | - | - | - |
| 13.3 ± 2.5 | ||||||||
| Crandall et al[23] | 22 | Fistulizing perianal disease, all biologic naive | Response in 41% (9/22, partial response-4, complete response-5) at week 2nd; 73% (16/22, partial-1, complete-15) at week 54th | 95.7% (serious 4.5%) | - | - | - | - |
| 13.3 ± 2.5 | ||||||||
| Hyams et al[26] | - | - | - | - | 52 | Moderate to severe, all biologic naive | CRes 27%: 6 months; CRes 38%: 12 months; CRes 21%: 24 months | None |
| 13.3 ± 2.6 | ||||||||
| deBruyn et al[31] | 147 | Moderate to severe; all biologic naive | SFCR 59% at 1 year, 59.5% at 2 years | 5.4% (serious 4.7%) | - | - | - | - |
| 14.3; 12.1-15.1 | ||||||||
| Adalimumab | ||||||||
| Hyams et al[30], IMAgINE trial | 188 | Moderate to severe, 44% infliximab exposed | CR 33.5% at week 26th | 89% drug related 41% (serious 22.3%) | - | - | - | - |
| 13.6 ± 2.5 | ||||||||
| Croft et al[34], ENVISION I trial, high dose vs standard dose vs placebo | - | - | - | - | 93 | Moderate to severe, all biologic-naive | Endoscopic remission at week 8th: High dose vs placebo-60% vs 20%, P = 0.0001; standard dose vs placebo-43% vs 20%, P = 0.38 | 78% (serious 23%) |
| 4-17 | At week 52th: High dose vs placebo: 45% vs 18%, P = 0.0001; standard dose vs placebo-29% vs 18%, P = 0.38) | |||||||
| deBruyn et al[31] | 147 | Moderate to severe; all biologic naive | SFCR: 63% at 1 year; 59% at 2 years | 1.3% (serious-none) | - | |||
| 13.4;11.6-14.9 | ||||||||
| Rinawi et al[114] | 65 | Moderate to severe, all biologic-naive | CR 60% at week 24th | NA | - | - | - | - |
| 12.1; 10.5-13.8 | ||||||||
The REACH trial, a multicentre, randomized, open-label trial evaluating the efficacy of IFX in 112 children with moderate-severe CD reported a clinical response (Cres) of 88% and CR of 59% at week 10, with 55.8% cases maintaining CR at week 54[21]. In another study, Nobile et. al. showed that 44.4% of CD patients showed endoscopic response and 22.2% achieved mucosal healing with IFX without significant adverse effects at week 52[22]. In the post-hoc analysis on the efficacy of IFX in perianal CD, in a subpopulation of 22 children from the REACH trial, Crandall et al[23] showed that 41% (9/22, partial response-4, complete response-5) cases showed response by week 2 and 73% (16/22, partial-1, complete-15) by week 54. In a multicentre cohort study of 85 children with fistulizing perianal CD, El-Matary et al[24] reported that 52% cases had healing of fistula at week 24. Regarding IFX use in children with UC, many clinical studies have shown promising results, with lower relapse rates and a reduction in the need of colectomy with increasing IFX use[25-29]. In a study of 52 children with steroid-refractory active UC treated with IFX, 38% and 21% respectively had CS-free inactive disease at 1 and 2 years with 61% cases remaining free of colectomy at 2 years[26].
It was approved in the Unites States and Europe for pediatric CD in 2012, based on the encouraging results of the IMAgINE 1 trial[30]. In the only propensity-matched comparison study between IFX and ADA in pediatric CD, no significant difference in Cres or safety profile was reported[31]. In 36 children with perianal fistulizing CD from the IMAgINE 1 study, fistula improvement and closure were observed in 52.8% and 44.4% respectively at week 12 and this improvement and closure was maintained throughout the 292 weeks of follow-up[32]. Although ADA has been used in adults with UC with good efficacy and a favorable safety profile, pediatric studies are limited[33,34]. ADA has not yet been Food and Drug Administration approved for treatment of UC in children. Croft et al[34] in a RCT of 93 children with moderate to severe UC (all biologic naive), compared long-term efficacy of high-dose induction ADA (2.4 mg/kg at weeks 0 and 1) or standard-dose induction (2.4 mg/kg at week 0 and placebo at week 1); thereafter both groups received 1.2 mg/kg at week 2 and 0.6 mg/kg at weeks 4 and 6. Thereafter, they got either high-dose maintenance (0.6 mg/kg weekly), standard-dose maintenance (0.6 mg/kg every other week) ADA, or placebo up to week 52. They showed a significantly higher rate of endoscopic remission (improvement in Mayo score ≥ 2 points) with high-dose ADA vs placebo (60% vs 19.8% at week 8, P = 0.0001; 45% vs 18.4% at week 52, P = 0.0001). Standard-dose ADA had a higher response than placebo but it was not significant (43% vs 19.8% at week 8, P = 0.38; 29% vs 18.4% at week 52, P = 0.38). Remission rates in the pooled ADA groups were significantly better than placebo[34].
There is an ongoing debate about the efficacy of ADA in patients who failed IFX therapy. In a large study of 1122 adults with IBD, who got second anti-TNF after failure of first anti TNF, 55% achieved CR but there was a high loss of response (LOR) of approximately 20%/year[35]. High-quality pediatric data on this issue is lacking. In a study of 53 children with IFX refractory CD, remission was obtained with ADA in 64% patients and it was sustained at 2 years in half of them. More IFX non-responders failed ADA than patients who lost response to IFX[36] Aloi et al[33] in a retrospective study of 32 children with UC on ADA (43% IFX-intolerant, 50% IFX non-responder), reported a clinical and endoscopic remission rate of 41% and 28% respectively at 52-week with no significant difference in efficacy between IFX intolerant or non-responders (P = 1.0). Recent ECCO-ESPGHAN guidelines recommend using ADA in children with intolerance or LOR to IFX with therapeutic drug monitoring (TDM) but not in those with primary non-response[13].
Despite the efficacy of IFX and ADA, their use is limited because of high cost, especially in the developing world. However, availability of low-cost biosimilars can resolve this issue. After expiry of the patent on IFX in 2013, many companies launched their biosimilars. CT-P13 was the first to be approved. Many commercial brands are available for these biosimilars viz. Remsima© (Celltrion) and Inflectra© (Hospira) for CT-P13, or Flixabi© (Biogen) and Renflexis® (Merck) for SB2[37]. ABP501 (Amgevita©, Amgen) was the first approved biosimilar of ADA[37]. Data on the efficacy and safety of biosimilars is steadily increasing[37-43] (Table 4). More recently, the first subcutaneous (SC) formulation of IFX, CT-P13 SC, received approval for adults in Europe[44]. It had good patient acceptance and satisfaction with ability to maintain remission following the switch from intravenous IFX[44]. In the only real-life experience of 7 children (CD-5, UC-2) receiving SC IFX (120 mg, 2 weekly), Gianolio et al[45] showed that all patients remained in CR with no significant change in median IFX trough levels (12.3 μg/mL at baseline; 13.9 μg/mL and 14.0 μg/Ml at 6 weeks and 40 weeks respectively) and no adverse events[45]. This study highlights the feasibility of an elective switch to SC-IFX in CD during maintenance, with advantages of reduced cost, no hospitalization and increased patient satisfaction[45].
| Ref. | Biosimilar | Patient population | Control group | Main outcomes |
| Age (years), mean ± SD/median age (years); IQR | Age (years), mean ± SD/median age (years); IQR | |||
| Infliximab biosimilar | ||||
| Sieczkowska-Golub et al[38], prospective | CT-P13 | 36 CD; 27 anti-TNF naive | No | 86% CRes; 67% clinical remission at week 14 |
| 11.8 ± 4 | ||||
| Chanchlani et al[39], prospective | Inflectra and remsima | 82 (63 CD, 14 UC, 5 IBD-U) | 175 (148 CD, 33 UC, 15 IBD-U) children on originator infliximab | Clinical remission 79% for biosimilar and 68% for the originator at week 12 |
| 12; 10-14 | 12; range 10-14 | |||
| Nikkonen et al[40], retrospective | CT-P13 | 28 (16 CD, 3 UC, 9 IBD-U) | 23 (17 CD, 2 UC, 4 IBD-U) on originator infliximab | 90% CRes; no difference between the two groups at week 12 |
| 12; 4-16 | 12.0; 5.4-16 | 65% vs 61% patients on maintenance treatment at 1 year | ||
| Sieczkowska et al[41], prospective | CT-P13 | 39 (32 CD, 7 UC) elected to switch | No | 80% and 100% clinical remission at the last follow-up assessment (median 8 months) for CD and UC patients, respectively |
| CD: 11.1 ± 3.3; UC: 12.3 ± 2.3 | ||||
| Kang et al[42], prospective | CT-P13 | 38 (32 CD, 6 UC) elected to switch | 36 (28 CD, 8 UC) on originator infliximab | Clinical remission rate 77.8% and 78.9% for biosimilar and originator IFX at 1 year |
| 17.5 ± 4.0 | 17.3 ± 3.4 | |||
| Gianolio et al[45], retrospective | IFX-SC | 7 CD | No | All (100%) remained in clinical remission at week 40 |
| 16.5 ± 0.8 | ||||
| Adalimumab biosimilar | ||||
| Dipasquale et al[43], retrospective | ABP501 | 41 (39 CD, 2 UC) | No | Clinical remission rate 70.7% at week 14 and 72% at week 52 |
| 13.6; 11.3-15.8 | ||||
Evidence regarding efficacy and safety of other biologics (VDZ, UST) and small molecules such as tofacitinib in PIBD is emerging and summarized in Table 5.
| Ref. | CD | UC | ||||||
| Number of cases | Details of cases | Efficacy | Adverse event | Number of cases | Details of cases | Efficacy | Adverse event | |
| Age (years), mean ± SD/median age (years); IQR | Age, (years) mean ± SD/median age (years); IQR | |||||||
| Vedolizumab | ||||||||
| Singh et al[47], retrospective | 30 | 87% anti TNFα agent exposed | Clinical remission 42% at week 14 | 14.3% | 22 | 95% anti TNFα agent exposed | Clinical remission 76% at week 14 | Nil |
| 15.2; 7-17 | 14.3; 7-17 | |||||||
| Hyams et al[49], HUBBLE trail | 45 | Refractory to steroid, immunomodulator and/or anti-TNFα agents; biologic exposed 64% | CRes 33%-63%; remission 16%-54% at week 14 | 90% (serious 30%) | 44 | Refractory to steroid, immunomodulator and/or anti-TNFα agents; biologic exposed 34% | CRes 40%-69%; remission 30%-60% at week 14 | 82% (serious 22%) |
| 2-17 | 2-17 | |||||||
| Atia et al[50], VEDOKIDS | 65 | Refractory to steroid, immunomodulator and/or anti-TNFα agents | Clinical remission 32% at week 14 | 28% (none serious) | 77 | Refractory to steroid, immunomodulator and/or anti-TNFα agents | Clinical remission 49% at week 14 | 18% (none serious) |
| 15 ± 2.3 | 14.2 ± 2.9 | |||||||
| Fang et al[51], systematic review | 216 | Refractory to steroid, immunomodulator and/or anti-TNFα agents | Clinical remission 18%-37% at week 14; 36%-57% at 1 year | serious 6%a | 239 | Refractory to steroid, immunomodulator and/or anti-TNFα agents | Clinical remission 31%-65% at week 14; 35%-54% at 1 year | Serious; 6%a |
| < 21 | < 21 | |||||||
| Ustekinumab | ||||||||
| Turner et al[53], UniStar study, Prospective | 34 | Moderate to severe CD, 94% anti-TNFα exposed | Clinical remission 41% at week 48 | 88% (serious 14.7%) | - | - | - | - |
| 13; 12-16 | ||||||||
| Yerushalmy-Feler et al[54], ESPGHAN Porto group, retrospective | 69 | 98.6% anti-TNFα exposed | CRes 67% at week 12; remission 42% at week 12 | 8.7% (none serious) | - | - | - | - |
| 15.8; 13.8-16.9 | ||||||||
| Koudsi et al[55], GETAID, retrospective | 48 | All anti-TNFα exposed | PCDAI reduced from 28.7 to 18.7 at week 12 | 17%a | 5 | Refractory, all anti-TNFα exposed | PUCAI 47 to 25 at week 12 | 17%a |
| 15.0; 8.7-18 | 15.0; 8.7-18 | |||||||
| Fang et al[56], systematic review | 204 | Refractory CD, 90% anti-TNFα exposed | Clinical remission 34% at week 8-16, 46% at 1 year | 3.5%a | 166 | Refractory UC, 86% anti TNFα exposed | Clinical remission 24% at week 8-16, 46% by 1 year | 3.5%a |
| Tofacitinib | ||||||||
| Moore et al[57], retrospective | - | - | - | - | 21 | 20 exposed to infliximab, 9 to adalimumab, 2 to ustekinumab, 13 to vedolizumab | CRes 43% at week 12; clinical remission 33% at week 12, 41% at week 52 | 71% (serious 52%, possibly non-drug related) |
| 18.4; 15-19.9 | ||||||||
| Ledder et al[58], retrospective | - | - | - | - | 101 | All at least 1 biologic exposed; 36% exposed to 3 biologics | CRes 46% at week 8; clinical remission 16% at week 8, 23% at week 24 | 3% (herpes zoster, dyslipidemia) |
| 12.8 ± 2.8 | ||||||||
VDZ is a gut-selective anti-α4β7 integrin monoclonal antibody that inhibits migration of T lymphocytes into the inflamed intestinal tissue. It has emerged as a promising biologic for subjects with corticosteroid, immunomodulator, or anti-TNFα refractory disease[46,47]. VDZ is effective in both CD and UC but it is more effective in UC[47]. The efficacy of VDZ is lower in the anti-TNFα exposed patients which may be due to the refractory disease phenotype or secondary to the downregulation of mucosal addressin cell adhesion molecule 1 expression by the anti-TNFα agents. VDZ is a slowly-acting medication and takes approximately 4-8 weeks for response and hence some centres prescribe oral corticosteroids as “bridging therapy” while awaiting the effects of VDZ. Higher rates of CRes are observed when VDZ is given as a first-line biologic[47-48]. Real-world clinical studies on safety and effectiveness of VDZ in children are limited[47-51]. In a systematic review by Fang et. al. involving 10 studies with 455 children with CD and UC, the CR was 48% at week 14th and 45% at one year for UC while it was 28% at week 14th and 46% at one year for CD. At one-year follow-up, mucosal healing rate was 15%-34% for UC and 17%-39% for CD. Only 6% cases had serious adverse events[51]. Data regarding mucosal and histological remission is limited and heterogeneous. More long-term efficacy and safety data are required.
