Published online Dec 9, 2023. doi: 10.5409/wjcp.v12.i5.263
Peer-review started: July 25, 2023
First decision: August 31, 2023
Revised: October 5, 2023
Accepted: October 16, 2023
Article in press: October 16, 2023
Published online: December 9, 2023
Processing time: 136 Days and 2.1 Hours
Prediabetes, the precursor of type 2 diabetes mellitus, is an intermediate stage between normal glucose homeostasis and overt diabetes. This asymptomatic metabolic state is increasingly prevalent in pediatric population and is very difficult to detect without appropriate screening. Studies have shown that a certain proportion of children with prediabetes will develop diabetes in a few years. Even more alarming is the evidence that youth-onset diabetes has a more aggressive clinical course with progressive beta-cell decline and accelerated end-organ damage. Despite its importance, several aspects involving prediabetes in childhood are disputed or unknown. This review presents the latest insights into this challenging entity and outlines a simplified screening approach to aid clinical practice. In summary, childhood prediabetes is an important clinical condition indicating the need for proper screening and timely intervention.
Core Tip: Prediabetes, an intermediate stage before type 2 diabetes mellitus, has increased in parallel with the growing burden of pediatric obesity worldwide. However, child health practitioners are struggling with the definition, significance, diagnostic approach, trajectories, implications, outcomes, and management of prediabetes. This review aims to provide pediatricians and primary care providers with an updated overview of this important, yet controversial, condition.
- Citation: Ng HY, Chan LTW. Prediabetes in children and adolescents: An updated review. World J Clin Pediatr 2023; 12(5): 263-272
- URL: https://www.wjgnet.com/2219-2808/full/v12/i5/263.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v12.i5.263
Childhood obesity has long been a public health challenge worldwide and has emerged as one of the most significant concerns. It poses an enormous health burden. The prevalence of childhood obesity has increased exponentially in recent years. This is further exacerbated by the novel coronavirus disease 2019 (COVID-19) pandemic, which negatively impacted the lifestyle and nutritional habits of children[1-3].
Prediabetes is a prominent clinical condition characterized by asymptomatic prodromal phase before the onset of diabetes mellitus. In adult population, prediabetes is considered a precursor to type 2 diabetes mellitus (T2DM)[4]. It is therefore tempting to infer a dramatic rise in prediabetes among pediatric population, given the increase in the prevalence of both obesity and T2DM in childhood[5]. A recent systematic review and meta-analysis showed a rapid increase in the prevalence of prediabetes in children globally. The pooled prevalence of 48 community-based studies was up to 8.84%[6]. Notably, prediabetes in children is associated with youth-onset T2DM, which is regarded as a more aggressive entity with increased cardiovascular and metabolic risk[7,8]. Significant damage to beta-cells may also occur prior to the development of dysglycemia[9]. The various adverse health effects in adulthood can be traced to prediabetes in childhood[10]. Fortunately, the occurrence and progression of dysglycemia in T2DM is more insidious compared with type 1 diabetes mellitus (T1DM) allowing more time for prevention and intervention.
Early diagnosis and management via screening represents a unique opportunity to intervene and is both logical and appealing. However, our current understanding of childhood prediabetes is mainly based on studies involving adult population and is poorly characterized[10]. The diagnosis and management of this important clinical condition is still imperfect, and is often disputed and debated. Thus, the need for a literature review to identify the latest evidence, insight and knowledge gaps cannot be overstated.
With this background, a comprehensive literature search was conducted in an effort to provide an overview of current understanding regarding prediabetes. It covers the latest epidemiology, diagnostic means, related controversies, and the need for future research. The span of our review is limited to articles published within the last 10 years in an effort to provide pediatricians and primary care providers with recent updates of this complex yet important condition.
For this narrative review, a literature search was conducted using MEDLINE, EMBASE, RCA, and Google Scholar databases. Search terms included “prediabetes”, “hyperglycemia”, “dysglycemia”, “abnormal glucose homeostasis”, “children”, and “adolescents”. Articles published between January 2013 to March 2023 were considered with the exception of landmark studies or articles. Additional publications were also retrieved by snowballing.
Specifically, articles reporting prediabetic children younger than 18 years old were reviewed, with full-text available in English. Exclusion criteria included T1DM (autoimmune β-cell destruction), gestational diabetes mellitus (GDM), and other specific types of diabetes, such as monogenic diabetes syndromes, pancreatogenic diabetes, and drug-induced diabetes[11].
Childhood obesity has evolved into a major public health crisis both in developed and developing countries[12-14]. Globally, studies showed a high level of obesity and a rising trend particularly in low-and middle-income countries[15]. The United Nations Children’s Fund estimated that 380 million children below 19 years of age were overweight, with the rate increasing to 18% in 2018 from 10% in 2000 among 5- to 19-year-old individuals[16].
The situation is further complicated by the unprecedented public health challenge due to coronavirus disease 2019 (COVID-19) pandemic. Response to global COVID-19 pandemic by decision-makers had a further impact on the obesity landscape as more than 80% of children worldwide experienced school closures, movement restrictions, physical inactivity, and drastic changes to their way of life[13,17,18]. These changes in lifestyle, daily routines, and nutritional habits contributed to weight gain[3]. Consequently, a significant rise in childhood obesity is imminent and inevitable[19,20].
Recently, two systemic reviews and meta-analyses demonstrated a significant increase in weight gain, body mass index (BMI), and prevalence of obesity in children during the COVID-19 pandemic[21,22]. In another study evaluating the net impact of the COVID-19 Lockdown, Dietz[23] reported that the changes in obesity prevalence among children aged below 12 years were 28- to 37-fold higher than the annual expected changes observed in the National Health and Nutrition Examination Survey of United States. Particularly, the highest weight gain observed among youth with severe obesity was a cause for serious concern[24]. As the obesity rates and levels continue to rise in childhood, the prevalence of prediabetes and diabetes in children also increases rapidly with alarming trends worldwide[6].
In brief, the pathophysiology of T2DM involves insulin resistance (IR) accompanied by insufficient insulin release[8,9,25]. Clinical signs suggesting insulin resistance, such as acanthosis nigricans, are risk factors indicated in various guidelines. Acanthosis nigricans is closely associated with insulin resistance and provides a prominent visual cue that can aid in early intervention[25-28]. Although the underlying risk for IR is not completely understood, genetic and environmental factors are largely implicated[8,25].
Youth-onset T2DM is a more aggressive disease with rapid deterioration of beta-cell function and poor response to treatment. Eventually, it progresses to complications more rapidly and earlier than in adult-onset T2DM, impacting the most productive years of life[29-33]. A substantial number of patients with youth-onset T2DM exhibit micro-vascular and macro-vascular complications in the early stages of the disease, suggesting prior ongoing vascular damage[34]. In an observational study of 500 cases of youth-onset T2DM conducted over 10 years, the cumulative incidence of hyper
Prediabetes is a condition that is characterized by dysregulated glucose homeostasis[25]. Advocating prediabetes as a distinct pathological condition is controversial despite its recent inclusion in the ICD-10 coding[37,38]. While some authors caution against medicalization of prediabetes[39,40], others believe that