It is a fully-humanized monoclonal antibody against IL-12/23 that acts via binding to their shared p40 subunit and inhibiting downstream T helper 1 (Th1) and Th17 pathways. It acts faster, with response seen within 3 weeks, as compared to VDZ. Most of the evidence of UST is in CD, with variable CR rate of 24%-46%[52-54]. In a phase 1, multicentric, 16-week, induction-dose finding, double-blind study (UniSTar) in 44 children with moderate to severe CD, 22%/29% (lower/higher dose) cases had CR with UST at week 16 with a favorable safety profile and no antigenicity[52]. In the long-term extension of UniStar trial, 34/44 patients entered the extension and 41% (15/34) of them were in CR at week 48th[53]. In a retrospective, multicentric study from the pediatric IBD Porto group of 69 biologic-exposed children with CD, 67% and 42% respectively showed CRes and CR at 3 months of UST use. The concomitant endoscopic and transmural healing rates were 16% and 13% respectively[54]. In another retrospective, French cohort study of biologic refractory CD (n = 39) and UC (n = 5) patients, UST was able to significantly lower the PCDAI and PUCAI score at 3 months[55]. In a systematic review of 11 studies involving 370 children, the pooled cortico-steroid free CR was 23% at 8-16 weeks and 45% at 1 year for CD while the corresponding figures were 24% and 46% for UC[56]. The endoscopic remission rate was 0%-37.5% in CD and 63.6% in UC, with only 3.5% patients reporting serious adverse events[56]. UST has higher efficacy in biologic naive than exposed patients. According to the ECCO-ESPGHAN guidelines, UST can be considered in children with CD who fail anti-TNFα agents (IFX or ADA)[14]. Risankizumab and mirikizumab selectively target the IL-23, which is critical for the functioning of Th17 cells and production of proinflammatory cytokines. The pediatric data on these medications is limited.
Tofacitinib is an oral pan JAK inhibitor, which has recently been approved for the treatment of moderate-to-severe UC in adults, but data in children is limited[57,58]. Oral administration, rapid onset of action, a shorter half-life making immunosuppression reversible after drug stoppage, lack of immunogenicity and relatively lower cost makes it an attractive therapeutic option. Table 5 shows the studies with tofacitinib in children. In a multicentric cohort of 101 children with refractory UC (all had failed biologics), 23% achieved corticosteroid-free CR at week 24 without serious drug-related adverse events[58]. More long-term prospective studies are required to establish efficacy and safety in PIBD.
ASUC is a “medical emergency” which needs to be treated aggressively. Supportive care, treatment of infections (if present), and intravenous corticosteroids are the first-line of therapy[15]. Patients not responding to steroids by day 3, are counselled about the second-line therapy [medical (cyclosporine or IFX) vs surgical colectomy][15]. IFX has emerged as a useful rescue therapy for subjects with ASUC and corticosteroid non-response[15]. In a prospective study, 33/37 children with steroid failure got IFX and 76% (25/33) responded. Only 28% (7/25) of the responders required colectomy in follow-up[59].
ASUC is characterized by high circulating levels of TNFα due to intense inflammation, lower serum albumin and a higher fecal clearance of the anti-TNFα agent[60]. Further, younger children more often have lower trough levels of IFX with conventional doses, owing to higher volume of distribution. These patients often need higher doses or more frequent administration of IFX to achieve response[61]. In a recent study of 105 children with ASUC, 54 (51%) steroid refractory patients received IFX with 87% getting the intensified regimen (induction: mean dose ≥ 7 mg/kg or interval ≤ 5 weeks between doses 1 and 3, maintenance 4 weekly doses). At 1 year follow-up, 63% of them had corticosteroid-free CR. Overall 74% of ASUC cases were on IFX by 1 year. The predictors of IFX use being higher PUCAI, hypoalbuminemia, older age and male gender, suggesting a role of these factors in choosing the optimal maintenance therapy in children with ASUC[62]. In a multicentric, prospective cohort of 38 hospitalized children receiving a higher dose of IFX (median 9.9 mg/kg) for ASUC, day-7 CRes, week-8 CR, and week-26 steroid-free CR was 71%, 55%, and 43% respectively with a colectomy rate of only 2.7% at 26-week and 10.8% at 2 years[63]. The authors concluded that an accelerated regimen of IFX dosing may optimize early clinical outcome in ASUC. However, more studies are required to determine if sustained intensification can overcome rapid clearance and improve late outcome also[63]. In contrast, a systematic review of outcome of accelerated regimen in 705 adults and pediatric patients (308 received intensified IFX therapy) showed no difference in the short or long-term colectomy rates in hospitalized ASUC patients[64]. The authors hypothesized that despite pharmacokinetic and pharmacodynamic data suggesting the potential need for accelerated induction to achieve better remission rates in ASUC, certain factors might have led to its apparent failure like difference in severity of disease, timing of accelerated therapy, variation of IFX dosing and possible provider bias in these retrospective studies[64]. Further pharmacokinetic studies are needed to determine the role of high-dose IFX regimens in improving the long-term outcomes in children with ASUC. Pending that, it might be prudent to try the accelerated IFX regimen with TDM before giving up on this important therapy.
The most common adverse event following anti-TNFα agents is infections, followed by infusion reaction, joint pain, muscle pain and dermatological conditions (notably psoriasis) (Tables 2 and 3)[65]. TNFα plays a key role in macrophage activation, and neutrophil recruitment and the use of anti-TNFα agents has been linked to an increased risk of infection, including TB[66]. Various pediatric studies have reported adverse events in 3%-95% of cases, most being mild with serious adverse events in a minority (0%-23%)[21,23,26,30,31,34]. A systematic review of 65 pediatric studies [9516 patient-years (PYs) of follow-up (PYF)] reported that the rate of serious infections in children with IBD exposed to anti-TNFα agents (352 per 10000 PYs) was similar to that of children receiving immunomodulator monotherapy (333 per 10000 PYs) but significantly lower than those receiving steroids (730 per 10000 PYs)[67]. Another systematic review of 14 studies involving 664 children showed that although half of the patients had adverse events; only 11.5% of them were serious[68]. Infections, abscess, allergic, hepatic and hematologic effects being the common adverse events[68]. Skin lesions especially like psoriasis can be seen in 2%-8% patients with anti-TNFα use. These occur independent of the type of anti-TNFα and regress on stopping the drug[69]. Studies have raised concerns for an increased risk of lymphoproliferative disorders [e.g. Epstein-Barr virus (EBV)-associated lymphomas, hemophagocytic lymphohistiocytosis or hepatosplenic T-cell lymphomas], especially in patients receiving combination therapy with anti-TNFα and thiopurines. However, the systematic review by Dulai et al[67] did not support this fact and showed that the incidence of lymphoma [2.1/10000 PYF] was similar to that seen in the general pediatric population (0.58/10000 PYF; standardized incidence ratio (SIR) = 3.5; 95%CI: 0.35-19.6), and lower than children receiving thiopurine monotherapy (4.5/10000 PYF; SIR = 0.47; 95%CI: 0.03-6.44). The systematic review by Hyams et al[70] also did not show any increased risk of malignancy among patients receiving IFX (SIR = 1.69; 95%CI: 0.46-4.32) as compared to those not receiving biologics (SIR = 2.17; 95%CI: 0.59-5.56), even when patients were stratified by thiopurine exposure. IFX use was not associated with an increased risk of lymphohistiocytosis[70].
Given its gut-specific action, VDZ has a relatively favorable safety profile compared to anti-TNFα agents. Pediatric studies have reported adverse events in 6%-90%, with serious side effects in up to a third of cases (Table 5)[47,49-51]. Safety data from the VEDOKIDS registry reported an adverse event incidence rate of 23.6%, with headache (4%), myalgia (3%), and fever (2%) being most common. There were no serious adverse events and only 1% cases discontinued treatment due to adverse events[50]. VDZ may be preferred in patients predisposed to infections or those from high TB endemic areas. Studies have not shown any increased risk of malignancies with VDZ[50,51,71]. As non-classical monocytes also express α4β7 integrin and they are the progenitors of wound healing macrophages, concerns have been raised about risk of postoperative complications like wound dehiscence with VDZ use. However, real world studies have not confirmed this finding[51,71].
While long-term safety data for UST are limited in children with IBD, safety data from psoriasis and psoriatic arthritis registry suggest no increased risk of serious infections, malignancy, or mortality[72]. The multicentric study from the pediatric IBD Porto group of ESPGHAN[54] and the recent GETAID registry[55] reported minor adverse events in 8.7%-17% cases with no serious adverse events. In the UniStar trial of 44 children, 88% reported adverse events; upper respiratory tract infection, nasopharyngitis, headache, fatigue, and fever being common[53]. The meta-analysis by Fang et al[56] reported serious adverse events in 3.5% cases, highlighting the favorable safety profile of UST in children.
The main adverse events, reported in adults include infections (mostly upper respiratory tract infection, varicella zoster, EBV), deep vein thrombosis, dyslipidemia and risk of malignancy[73]. Pediatric literature is limited. Adverse events have been reported in up to 3%-71% cases, with mild infection being the most common including herpes zoster and no report of malignancy[57,58,74,75]. Further robust data on the long-term safety profile of biologics and tofacitinib is needed in children with IBD. Till then, we need to carefully monitor children receiving biologics for possible adverse events.
Various studies have evaluated the outcome of combination vs monotherapy with biologics in adults and children with IBD[76-80]. The combined use of anti-TNFα agents and IM (AZA/MTX) has been shown to reduce the formation of antidrug antibody and increase effectiveness[77]. In the landmark sonic trial of 508 adult CD patients (all biologic-naive) comparing IFX plus AZA vs. IFX alone vs AZA alone, the CR (57% vs 44% vs 30%) and endoscopic remission (44% vs 30% vs 16.5%) at 26 weeks was significantly higher with combination therapy as compared to IFX or AZA alone[76]. However, post-hoc analysis of the sonic trial revealed that among patients with similar IFX trough levels, there was no advantage of combination therapy in regards to steroid-free CR at 26 weeks[77]. In the commit trial of 126 adult CD patients, the combination of IFX plus MTX was associated with a lower risk for antidrug antibody formation (4% vs 20%; P = 0.01) and higher IFX trough levels (6.35 μg/mL vs 3.75 μg/mL; P = 0.08), though there was no statistically significant difference in the clinical efficacy at week 50[78].
Pediatric data is scarce[79-81]. The open-label RCT in 99 CD children, evaluated the role of maintenance therapy with combination (IFX plus AZA or MTX) vs IFX monotherapy after 6 months of combination therapy in both groups. There was no significant difference between the groups in outcomes as assessed by PCDAI, simple endoscopic score for CD, and therapy escalation at week 54th. They suggested that 6 months is a safe duration for combination therapy[79]. Grossi et al[80] (n = 502 with CD) showed that longer use of concomitant IM increased the durability of response and probability of continuing IFX at 5 years. Church et al[81] (n = 125 with CD) similarly showed increased durability and reduced LOR to IFX with ≥ 30 weeks of concurrent IM.
Data evaluating combination vs monotherapy in UC is limited. In adults with moderate-severe UC, a RCT of combination therapy vs IFX vs AZA showed that combination therapy had significantly higher CS free CR at week 16 (39.7% vs 22.1% vs 23.7%)[82]. Combination therapy may be preferred in patients with increased risk of disease complications, and those with immunogenic LOR to previous anti-TNFα agents. It should be avoided in young men (especially thiopurines) and EBV-naive subjects. The benefit of combined biologics and IM should be balanced against the increased risk of complications like infection, hepatosplenic T-cell lymphoma and skin cancers with dual therapy[83]. The latest ECCO-ESPGHAN guidelines recommend starting combination therapy with IM and IFX and thereafter stopping the IM after 6-12 months once endoscopic healing is achieved and the IFX trough levels are within the target range[13].
In comparison to IFX, the evidence to support combination therapy with ADA is less convincing[30,83-85]. In the pediatric CD adalimumab level-based optimization treatment trial (78 biologic naive children with CD)[84] and the personalised anti-TNF therapy in CD study trial (1610 patients with CD, 10% children, all biologic naive)[85], there was no significant difference in remission rates between patients receiving combination (ADA+IM) vs ADA monotherapy. However, antidrug antibody formation was lower in the combination group[85]. The ECCO-ESPGHAN guidelines suggest that ADA monotherapy would be appropriate when started as first-line therapy in patients naive to anti-TNF agents[13]. Compared to anti-TNF agents, both VDZ and UST have low immunogenicity (< 5%), which is often transient, explaining the observed lack of benefit of combination therapy[86-88]. Hence, concomitant use of an IM is not currently recommended for these drugs including tofacitinib[57].
Despite the availability and use of biologics and SMDs, there are patients with IBD who are refractory to all current therapeutic options. For this subset of patients, a combination of biological agents or a biologic with SMD as dual-targeted therapy (DTT), is a potential therapeutic option[89]. Several combination therapies have been tried as shown in Table 6 in children with refractory IBD[89-94]. The main combinations are anti-TNFα agents with VDZ or with UST, VDZ with UST, and biologics with tofacitinib[89,91]. Studies in adults have reported good clinical efficacy with DTT. It is important to stop the immunomodulator before starting DTT[95]. Most pediatric studies have reported on clinical efficacy (35%-100%) and not endoscopic or histologic remission[89-94]. Adverse events were reported in 7%-47% of cases, mostly mild but few had serious side effects like deep vein thrombosis, cellulitis, infusion reaction and severe skin eruptions[89-94]. DTT is a valid option for children with difficult-to-treat IBD. However, it should be given by experts under strict vigilance and the benefits should be weighed against the risk of serious adverse events and increased medication cost.
| Ref. | Number of cases | Details of case | Medications used | Efficacy-clinical remission | Adverse events |
| Median age (years); IQR | |||||
| Dolinger et al[89], retrospective | 16 (CD 7, UC 9) | All failed ≥ 2 biologics | VDZ + TOFA 9, VDZ + UST 4, UST + TOFA 3 | 75% at 6 months | 6.25% (arthritis, deep vein thrombosis) |
| 15.9; 13.5-16.8 | |||||
| Wlazło et al[90], retrospective | 14 ( CD 4, UC 10) | All failed single biologic | UST + ADA 5, VDZ + ADA 5, IFX + VDZ 3, VDZ + ADA/UST + ADA-1 | 73% at 4 months | 7% (mild infection) |
| 11.7; 3-17 | |||||
| Yerushalmy-Feler et al[91], retrospective | 62 (CD 35, UC 27) | All failed 1 biologic, 76% failed ≥ 2 biologics | IFX + VDZ 18, IFX + UST 8, ADA + UST 11, ADA + VDZ 11, VDZ + UST 8, TOFA + IFX 2, TOFA + VDZ 1 | 35% at 3 months, 50% at 6 months, 63% at 1 year | 47% (7% serious, infection, thrombosis) |
| 15.5; 13-16.8 | |||||
| Olbjørn et al[92], retrospective | 13 (9 CD, 4 UC) | All failed anti-TNFα agents | IFX + VDZ 8 (4 CD, 4UC), IFX + UST 5 (all CD) | IFX + VDZ, 75% remission at 6 months in UC, 25% in CD, IFX + UST 100% remission at 2 year | IFX + VDZ 2/8 (25%), IFX + UST 1/5 (20%) |
| 8; 4-17.5 | |||||
| Goyal et al[93], retrospective | 9 children with CD | All failed anti-TNFα agents | IFX + VDZ + metho 1, IFX + anakinra 1, ADA + VDZ 1, ADA + VDZ+ PEN 1, ADA + VDZ + metho 3, UST + VDZ 2 | Complete response 45%, partial response 22% at 4 months | 3/9 (33%), skin infection, bleeding, fracture, unrelated |
| 16; 8-19 | |||||
| Wlazlo et al[94], retrospective | 14 (CD 4, UC 10) | All failed anti-TNFα agents | ADA + VDZ 5, ADA + UST 6, IFX + VDZ 3 | 73% CRes by 4 months | Not reported |
| 11.7; 3-17 |
A considerable proportion of patients do not respond to biologics (primary non-response, approximately 1/3rd) or have a secondary LOR after initial response[96]. In adults with CD, the pooled incidence of LOR with anti-TNFα was 33% (95%CI: 29%-38%) and need for dose intensification was 34% (95%CI: 28%-41%) over 1 year follow-up (similar for IFX and ADA)[97]. Numerous real-world studies have shown that approximately half of the children on IFX maintain CR over 1-5 years of therapy[21,98-101]. The cumulative probability of losing response to anti-TNFα therapy (IFX/ADA) was 17%, 38% and 49 % at 1 year, 3 years and 5 years respectively[102]. The CR rate obtained with 1 year of ADA therapy in IMAgINE-1 was maintained in about half of the cases by week 240 of IMAgINE-2[103].
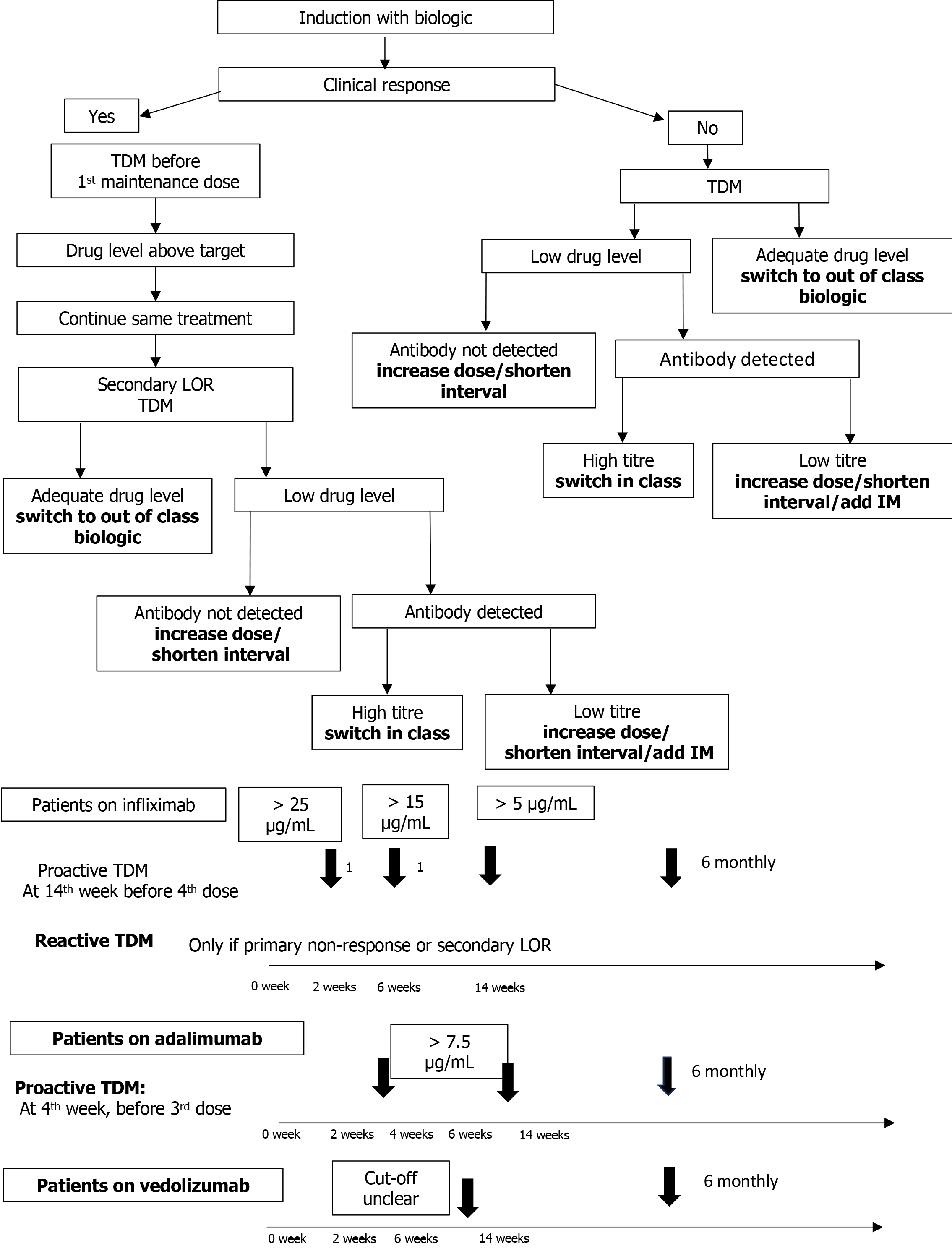
Routine use of TDM to systematically measure the drug concentration and guide clinical decision-making and dose optimization before (proactive) or after (reactive) experiencing gastrointestinal symptoms suggestive of inadequate response has been shown to improve the rate of sustained remission and intestinal healing[104-109]. Proactive monitoring has the advantage of providing sustained drug levels in the target range, which translates into superior efficacy as “drug levels” directly relate to “mucosal healing”. Many prospective pediatric studies have provided convincing evidence for the utility of proactive TDM during maintenance therapy, showing a positive association between adequate drug exposure and better clinical-endoscopic outcome[108-110]. An RCT conducted in children with CD comparing proactive (n = 31) vs reactive (n = 19) TDM showed that sustained corticosteroid-free CR at all visits (week 8th through 72) was significantly higher in the proactive than in the reactive group (82% vs 48%, P = 0.002) with 87% requiring ADA dose escalation in proactive, compared to 60% in the reactive group (P = 0.001)[111]. The approach to decision making after TDM is shown in Figure 2.
Clarkston et al[112] in a study of 72 children with CD on IFX undergoing proactive TDM, demonstrated that responders had higher IFX levels at second/third infusion than non-responders. IFX concentrations > 18 μg/mL at infusion 3 (week 6th) was associated with improved early outcome and start of maintenance concentration of > 5 μg/mL. For, ADA, it is suggested to maintain a drug concentration of > 8-9 μg/mL during maintenance therapy[113]. Rinawi et al[114] showed that ADA level of > 22.5 μg/mL at week 4th and > 12.5 μg/mL at week 8th was useful for predicting clinical and BR at week 24th.
However, there is an ongoing debate about the preferred TDM modality (reactive vs proactive), frequency of monitoring (during induction vs maintenance or both) and optimal trough levels in different disease settings. The extra cost and limited availability of drug level measurement in routine clinical settings further limits its use. Regarding TDM for VDZ, studies in adults have demonstrated an exposure-response relationship[115-120]. Dreesen et. al. showed that VDZ ≥ 24 μg/mL at week 6th and ≥ 14 μg/mL during the maintenance phase was associated with significantly higher rate of CR[121]. Pediatric literature is limited[122,123]. Rowland et al[122] in a prospective cohort study of 59 children (31 CD, 28 UC), showed that proactive TDM and early dose optimization with VDZ was helpful in improving drug durability and clinical outcomes at week 52nd. The authors had taken target drug levels of > 20 μg/mL at week 6th and > 12 μg/mL during maintenance based on existing data. In contrast, the study by Hemming-Harlo et al[123] in 50 PIBD cases on VDZ did not show any benefit of dose enhancement over 1 year follow-up and concluded that an optimal trough level for CR could not be determined. Robust evidence recommending routine use of TDM for VDZ and its optimal levels is currently lacking in children[13].
Although evidence from studies in adults with CD supports the existence of exposure-response for UST[124-126], pediatric data on optimal dosing, and target drug levels is limited[127,128]. Bouhuys et al[128], in a series of 6 children on UST, reported that dose escalation guided by TDM was successful in inducing clinical and BR in 2 (40%) cases. Various commercial assays like enzyme linked immune sorbent assay, radio immune assay, and electrochemiluminescence immune assay etc., are used for TDM. In general, there is agreement across tests for the drug levels but the anti-drug antibody levels are more variable. Thus, it is important to use the same assay for serial monitoring in any given patient. Several factors like body weight, younger age, low serum albumin, extensive disease, development of antidrug antibody, and a high inflammatory burden (as evidenced by increased erythrocyte sedimentation rate and neutrophil CD64 surface expression) control the IFX clearance and thus affect the trough levels[129,130]. These factors need to be considered during TDM. Precision dosing based on pharmacokinetic models are increasingly being studied to predict subsequent dose requirement[131]. In addition, point-of-care assays of IFX, ADA and anti-IFX antibodies are commercially available. These can help reduce the turn-over time and aid in providing clinically relevant, real-time values for better clinical decision making[132]. However, they have limitations of standardization, cost and lack of validation studies[132,133].
Although biologics have shown good clinical efficacy, their long-term use is expensive and associated with adverse effects. Firstly, patients are at an increased risk of infection, more so in those receiving combination therapy with immunomodulators[134-136]. Although serious infections are relatively infrequent, patients often have minor infections which affect the quality of life. Secondly, there is an increased risk of malignancy with biologics (skin cancer, lymphoma mainly associated with anti-TNFα agents and thiopurines)[137]. In a recent non-IBD study, tofacitinib was associated with an increased risk of venous thromboembolism and malignancy[138]. Hence, clinicians are always tempted to try a temporary cessation of biologics in select patients[139]. The main risk of stopping therapy is a flare of the disease.
Many RCTs and meta-analysis have assessed the effect of biologic withdrawal both in UC and CD[140-142]. The HAYABUSA trial in 92 adult UC patients (in corticosteroid free CR > 6 months and Mayo endoscopic score of 0/1) found that 46% subjects who stopped IFX had a relapse at week 48th in comparison to 20% in those who continued IFX[141]. Two other RCTs, a multicentre European spare trial of 211 adult CD patients (steroid-free CR > 6 months)[142] and STOP-IT trial of 115 adult patients with CD (in clinical, biochemical and endoscopic remission for ≥ 12 months)[143] reported similar results of higher relapse in patients discontinuing biologics than those continuing on it. In light of lower relapse with stopping IM rather than stopping IFX while de-escalating from combination (IFX + IM) therapy, stopping IM was the preferable de-escalating strategy[142].
On the contrary, the Spanish EXIT study found no increased risk of relapse in a RCT of anti-TNF (IFX or ADA) withdrawal in 140 adults with IBD in clinical, endoscopic, and radiologic remission[144]. The relapse rate was 24% in the anti-TNF withdrawal group and 16% in the continued group (P = not significant). Most of the available data on biologic withdrawal is with anti-TNFα agents only. Pediatric data is limited. In a retrospective series of 20 CD children on IFX induction and maintenance therapy, 11/20 (55%) achieved CR and 8/11 (72%) relapsed within 1 year of stopping IFX[145]. Another series of 75 Korean children with CD on combination therapy and in deep remission for 2 years, 44 continued to be treated while 21 stopped IFX. 71% (15/21) in the IFX discontinuation group had relapse in the next 28 months vs 36.4% (16/44) in the continuation group[146]. Many factors have been associated with a higher risk of relapse following biologic discontinuation. These include younger age of disease onset (< 16 years), male gender, longer disease duration at first administration of biologic, penetrating or fistulizing disease, ileocolonic disease, isolated upper gastrointestinal disease, previous surgical resection and clinical symptoms at discontinuation[147-150]. Cotreatment with an immunomodulator appears to protect against relapse[147].
Interestingly, studies have also shown that patients with relapse after biologic discontinuation often respond well to the same biologics, despite the concern of higher antigenic reaction after restarting biologic. In the HAYABUSA study, 8/12 (66.7%, 95%CI: 34.9-90.1) patients in the IFX-discontinuation group who were re-treated with IFX after relapse, obtained remission within 8 weeks of re-treatment and none had infusion reactions[141]. Available evidence suggests that 70%-90% of patients recommencing IFX regain CR[149]. Therefore, it is sensible to restart treatment with the same agent that was electively discontinued. A concept of cyclic biologic therapy, which will reduce the total exposure to biologics while maintaining good disease control in patients at low risk of relapse is emerging. Keeping this dilemma in mind, the ECCO has recently published a position statement and proposed that the decision to discontinue biologics should be individualized after careful consideration of the risk factors for relapse and under close monitoring[151]. Discontinuation should be attempted only when the patient is in clinical and BR with mucosal healing. Patients should be counselled about the risks of treatment discontinuation especially since not everyone recaptures response and remission on retreatment. As pediatric IBD is usually more aggressive and extensive, the ECCO suggested that biologic discontinuation should not be attempted in children below 18 years[13].
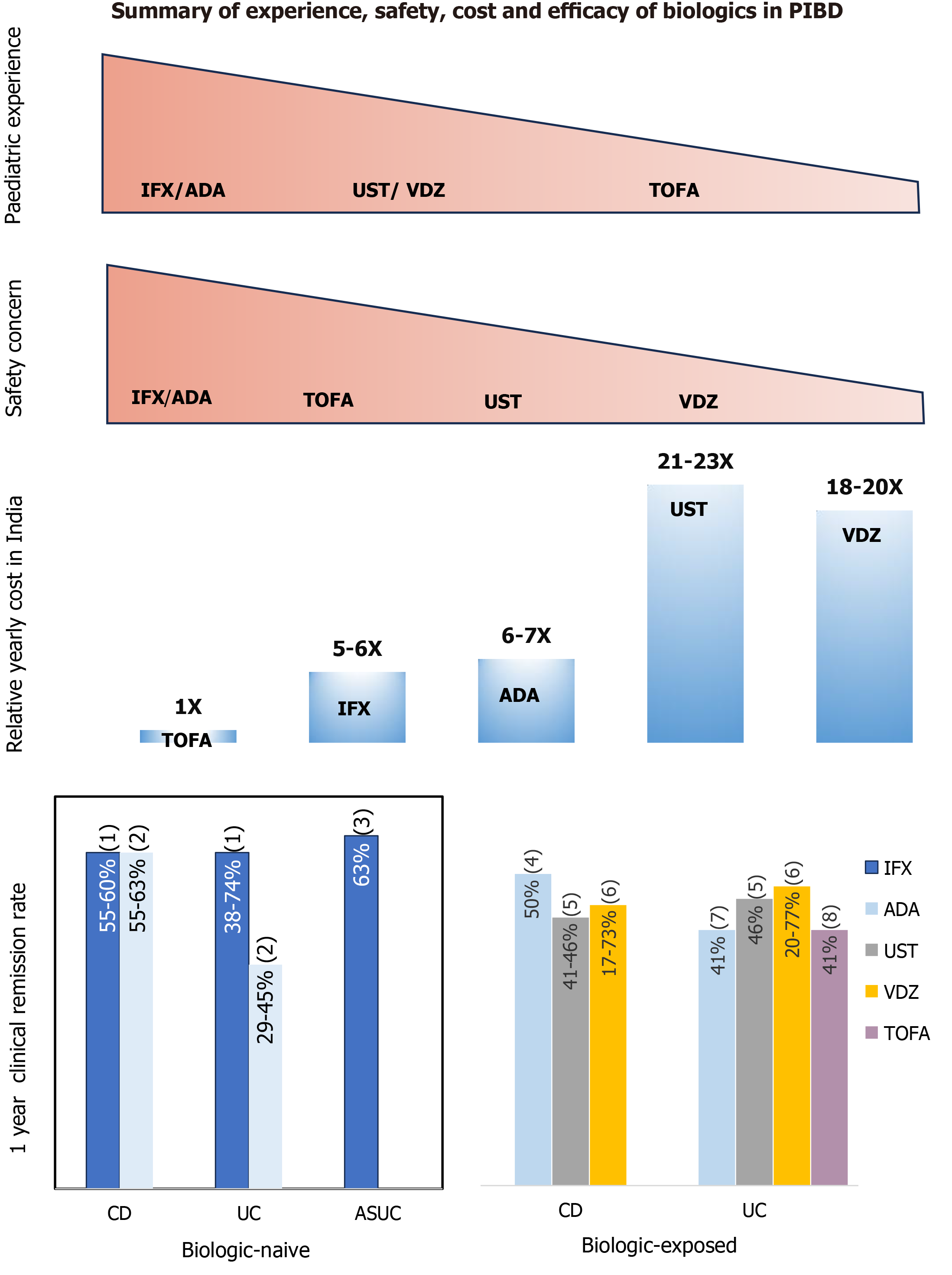
Biologics are often not used or given late in the disease to patients from the developing world largely because of the cost of therapy and issues with restricted availability. This is evident by the fact that only 18% patients received biologics in the multicentre Indian study[4]. The other concern is the higher risk/flare of infections with anti-TNFα agent especially TB in the developing world[152]. Figure 3 depicts the comparative safety profile, relative annual cost (in India) and efficacy of various biologics used in PIBD. The following measures may be considered while using biologics in the developing world to optimize patient outcome: (1) Early diagnosis of IBD and optimization of other treatments; (2) Thorough evaluation and differentiation of CD from intestinal TB. When in diagnostic dilemma, a trial of empirical anti-tubercular therapy may be given as the first step with clinical and endoscopic monitoring for healing; (3) Extensive evaluation to exclude active or latent TB infection in IBD patients before starting biologics and patients should be monitored closely during treatment[153]; (4) Prefer accelerated step-up approach to biologics after considering risk factors and factors predicting poor outcome, rather than a “top down” approach in all[154]; (5) Prefer biosimilar over original compound to reduce cost; (6) Use biologics (anti-TNFα agents) with immunomodulators (AZA or MTX) and then step down to immunomodulator alone after obtaining response in carefully selected patients; and (7) Involve non-government organizations/support groups for financial support.
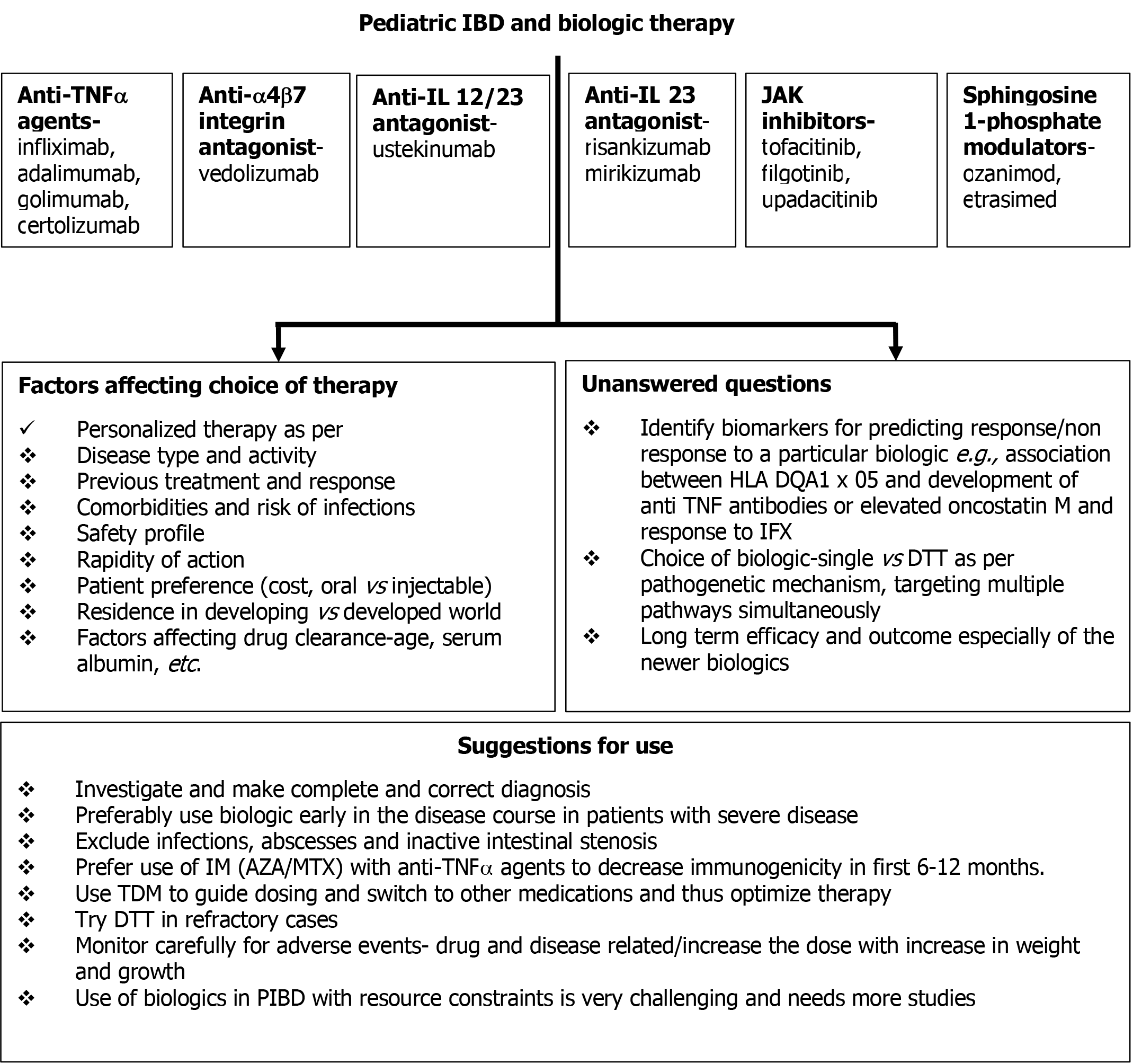
Although we have come a long way, there is still a lot that needs to be done to improve the treatment and outcome of children with IBD. Figure 4 shows the available biologics, factors affecting the choice of biologic and the unanswered questions related to their use. Studies exploring multi-omics (intestinal transcriptome, proteome, metabolome, and microbiome profile) are ongoing in IBD patients to identify the molecular signatures associated with biologic response. Given the chronic nature of IBD and the lack of curative treatments, we need to be better equipped to “identify” IBD cases in terms of responders vs non-responders, patients who may discontinue biologics after obtaining response and the parameters/frequency of monitoring that allow for timely recapture of response in case of flare. This is especially important for the subjects from the developing world. Conducting clinical trials in children with IBD is often fraught with several challenges like limited eligible population, ethical concerns, and long delays in drug approval. We need to follow the international recommendations regarding drug trials to facilitate successful RCTs and hasten the approval of drugs for children[155]. Finally, coupled with point-of-care TDM and biomarker monitoring, model-based precision medicine for dose individualization of biologics may change the way PIBD is treated in the days to come.
Biologics are a useful group of medicines for treatment of IBD in children. Keeping in mind the limited approved options for therapy, especially in children, all efforts should be made to optimize each medication. Proper selection of cases, use of “correct” dose with optimization of target drug levels by TDM and monitoring for side effects can help us achieve the holistic target of “healing”. Dual biologics hold potential in refractory cases. Development of cheaper biosimilars and identification of biomarkers determining response will facilitate the use of biologics in the developing world.
| 1. | Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 725] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 2. | Huang JG, Wong YKY, Chew KS, Tanpowpong P, Calixto Mercado KS, Reodica A, Rajindrajith S, Chang KC, Ni YH, Treepongkaruna S, Lee WS, Aw MM. Epidemiological characteristics of Asian children with inflammatory bowel disease at diagnosis: Insights from an Asian-Pacific multi-centre registry network. World J Gastroenterol. 2022;28:1830-1844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Choe JY, Choi S, Song KH, Jang HJ, Choi KH, Yi DY, Hong SJ, Hwang JH, Cho SM, Kim YJ, Choe BH, Kang B. Incidence and Prevalence Trends of Pediatric Inflammatory Bowel Disease in the Daegu-Kyungpook Province From 2017 to 2020. Front Pediatr. 2021;9:810173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Srivastava A, Sathiyasekharan M, Jagadisan B, Bolia R, Peethambaran M, Mammayil G, Acharya B, Malik R, Sankaranarayanan S, Biradar V, Malhotra S, Philip M, Poddar U, Yachha SK. Paediatric inflammatory bowel disease in India: a prospective multicentre study. Eur J Gastroenterol Hepatol. 2020;32:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Poddar U, Yachha SK, Srivastava A, Kumari N. Pediatric inflammatory bowel disease: Is it really uncommon in Asian children? JGH Open. 2020;4:860-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Lee WS, Arai K, Alex G, Treepongkaruna S, Kim KM, Choong CL, Mercado KS, Darma A, Srivastava A, Aw MM; APPSPGHAN PIBD Working Group, Huang J, Ni YH, Malik R, Tanpowpong P, Tran HN, Ukarapol N. Medical Management of Pediatric Inflammatory Bowel Disease (PIBD) in the Asia Pacific Region: A Position Paper by the Asian Pan-Pacific Society for Pediatric Gastroenterology, Hepatology, and Nutrition (APPSPGHAN) PIBD Working Group. J Gastroenterol Hepatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Jefremow A, Neurath MF. Novel Small Molecules in IBD: Current State and Future Perspectives. Cells. 2023;12:1730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Lev-Tzion R, Turner D. Is pediatric IBD treatment different than in adults? Minerva Gastroenterol Dietol. 2012;58:137-150. [PubMed] |
| 9. | Heuschkel R, Salvestrini C, Beattie RM, Hildebrand H, Walters T, Griffiths A. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Lee WJ, Briars L, Lee TA, Calip GS, Suda KJ, Schumock GT. Top-down Versus Step-up Prescribing Strategies for Tumor Necrosis Factor Alpha Inhibitors in Children and Young Adults with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:2410-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Kugathasan S, Denson LA, Walters TD, Kim MO, Marigorta UM, Schirmer M, Mondal K, Liu C, Griffiths A, Noe JD, Crandall WV, Snapper S, Rabizadeh S, Rosh JR, Shapiro JM, Guthery S, Mack DR, Kellermayer R, Kappelman MD, Steiner S, Moulton DE, Keljo D, Cohen S, Oliva-Hemker M, Heyman MB, Otley AR, Baker SS, Evans JS, Kirschner BS, Patel AS, Ziring D, Trapnell BC, Sylvester FA, Stephens MC, Baldassano RN, Markowitz JF, Cho J, Xavier RJ, Huttenhower C, Aronow BJ, Gibson G, Hyams JS, Dubinsky MC. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet. 2017;389:1710-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 524] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 12. | Zhang L, Jin Z, Hao J. Efficacy of early biologic therapy versus late/conventional therapy in children and adolescents with Crohn's disease: A systematic review and meta-analysis. Saudi J Gastroenterol. 2023;29:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, Gasparetto M, Gerasimidis K, Griffiths A, Henderson P, Koletzko S, Kolho KL, Levine A, van Limbergen J, Martin de Carpi FJ, Navas-López VM, Oliva S, de Ridder L, Russell RK, Shouval D, Spinelli A, Turner D, Wilson D, Wine E, Ruemmele FM. The Medical Management of Paediatric Crohn's Disease: an ECCO-ESPGHAN Guideline Update. J Crohns Colitis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 408] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 14. | Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, Veres G, Aloi M, Strisciuglio C, Braegger CP, Assa A, Romano C, Hussey S, Stanton M, Pakarinen M, de Ridder L, Katsanos K, Croft N, Navas-López V, Wilson DC, Lawrence S, Russell RK. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care-An Evidence-based Guideline From European Crohn's and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67:257-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 358] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 15. | Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, Veres G, Aloi M, Strisciuglio C, Braegger CP, Assa A, Romano C, Hussey S, Stanton M, Pakarinen M, de Ridder L, Katsanos KH, Croft N, Navas-López VM, Wilson DC, Lawrence S, Russell RK. Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis-An Evidence-based Consensus Guideline From the European Crohn's and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67:292-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 16. | Mack DR, Benchimol EI, Critch J, deBruyn J, Tse F, Moayyedi P, Church P, Deslandres C, El-Matary W, Huynh H, Jantchou P, Lawrence S, Otley A, Sherlock M, Walters T, Kappelman MD, Sadowski D, Marshall JK, Griffiths A. Canadian Association of Gastroenterology Clinical Practice Guideline for the Medical Management of Pediatric Luminal Crohn's Disease. Gastroenterology. 2019;157:320-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R, Esteve M, Katsanos K, Lees CW, Macmahon E, Moreels T, Reinisch W, Tilg H, Tremblay L, Veereman-Wauters G, Viget N, Yazdanpanah Y, Eliakim R, Colombel JF; European Crohn's and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 759] [Article Influence: 63.3] [Reference Citation Analysis (1)] |
| 18. | Mantri AK, Meena P, Puri AS, Kumar A, Sachdeva S, Srivastava S, Arivarasan K, Varakanahali S. Comparison of Interferon-Gamma Release Assay and Tuberculin Skin Test for the Screening of Latent Tuberculosis in Inflammatory Bowel Disease Patients: Indian Scenario. Tuberc Res Treat. 2021;2021:6682840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Banerjee R, Ali RAR, Wei SC, Adsul S. Biologics for the Management of Inflammatory Bowel Disease: A Review in Tuberculosis-Endemic Countries. Gut Liver. 2020;14:685-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 20. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1921] [Article Influence: 384.2] [Reference Citation Analysis (1)] |
| 21. | Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, Liu G, Travers S, Heuschkel R, Markowitz J, Cohen S, Winter H, Veereman-Wauters G, Ferry G, Baldassano R; REACH Study Group. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132:863-73; quiz 1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 650] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 22. | Nobile S, Gionchetti P, Rizzello F, Calabrese C, Campieri M. Mucosal healing in pediatric Crohn's disease after anti-TNF therapy: a long-term experience at a single center. Eur J Gastroenterol Hepatol. 2014;26:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Crandall W, Hyams J, Kugathasan S, Griffiths A, Zrubek J, Olson A, Liu G, Heuschkel R, Markowitz J, Cohen S, Winter H, Veereman-Wauters G, Ferry G, Baldassano RN. Infliximab therapy in children with concurrent perianal Crohn disease: observations from REACH. J Pediatr Gastroenterol Nutr. 2009;49:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | El-Matary W, Walters TD, Huynh HQ, deBruyn J, Mack DR, Jacobson K, Sherlock ME, Church P, Wine E, Carroll MW, Benchimol EI, Lawrence S, Griffiths AM. Higher Postinduction Infliximab Serum Trough Levels Are Associated With Healing of Fistulizing Perianal Crohn's Disease in Children. Inflamm Bowel Dis. 2019;25:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (2)] |
| 25. | Kim MJ, Kim E, Kang B, Choe YH. Infliximab Therapy for Children with Moderate to Severe Ulcerative Colitis: A Step-Up versus a Top-Down Strategy. Yonsei Med J. 2021;62:608-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Stephens M, Evans J, Otley A, Carvalho R, Mack D, Bousvaros A, Rosh J, Grossman A, Tomer G, Kay M, Crandall W, Oliva-Hemker M, Keljo D, LeLeiko N, Markowitz J; Pediatric Inflammatory Bowel Disease Collaborative Research Group. Outcome following infliximab therapy in children with ulcerative colitis. Am J Gastroenterol. 2010;105:1430-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Larsen MD, Qvist N, Nielsen J, Kjeldsen J, Nielsen RG, Nørgård BM. Use of Anti-TNFα Agents and Time to First-time Surgery in Paediatric Patients with Ulcerative Colitis and Crohn's Disease. J Crohns Colitis. 2016;10:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Turner D, Leach ST, Mack D, Uusoue K, McLernon R, Hyams J, Leleiko N, Walters TD, Crandall W, Markowitz J, Otley AR, Griffiths AM, Day AS. Faecal calprotectin, lactoferrin, M2-pyruvate kinase and S100A12 in severe ulcerative colitis: a prospective multicentre comparison of predicting outcomes and monitoring response. Gut. 2010;59:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Bolia R, Rajanayagam J, Hardikar W, Alex G. Impact of Changing Treatment Strategies on Outcomes in Pediatric Ulcerative Colitis. Inflamm Bowel Dis. 2019;25:1838-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Hyams JS, Griffiths A, Markowitz J, Baldassano RN, Faubion WA Jr, Colletti RB, Dubinsky M, Kierkus J, Rosh J, Wang Y, Huang B, Bittle B, Marshall M, Lazar A. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology. 2012;143:365-74.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 31. | deBruyn JC, Huynh HQ, Griffiths AM, Jacobson K, Mack D, Deslandres C, El-Matary W, Otley AR, Church PC, Lawrence S, Wine E, Sherlock M, Critch J, Benchimol EI, Jantchou P, Rashid M, Carroll MW, Bax K, Ricciuto A, Carman N, Walters TD; Canadian Children IBD Network. Adalimumab vs Infliximab in Luminal Pediatric Crohn's Disease: Comparable Outcomes in a Prospective Multicenter Cohort Study. Am J Gastroenterol. 2024;119:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Ruemmele FM, Rosh J, Faubion WA, Dubinsky MC, Turner D, Lazar A, Eichner S, Maa JF, Alperovich G, Robinson AM, Hyams JS. Efficacy of Adalimumab for Treatment of Perianal Fistula in Children with Moderately to Severely Active Crohn's Disease: Results from IMAgINE 1 and IMAgINE 2. J Crohns Colitis. 2018;12:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (2)] |
| 33. | Aloi M, Bramuzzo M, Arrigo S, Romano C, D'Arcangelo G, Lacorte D, Gatti S, Illiceto MT, Zucconi F, Dilillo D, Zuin G, Knafelz D, Ravelli A, Cucchiara S, Alvisi P; SIGENP IBD Working Group. Efficacy and Safety of Adalimumab in Pediatric Ulcerative Colitis: A Real-life Experience from the SIGENP-IBD Registry. J Pediatr Gastroenterol Nutr. 2018;66:920-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Croft NM, Faubion WA Jr, Kugathasan S, Kierkus J, Ruemmele FM, Shimizu T, Mostafa NM, Venetucci M, Finney-Hayward T, Sanchez Gonzalez Y, Bereswill M, Lazar A, Turner D. Efficacy and safety of adalimumab in paediatric patients with moderate-to-severe ulcerative colitis (ENVISION I): a randomised, controlled, phase 3 study. Lancet Gastroenterol Hepatol. 2021;6:616-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 35. | Casanova MJ, Chaparro M, Mínguez M, Ricart E, Taxonera C, García-López S, Guardiola J, López-San Román A, Iglesias E, Beltrán B, Sicilia B, Vera MI, Hinojosa J, Riestra S, Domènech E, Calvet X, Pérez-Calle JL, Martín-Arranz MD, Aldeguer X, Rivero M, Monfort D, Barrio J, Esteve M, Márquez L, Lorente R, García-Planella E, de Castro L, Bermejo F, Merino O, Rodríguez-Pérez A, Martínez-Montiel P, Van Domselaar M, Alcaín G, Domínguez-Cajal M, Muñoz C, Gomollón F, Fernández-Salazar L, García-Sepulcre MF, Rodríguez-Lago I, Gutiérrez A, Argüelles-Arias F, Rodriguez C, Rodríguez GE, Bujanda L, Llaó J, Varela P, Ramos L, Huguet JM, Almela P, Romero P, Navarro-Llavat M, Abad Á, Ramírez-de la Piscina P, Lucendo AJ, Sesé E, Madrigal RE, Charro M, García-Herola A, Pajares R, Khorrami S, Gisbert JP. Effectiveness and Safety of the Sequential Use of a Second and Third Anti-TNF Agent in Patients With Inflammatory Bowel Disease: Results From the Eneida Registry. Inflamm Bowel Dis. 2020;26:606-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Cozijnsen M, Duif V, Kokke F, Kindermann A, van Rheenen P, de Meij T, Schaart M, Damen G, Norbruis O, Pelleboer R, Van den Neucker A, van Wering H, Hummel T, Oudshoorn J, Escher J, de Ridder L; Dutch PIBD Working Group Kids with Crohn and Colitis. Adalimumab therapy in children with Crohn disease previously treated with infliximab. J Pediatr Gastroenterol Nutr. 2015;60:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Dipasquale V, Cicala G, Spina E, Romano C. Biosimilars in Pediatric Inflammatory Bowel Diseases: A Systematic Review and Real Life-Based Evidence. Front Pharmacol. 2022;13:846151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Sieczkowska-Golub J, Meglicka M, Plocek A, Banaszkiewicz A, Jarzębicka D, Toporowska-Kowalska E, Gawronska A, Oracz G, Kierkus J. Induction Therapy With Biosimilar Infliximab in Children With Crohn Disease. J Pediatr Gastroenterol Nutr. 2017;65:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Chanchlani N, Mortier K, Williams LJ, Muhammed R, Auth MKH, Cosgrove M, Fagbemi A, Fell J, Chong S, Zamvar V, Hyer W, Bisset WM, Morris MA, Rodrigues A, Mitton SG, Bunn S, Beattie RM, Willmott A, Wilson DC, Russell RK. Use of Infliximab Biosimilar Versus Originator in a Pediatric United Kingdom Inflammatory Bowel Disease Induction Cohort. J Pediatr Gastroenterol Nutr. 2018;67:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Nikkonen A, Kolho KL. Infliximab and its biosimilar produced similar first-year therapy outcomes in patients with inflammatory bowel disease. Acta Paediatr. 2020;109:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Sieczkowska J, Jarzębicka D, Banaszkiewicz A, Plocek A, Gawronska A, Toporowska-Kowalska E, Oracz G, Meglicka M, Kierkus J. Switching Between Infliximab Originator and Biosimilar in Paediatric Patients with Inflammatory Bowel Disease. Preliminary Observations. J Crohns Colitis. 2016;10:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 42. | Kang B, Lee Y, Lee K, Choi YO, Choe YH. Long-term Outcomes After Switching to CT-P13 in Pediatric-Onset Inflammatory Bowel Disease: A Single-Center Prospective Observational Study. Inflamm Bowel Dis. 2018;24:607-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Dipasquale V, Pellegrino S, Ventimiglia M, Citrano M, Graziano F, Cappello M, Busacca A, Orlando A, Accomando S, Romano C, Sicilian Network For Inflammatory Bowel Disease. Adalimumab Biosimilar in Pediatric Inflammatory Bowel Disease: A Retrospective Study from the Sicilian Network for Inflammatory Bowel Disease (SN-IBD). Healthcare (Basel). 2024;12:404. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 44. | Richter V, Cohen DL, Kriger-Sharabi O, Zelnik Yovel D, Kochen N, Broide E, Shirin H. Switching from Intravenous to Subcutaneous Biological Therapy for Inflammatory Bowel Disease Patients Remains a Challenge. J Clin Med. 2024;13:1357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 45. | Gianolio L, Armstrong K, Swann E, Shepherd R, Henderson P, Wilson DC, Russell RK. Effectiveness of Switching to Subcutaneous Infliximab in Pediatric Inflammatory Bowel Disease Patients on Intravenous Maintenance Therapy. J Pediatr Gastroenterol Nutr. 2023;77:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Conrad MA, Stein RE, Maxwell EC, Albenberg L, Baldassano RN, Dawany N, Grossman AB, Mamula P, Piccoli DA, Kelsen JR. Vedolizumab Therapy in Severe Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:2425-2431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 47. | Singh N, Rabizadeh S, Jossen J, Pittman N, Check M, Hashemi G, Phan BL, Hyams JS, Dubinsky MC. Multi-Center Experience of Vedolizumab Effectiveness in Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:2121-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 48. | Jossen J, Kiernan BD, Pittman N, Dubinsky MC. Anti-tumor Necrosis Factor-alpha Exposure Impacts Vedolizumab Mucosal Healing Rates in Pediatric Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2020;70:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Hyams JS, Turner D, Cohen SA, Szakos E, Kowalska-Duplaga K, Ruemmele F, Croft NM, Korczowski B, Lawrence P, Bhatia S, Kadali H, Chen C, Sun W, Rosario M, Kabilan S, Treem W, Rossiter G, Lirio RA. Pharmacokinetics, Safety and Efficacy of Intravenous Vedolizumab in Paediatric Patients with Ulcerative Colitis or Crohn's Disease: Results from the Phase 2 HUBBLE Study. J Crohns Colitis. 2022;16:1243-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 50. | Atia O, Shavit-Brunschwig Z, Mould DR, Stein R, Matar M, Aloi M, Ledder O, Focht G, Urlep D, Hyams J, Broide E, Weiss B, Levine J, Russell RK, Turner D. Outcomes, dosing, and predictors of vedolizumab treatment in children with inflammatory bowel disease (VEDOKIDS): a prospective, multicentre cohort study. Lancet Gastroenterol Hepatol. 2023;8:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 51. | Fang S, Song Y, Zhang C, Wang L. Efficacy and safety of vedolizumab for pediatrics with inflammatory bowel disease: a systematic review. BMC Pediatr. 2022;22:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Rosh JR, Turner D, Griffiths A, Cohen SA, Jacobstein D, Adedokun OJ, Padgett L, Terry NA, O'Brien C, Hyams JS. Ustekinumab in Paediatric Patients with Moderately to Severely Active Crohn's Disease: Pharmacokinetics, Safety, and Efficacy Results from UniStar, a Phase 1 Study. J Crohns Colitis. 2021;15:1931-1942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 53. | Turner D, Rosh JR, Cohen SA, Griffiths AM, Hyams JS, Kierkuś J, Adedokun OJ, Strauss R, Kim L, Volger S; UniStar Study Group. Ustekinumab in paediatric patients with moderately to severely active Crohn's disease: UniStar study long-term extension results. J Pediatr Gastroenterol Nutr. 2024;79:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 54. | Yerushalmy-Feler A, Pujol-Muncunill G, Martin-de-Carpi J, Kolho KL, Levine A, Olbjørn C, Granot M, Bramuzzo M, Rolandsdotter H, Mouratidou N, Hradsky O, Scarallo L, Matar M, Rimon RM, Rinawi F, Shalem T, Najajra H, de Meij T, Aloi M, Rodríguez-Belvís MV, Alvisi P, Schneider AM, van Rheenen P, Navas-López VM, Kiparissi F, Barrio J, Turner D, Cohen S. Safety and Potential Efficacy of Escalating Dose of Ustekinumab in Pediatric Crohn Disease (the Speed-up Study): A Multicenter Study from the Pediatric IBD Porto Group of ESPGHAN. J Pediatr Gastroenterol Nutr. 2022;75:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 55. | Koudsi M, Martinez-Vinson C, Pigneur B, Willot S, Djamal D, Enaud R, Rebeuh J, Dupont C, Dabadie A, Bertrand V, Hugot JP, Lachaux A, Ruemmele F, Viala J, Duclaux-Loras R, Pédiatrique G. Ustekinumab Use in Pediatric Inflammatory Bowel Disease: A French Multicenter Study From the Pediatric GETAID. J Pediatr Gastroenterol Nutr. 2023;76:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 56. | Fang S, Zhang S, Zhang C, Wang L. Effectiveness and Safety of Ustekinumab for Pediatric Inflammatory Bowel Disease: A Systematic Review. Paediatr Drugs. 2023;25:499-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 57. | Moore H, Dubes L, Fusillo S, Baldassano R, Stein R. Tofacitinib Therapy in Children and Young Adults With Pediatric-onset Medically Refractory Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2021;73:e57-e62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 58. | Ledder O, Dolinger M, Dubinsky MC, Stein R, Vellanki S, Buckuk R, Fatima A, Suskind DL, Scarlett J, Röser D, Shouval DS, Meyer G, Rios ZM, Pujol-Muncunill G, Lozano A, Kolho KL, Rohani P, Hussey S, de Mejj T, Ayers T, Navas-López VM, Turner D, Tzivinikos C. Tofacitinib in Pediatric Ulcerative Colitis: A Retrospective Multicenter Experience. Inflamm Bowel Dis. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 59. | Turner D, Mack D, Leleiko N, Walters TD, Uusoue K, Leach ST, Day AS, Crandall W, Silverberg MS, Markowitz J, Otley AR, Keljo D, Mamula P, Kugathasan S, Hyams J, Griffiths AM. Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010;138:2282-2291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 60. | Brandse JF, van den Brink GR, Wildenberg ME, van der Kleij D, Rispens T, Jansen JM, Mathôt RA, Ponsioen CY, Löwenberg M, D'Haens GR. Loss of Infliximab Into Feces Is Associated With Lack of Response to Therapy in Patients With Severe Ulcerative Colitis. Gastroenterology. 2015;149:350-5.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 324] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 61. | Jongsma MME, Winter DA, Huynh HQ, Norsa L, Hussey S, Kolho KL, Bronsky J, Assa A, Cohen S, Lev-Tzion R, Van Biervliet S, Rizopoulos D, de Meij TGJ, Shouval DS, Wine E, Wolters VM, Martinez-Vinson C, de Ridder L; Paediatric IBD Porto Group of ESPGHAN. Infliximab in young paediatric IBD patients: it is all about the dosing. Eur J Pediatr. 2020;179:1935-1944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 62. | Dhaliwal J, Tertigas D, Carman N, Lawrence S, Debruyn JC, Wine E, Church PC, Huynh HQ, Rashid M, El-Matary W, Deslandres C, Critch J, Ricciuto A, Carroll MW, Benchimol EI, Muise A, Jacobson K, Otley AR, Vallance B, Mack DR, Walters TD, Surette MG, Griffiths AM. Outcomes Following Acute Severe Colitis at Initial Presentation: A Multi-centre, Prospective, Paediatric Cohort Study. J Crohns Colitis. 2024;18:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Whaley KG, Xiong Y, Karns R, Hyams JS, Kugathasan S, Boyle BM, Walters TD, Kelsen J, LeLeiko N, Shapiro J, Waddell A, Fox S, Bezold R, Bruns S, Widing R, Haberman Y, Collins MH, Mizuno T, Minar P, D'Haens GR, Denson LA, Vinks AA, Rosen MJ. Multicenter Cohort Study of Infliximab Pharmacokinetics and Therapy Response in Pediatric Acute Severe Ulcerative Colitis. Clin Gastroenterol Hepatol. 2023;21:1338-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 64. | Sebastian S, Myers S, Nadir S, Subramanian S. Systematic Review: Efficacy and Safety of Accelerated Induction Regimes in Infliximab Rescue Therapy for Hospitalized Patients with Acute Severe Colitis. Dig Dis Sci. 2019;64:1119-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 65. | Breton J, Kastl A, Conrad MA, Baldassano RN. Positioning Biologic Therapies in the Management of Pediatric Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2020;16:400-414. [PubMed] |
| 66. | Raychaudhuri SP, Nguyen CT, Raychaudhuri SK, Gershwin ME. Incidence and nature of infectious disease in patients treated with anti-TNF agents. Autoimmun Rev. 2009;9:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Dulai PS, Thompson KD, Blunt HB, Dubinsky MC, Siegel CA. Risks of serious infection or lymphoma with anti-tumor necrosis factor therapy for pediatric inflammatory bowel disease: a systematic review. Clin Gastroenterol Hepatol. 2014;12:1443-51; quiz e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 68. | Dziechciarz P, Horvath A, Kierkuś J. Efficacy and Safety of Adalimumab for Paediatric Crohn's Disease: A Systematic Review. J Crohns Colitis. 2016;10:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Conrad C, Di Domizio J, Mylonas A, Belkhodja C, Demaria O, Navarini AA, Lapointe AK, French LE, Vernez M, Gilliet M. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 70. | Hyams JS, Dubinsky MC, Baldassano RN, Colletti RB, Cucchiara S, Escher J, Faubion W, Fell J, Gold BD, Griffiths A, Koletzko S, Kugathasan S, Markowitz J, Ruemmele FM, Veereman G, Winter H, Masel N, Shin CR, Tang KL, Thayu M. Infliximab Is Not Associated With Increased Risk of Malignancy or Hemophagocytic Lymphohistiocytosis in Pediatric Patients With Inflammatory Bowel Disease. Gastroenterology. 2017;152:1901-1914.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 71. | Ledder O, Assa A, Levine A, Escher JC, de Ridder L, Ruemmele F, Shah N, Shaoul R, Wolters VM, Rodrigues A, Uhlig HH, Posovszky C, Kolho KL, Jakobsen C, Cohen S, Shouval DS, de Meij T, Martin-de-Carpi J, Richmond L, Bronsky J, Friedman M, Turner D. Vedolizumab in Paediatric Inflammatory Bowel Disease: A Retrospective Multi-Centre Experience From the Paediatric IBD Porto Group of ESPGHAN. J Crohns Colitis. 2017;11:1230-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 72. | Gottlieb AB, Kalb RE, Langley RG, Krueger GG, de Jong EM, Guenther L, Goyal K, Fakharzadeh S, Chevrier M, Calabro S, Langholff W, Menter A. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448. [PubMed] |
| 73. | Lichtenstein GR, Bressler B, Francisconi C, Vermeire S, Lawendy N, Salese L, Sawyerr G, Shi H, Su C, Judd DT, Jones T, Loftus EV. Assessment of Safety and Efficacy of Tofacitinib, Stratified by Age, in Patients from the Ulcerative Colitis Clinical Program. Inflamm Bowel Dis. 2023;29:27-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 74. | Costaguta GA, Girard C, Groleau V, Grzywacz K, Dirks MH, Deslandres C. The Role of Tofacitinib in the Treatment of Acute Severe Colitis in Children. J Can Assoc Gastroenterol. 2024;7:196-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Ryan N, Cooper S, Dominik A, Quinn S, Broderick A, Bourke B, Hussey S. Outcomes of Tofacitinib Use in an Irish Pediatric Cohort. JPGN Rep. 2023;4:e332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 76. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2441] [Article Influence: 152.6] [Reference Citation Analysis (1)] |
| 77. | Colombel JF, Adedokun OJ, Gasink C, Gao LL, Cornillie FJ, D'Haens GR, Rutgeerts PJ, Reinisch W, Sandborn WJ, Hanauer SB. Combination Therapy With Infliximab and Azathioprine Improves Infliximab Pharmacokinetic Features and Efficacy: A Post Hoc Analysis. Clin Gastroenterol Hepatol. 2019;17:1525-1532.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 78. | Feagan BG, McDonald JW, Panaccione R, Enns RA, Bernstein CN, Ponich TP, Bourdages R, Macintosh DG, Dallaire C, Cohen A, Fedorak RN, Paré P, Bitton A, Saibil F, Anderson F, Donner A, Wong CJ, Zou G, Vandervoort MK, Hopkins M, Greenberg GR. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn's disease. Gastroenterology. 2014;146:681-688.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 252] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 79. | Kierkuś J, Iwańczak B, Wegner A, Dadalski M, Grzybowska-Chlebowczyk U, Łazowska I, Maślana J, Toporowska-Kowalska E, Czaja-Bulsa G, Mierzwa G, Korczowski B, Czkwianianc E, Żabka A, Szymańska E, Krzesiek E, Więcek S, Sładek M. Monotherapy with infliximab versus combination therapy in the maintenance of clinical remission in children with moderate to severe Crohn disease. J Pediatr Gastroenterol Nutr. 2015;60:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Grossi V, Lerer T, Griffiths A, LeLeiko N, Cabrera J, Otley A, Rick J, Mack D, Bousvaros A, Rosh J, Grossman A, Saeed S, Kay M, Boyle B, Oliva-Hemker M, Keljo D, Pfefferkorn M, Faubion W, Kappelman MD, Sudel B, Markowitz J, Hyams JS. Concomitant Use of Immunomodulators Affects the Durability of Infliximab Therapy in Children With Crohn's Disease. Clin Gastroenterol Hepatol. 2015;13:1748-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 81. | Church PC, Guan J, Walters TD, Frost K, Assa A, Muise AM, Griffiths AM. Infliximab maintains durable response and facilitates catch-up growth in luminal pediatric Crohn's disease. Inflamm Bowel Dis. 2014;20:1177-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 82. | Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, van Hoogstraten HJ, Chen AC, Zheng H, Danese S, Rutgeerts P. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392-400.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 712] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 83. | Day AS, Gulati AS, Patel N, Boyle B, Park KT, Saeed SA. The Role of Combination Therapy in Pediatric Inflammatory Bowel Disease: A Clinical Report from the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Matar M, Shamir R, Turner D, Broide E, Weiss B, Ledder O, Guz-Mark A, Rinawi F, Cohen S, Topf-Olivestone C, Shaoul R, Yerushalmi B, Ben-Horin S, Assa A. Combination Therapy of Adalimumab With an Immunomodulator Is Not More Effective Than Adalimumab Monotherapy in Children With Crohn's Disease: A Post Hoc Analysis of the PAILOT Randomized Controlled Trial. Inflamm Bowel Dis. 2020;26:1627-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, Thomas A, Nice R, Perry MH, Bouri S, Chanchlani N, Heerasing NM, Hendy P, Lin S, Gaya DR, Cummings JRF, Selinger CP, Lees CW, Hart AL, Parkes M, Sebastian S, Mansfield JC, Irving PM, Lindsay J, Russell RK, McDonald TJ, McGovern D, Goodhand JR, Ahmad T; UK Inflammatory Bowel Disease Pharmacogenetics Study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 511] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 86. | Van den Berghe N, Verstockt B, Tops S, Ferrante M, Vermeire S, Gils A. Immunogenicity is not the driving force of treatment failure in vedolizumab-treated inflammatory bowel disease patients. J Gastroenterol Hepatol. 2019;34:1175-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Adedokun OJ, Xu Z, Gasink C, Jacobstein D, Szapary P, Johanns J, Gao LL, Davis HM, Hanauer SB, Feagan BG, Ghosh S, Sandborn WJ. Pharmacokinetics and Exposure Response Relationships of Ustekinumab in Patients With Crohn's Disease. Gastroenterology. 2018;154:1660-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 88. | Battat R, Kopylov U, Bessissow T, Bitton A, Cohen A, Jain A, Martel M, Seidman E, Afif W. Association Between Ustekinumab Trough Concentrations and Clinical, Biomarker, and Endoscopic Outcomes in Patients With Crohn's Disease. Clin Gastroenterol Hepatol. 2017;15:1427-1434.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 89. | Dolinger MT, Spencer EA, Lai J, Dunkin D, Dubinsky MC. Dual Biologic and Small Molecule Therapy for the Treatment of Refractory Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis. 2021;27:1210-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 90. | Wlazło M, Meglicka M, Wiernicka A, Osiecki M, Kierkuś J. Dual Biologic Therapy in Moderate to Severe Pediatric Inflammatory Bowel Disease: A Retrospective Study. Children (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 91. | Yerushalmy-Feler A, Olbjorn C, Kolho KL, Aloi M, Musto F, Martin-de-Carpi J, Lozano-Ruf A, Yogev D, Matar M, Scarallo L, Bramuzzo M, de Ridder L, Kang B, Norden C, Wilson DC, Tzivinikos C, Turner D, Cohen S. Dual Biologic or Small Molecule Therapy in Refractory Pediatric Inflammatory Bowel Disease (DOUBLE-PIBD): A Multicenter Study from the Pediatric IBD Porto Group of ESPGHAN. Inflamm Bowel Dis. 2024;30:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 92. | Olbjørn C, Rove JB, Jahnsen J. Combination of Biological Agents in Moderate to Severe Pediatric Inflammatory Bowel Disease: A Case Series and Review of the Literature. Paediatr Drugs. 2020;22:409-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 93. | Goyal A, Bass J. P090 Safety And Efficacy Of Combining Biologicals In Children With Inflammatory Bowel Disease. Gastroenterol. 2020;158:S122-S123. [DOI] [Full Text] |
| 94. | Wlazlo M, Meglicka M, Wiernicka A, Osiecki M, Matuszczyk M, Kierkus J. Combination biologic therapy in pediatric inflammatory bowel disease: Safety and efficacy over a minimum 12-month follow-up period. J Pediatr Gastroenterol Nutr. 2024;79:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 95. | McCormack MD, Wahedna NA, Aldulaimi D, Hawker P. Emerging role of dual biologic therapy for the treatment of inflammatory bowel disease. World J Clin Cases. 2023;11:2621-2630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (2)] |
| 96. | Sparrow MP, Papamichael K, Ward MG, Riviere P, Laharie D, Paul S, Roblin X. Therapeutic Drug Monitoring of Biologics During Induction to Prevent Primary Non-Response. J Crohns Colitis. 2020;14:542-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 97. | Qiu Y, Chen BL, Mao R, Zhang SH, He Y, Zeng ZR, Ben-Horin S, Chen MH. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn's disease. J Gastroenterol. 2017;52:535-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 98. | Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Kugathasan S, Evans J, Otley A, Carvalho R, Mack D, Bousvaros A, Rosh J, Mamula P, Kay M, Crandall W, Oliva-Hemker M, Keljo D, LeLeiko N, Markowitz J; Pediatric Inflammatory Bowel Disease Collaborative Research Group. Long-term outcome of maintenance infliximab therapy in children with Crohn's disease. Inflamm Bowel Dis. 2009;15:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 99. | Vahabnezhad E, Rabizadeh S, Dubinsky MC. A 10-year, single tertiary care center experience on the durability of infliximab in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 100. | De Bie CI, Hummel TZ, Kindermann A, Kokke FT, Damen GM, Kneepkens CM, Van Rheenen PF, Schweizer JJ, Hoekstra JH, Norbruis OF, Tjon A Ten WE, Vreugdenhil AC, Deckers-Kocken JM, Gijsbers CF, Escher JC, De Ridder L. The duration of effect of infliximab maintenance treatment in paediatric Crohn's disease is limited. Aliment Pharmacol Ther. 2011;33:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 101. | Crombé V, Salleron J, Savoye G, Dupas JL, Vernier-Massouille G, Lerebours E, Cortot A, Merle V, Vasseur F, Turck D, Gower-Rousseau C, Lémann M, Colombel JF, Duhamel A. Long-term outcome of treatment with infliximab in pediatric-onset Crohn's disease: a population-based study. Inflamm Bowel Dis. 2011;17:2144-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 102. | Assa A, Hartman C, Weiss B, Broide E, Rosenbach Y, Zevit N, Bujanover Y, Shamir R. Long-term outcome of tumor necrosis factor alpha antagonist's treatment in pediatric Crohn's disease. J Crohns Colitis. 2013;7:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 103. | Faubion WA, Dubinsky M, Ruemmele FM, Escher J, Rosh J, Hyams JS, Eichner S, Li Y, Reilly N, Thakkar RB, Robinson AM, Lazar A. Long-term Efficacy and Safety of Adalimumab in Pediatric Patients with Crohn's Disease. Inflamm Bowel Dis. 2017;23:453-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 104. | Bodini G, Giannini EG, Savarino V, Del Nero L, Lo Pumo S, Brunacci M, De Bortoli N, Jain A, Tolone S, Savarino E. Infliximab trough levels and persistent vs transient antibodies measured early after induction predict long-term clinical remission in patients with inflammatory bowel disease. Dig Liver Dis. 2018;50:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 105. | Barnes EL, Allegretti JR. Are Anti-Tumor Necrosis Factor Trough Levels Predictive of Mucosal Healing in Patients With Inflammatory Bowel Disease?: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2016;50:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 106. | Cornillie F, Hanauer SB, Diamond RH, Wang J, Tang KL, Xu Z, Rutgeerts P, Vermeire S. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721-1727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 304] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 107. | Dubinsky MC, Rosh J, Faubion WA Jr, Kierkus J, Ruemmele F, Hyams JS, Eichner S, Li Y, Huang B, Mostafa NM, Lazar A, Thakkar RB. Efficacy and Safety of Escalation of Adalimumab Therapy to Weekly Dosing in Pediatric Patients with Crohn's Disease. Inflamm Bowel Dis. 2016;22:886-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 108. | Stein R, Lee D, Leonard MB, Thayu M, Denson LA, Chuang E, Herskovitz R, Kerbowski T, Baldassano RN. Serum Infliximab, Antidrug Antibodies, and Tumor Necrosis Factor Predict Sustained Response in Pediatric Crohn's Disease. Inflamm Bowel Dis. 2016;22:1370-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 109. | Adedokun OJ, Xu Z, Padgett L, Blank M, Johanns J, Griffiths A, Ford J, Zhou H, Guzzo C, Davis HM, Hyams J. Pharmacokinetics of infliximab in children with moderate-to-severe ulcerative colitis: results from a randomized, multicenter, open-label, phase 3 study. Inflamm Bowel Dis. 2013;19:2753-2762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 110. | Singh N, Rosenthal CJ, Melmed GY, Mirocha J, Farrior S, Callejas S, Tripuraneni B, Rabizadeh S, Dubinsky MC. Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1708-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 111. | Assa A, Matar M, Turner D, Broide E, Weiss B, Ledder O, Guz-Mark A, Rinawi F, Cohen S, Topf-Olivestone C, Shaoul R, Yerushalmi B, Shamir R. Proactive Monitoring of Adalimumab Trough Concentration Associated With Increased Clinical Remission in Children With Crohn's Disease Compared With Reactive Monitoring. Gastroenterology. 2019;157:985-996.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 112. | Clarkston K, Tsai YT, Jackson K, Rosen MJ, Denson LA, Minar P. Development of Infliximab Target Concentrations During Induction in Pediatric Crohn Disease Patients. J Pediatr Gastroenterol Nutr. 2019;69:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 113. | Choi SY, Kang B. Adalimumab in Pediatric Inflammatory Bowel Disease. Front Pediatr. 2022;10:852580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 114. | Rinawi F, Ricciuto A, Church PC, Frost K, Crowley E, Walters TD, Griffiths AM. Association of Early Postinduction Adalimumab Exposure With Subsequent Clinical and Biomarker Remission in Children with Crohn's Disease. Inflamm Bowel Dis. 2021;27:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 115. | Rosario M, French JL, Dirks NL, Sankoh S, Parikh A, Yang H, Danese S, Colombel JF, Smyth M, Sandborn WJ, Feagan BG, Reinisch W, Sands BE, Sans M, Fox I. Exposure-efficacy Relationships for Vedolizumab Induction Therapy in Patients with Ulcerative Colitis or Crohn's Disease. J Crohns Colitis. 2017;11:921-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 116. | Osterman MT, Rosario M, Lasch K, Barocas M, Wilbur JD, Dirks NL, Gastonguay MR. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: determining the potential for dose optimisation. Aliment Pharmacol Ther. 2019;49:408-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 117. | Pouillon L, Vermeire S, Bossuyt P. Vedolizumab trough level monitoring in inflammatory bowel disease: a state-of-the-art overview. BMC Med. 2019;17:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 118. | Schulze H, Esters P, Hartmann F, Stein J, Christ C, Zorn M, Dignass A. A prospective cohort study to assess the relevance of vedolizumab drug level monitoring in IBD patients. Scand J Gastroenterol. 2018;53:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Yacoub W, Williet N, Pouillon L, Di-Bernado T, De Carvalho Bittencourt M, Nancey S, Lopez A, Paul S, Zallot C, Roblin X, Peyrin-Biroulet L. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther. 2018;47:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 120. | Ungaro RC, Yarur A, Jossen J, Phan BL, Chefitz E, Sehgal P, Kamal K, Bruss A, Beniwal-Patel P, Fox C, Patel A, Bahur B, Jain A, Stein D, Naik S, Dubinsky MC. Higher Trough Vedolizumab Concentrations During Maintenance Therapy are Associated With Corticosteroid-Free Remission in Inflammatory Bowel Disease. J Crohns Colitis. 2019;13:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 121. | Dreesen E, Verstockt B, Bian S, de Bruyn M, Compernolle G, Tops S, Noman M, Van Assche G, Ferrante M, Gils A, Vermeire S. Evidence to Support Monitoring of Vedolizumab Trough Concentrations in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2018;16:1937-1946.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 122. | Rowland P, McNicol M, Kiel A, Maltz RM, Donegan A, Dotson JL, Michel HK, Boyle B. Proactive therapeutic drug monitoring and vedolizumab dose optimization in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2024;78:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 123. | Hemming-Harlo M, Merras-Salmio L, Nikkonen A, Kolho KL. Drug levels of VEDOLIZUMAB in patients with pediatric-onset inflammatory bowel disease in a real-life setting. Eur J Pediatr. 2024;183:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 124. | Liefferinckx C, Hubert A, Thomas D, Bottieau J, Minsart C, Cremer A, Amininejad L, Vallée F, Toubeau JF, Franchimont D. Predictive models assessing the response to ustekinumab highlight the value of therapeutic drug monitoring in Crohn's disease. Dig Liver Dis. 2023;55:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 125. | Hirayama H, Morita Y, Imai T, Takahashi K, Yoshida A, Bamba S, Inatomi O, Andoh A. Ustekinumab trough levels predicting laboratory and endoscopic remission in patients with Crohn's disease. BMC Gastroenterol. 2022;22:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 126. | Gómez Espín R, Nicolás De Prado I, Gil Candel M, González Carrión M, Rentero Redondo L, Iniesta Navalón C. Association between ustekinumab trough concentrations and biochemical outcomes in patients with Crohn's disease. A real life study. Rev Esp Enferm Dig. 2021;113:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 127. | Carman N, Mack DR, Benchimol EI. Therapeutic Drug Monitoring in Pediatric Inflammatory Bowel Disease. Curr Gastroenterol Rep. 2018;20:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 128. | Bouhuys M, Mian P, van Rheenen PF. Ustekinumab trough levels in children with Crohn's disease refractory to anti-tumor necrosis factor agents: a prospective case series of off-label use. Front Pharmacol. 2023;14:1180750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 129. | Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012;91:635-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 390] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 130. | Brandse JF, Mathôt RA, van der Kleij D, Rispens T, Ashruf Y, Jansen JM, Rietdijk S, Löwenberg M, Ponsioen CY, Singh S, van den Brink GR, D'Haens GR. Pharmacokinetic Features and Presence of Antidrug Antibodies Associate With Response to Infliximab Induction Therapy in Patients With Moderate to Severe Ulcerative Colitis. Clin Gastroenterol Hepatol. 2016;14:251-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 131. | Samuels A, Whaley KG, Minar P. Precision Dosing of Anti-TNF Therapy in Pediatric Inflammatory Bowel Disease. Curr Gastroenterol Rep. 2023;25:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 132. | Levitte S. Point-of-Care Assays for Infliximab Therapeutic Drug Monitoring in Patients with IBD: Is Quicker Better? Dig Dis Sci. 2024;69:5-6. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 133. | Nasser Y, Labetoulle R, Harzallah I, Berger AE, Roblin X, Paul S. Comparison of Point-of-Care and Classical Immunoassays for the Monitoring Infliximab and Antibodies Against Infliximab in IBD. Dig Dis Sci. 2018;63:2714-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 134. | Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013;108:1268-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 135. | Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Chen DM, Pritchard ML, Sandborn WJ. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 639] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 136. | Toruner M, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, Colombel JF, Egan LJ. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 762] [Article Influence: 42.3] [Reference Citation Analysis (1)] |
| 137. | Lemaitre M, Kirchgesner J, Rudnichi A, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Association Between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients With Inflammatory Bowel Disease. JAMA. 2017;318:1679-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 464] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 138. | Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, Germino R, Menon S, Sun Y, Wang C, Shapiro AB, Kanik KS, Connell CA; ORAL Surveillance Investigators. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med. 2022;386:316-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 1152] [Article Influence: 288.0] [Reference Citation Analysis (0)] |
| 139. | Louis E. Stopping Biologics in IBD-What Is the Evidence? Inflamm Bowel Dis. 2018;24:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 140. | Zhang B, Gulati A, Alipour O, Shao L. Relapse From Deep Remission After Therapeutic De-escalation in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J Crohns Colitis. 2020;14:1413-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 141. | Kobayashi T, Motoya S, Nakamura S, Yamamoto T, Nagahori M, Tanaka S, Hisamatsu T, Hirai F, Nakase H, Watanabe K, Matsumoto T, Tanaka M, Abe T, Suzuki Y, Watanabe M, Hibi T; HAYABUSA Study Group. Discontinuation of infliximab in patients with ulcerative colitis in remission (HAYABUSA): a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 142. | Louis E, Resche-Rigon M, Laharie D, Satsangi J, Ding N, Siegmund B, D'Haens G, Picon L, Bossuyt P, Vuitton L, Irving P, Viennot S, Lamb CA, Pollok R, Baert F, Nachury M, Fumery M, Gilletta C, Almer S, Ben-Horin S, Bouhnik Y, Colombel JF, Hertervig E; GETAID and the SPARE-Biocycle research group. Withdrawal of infliximab or concomitant immunosuppressant therapy in patients with Crohn's disease on combination therapy (SPARE): a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2023;8:215-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 143. | Buhl S, Steenholdt C, Brynskov J, Christensen KR, Dorn-Rasmussen M, Thomsen OØ, Bendtzen K, Klausen TW, Dahlerup JF, Thorsgaard N, Jahnsen J, Molazahi A, Pedersen N, Kjeldsen J, Almer S, Dahl EE, Vind I, Cannon AG, Marsal J, Sipponen T, Agnholt JS, Kievit HAL, Aure SL, Martinsen L, Meisner S, Hansen JM, Ainsworth MA. Discontinuation of Infliximab Therapy in Patients with Crohn's Disease. NEJM Evid. 2022;1:EVIDoa2200061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 144. | Chaparro M, García Donday M, Riestra S, Lucendo AJ, Benítez JM, Navarro-llavat M, Barrio J, Morales-alvarado VJ, Rivero M, Busquets D, Leo Carnerero E, Merino Ochoa O, Nantes Castillejo O, Navarro P, Van Domselaar M, Gutiérrez Casbas A, Alonso-abreu I, Mejuto R, Fernández Salazar L, Iborra M, Martín-arranz MD, Pineda JR, Sampedro MJ, Serra Nilsson K, Bouhmidi Assakali A, Batista L, Muñoz Villafranca C, Rodríguez-lago I, Ceballos Santos DS, Guerra I, Mañosa M, Marín Jimenez I, Vera Mendoza I, Barreiro-de Acosta M, Domènech E, Esteve M, García-sánchez V, Nos P, Panés J, Gisbert JP. OP37 Is the withdrawal of anti-tumour necrosis factor in inflammatory bowel disease patients in remission feasible without increasing the risk of relapse? Results from the randomised clinical trial of GETECCU (EXIT). J Crohns Colitis. 2023;17:i50-i54. [DOI] [Full Text] |
| 145. | Wynands J, Belbouab R, Candon S, Talbotec C, Mougenot JF, Chatenoud L, Schmitz J, Cézard JP, Goulet O, Hugot JP, Ruemmele FM. 12-month follow-up after successful infliximab therapy in pediatric crohn disease. J Pediatr Gastroenterol Nutr. 2008;46:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 146. | Jeong TJ, Kim ES, Kwon Y, Kim S, Seo SW, Choe YH, Kim MJ. Discontinuation of Azathioprine could be considered in pediatric patients with Crohn's disease who have sustained clinical and deep remission. Sci Rep. 2022;12:507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 147. | Miyatani Y, Kobayashi T. Evidence-Based Approach to the Discontinuation of Immunomodulators or Biologics in Inflammatory Bowel Disease. Digestion. 2023;104:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 148. | Stoker AMH, Logghe L, van der Ende-van Loon MCM, Schoon EJ, Schreuder RM, Stronkhorst A, Gilissen LPL. Relapse rates after withdrawal versus maintaining biologic therapy in IBD patients with prolonged remission. Clin Exp Med. 2023;23:2789-2797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 149. | Selinger CP, Rosiou K, Lenti MV. Biological therapy for inflammatory bowel disease: cyclical rather than lifelong treatment? BMJ Open Gastroenterol. 2024;11:e001225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 150. | Gisbert JP, Marín AC, Chaparro M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment Pharmacol Ther. 2015;42:391-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 151. | Noor NM, Sousa P, Bettenworth D, Gomollón F, Lobaton T, Bossuyt P, Casanova MJ, Ding NS, Dragoni G, Furfaro F, van Rheenen PF, Chaparro M, Gisbert JP, Louis E, Papamichail K. ECCO Topical Review on Biological Treatment Cycles in Crohn's Disease. J Crohns Colitis. 2023;17:1031-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 152. | Banerjee R, Pal P, Mak JWY, Ng SC. Challenges in the diagnosis and management of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol Hepatol. 2020;5:1076-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 153. | Fehily SR, Al-Ani AH, Abdelmalak J, Rentch C, Zhang E, Denholm JT, Johnson D, Ng SC, Sharma V, Rubin DT, Gibson PR, Christensen B. Review article: latent tuberculosis in patients with inflammatory bowel diseases receiving immunosuppression-risks, screening, diagnosis and management. Aliment Pharmacol Ther. 2022;56:6-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 154. | Ooi CJ, Hilmi I, Banerjee R, Chuah SW, Ng SC, Wei SC, Makharia GK, Pisespongsa P, Chen MH, Ran ZH, Ye BD, Park DI, Ling KL, Ong D, Ahuja V, Goh KL, Sollano J, Lim WC, Leung WK, Ali RAR, Wu DC, Ong E, Mustaffa N, Limsrivilai J, Hisamatsu T, Yang SK, Ouyang Q, Geary R, De Silva JH, Rerknimitr R, Simadibrata M, Abdullah M, Leong RW; Asia Pacific Association of Gastroenterology (APAGE) Working Group on Inflammatory Bowel Disease and Asian Organization for Crohn’s and Colitis. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn's disease in Asia. Intest Res. 2019;17:285-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 155. | Turner D, Griffiths AM, Wilson D, Mould DR, Baldassano RN, Russell RK, Dubinsky M, Heyman MB, de Ridder L, Hyams J, Martin de Carpi J, Conklin L, Faubion WA, Koletzko S, Bousvaros A, Ruemmele FM. Designing clinical trials in paediatric inflammatory bowel diseases: a PIBDnet commentary. Gut. 2020;69:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |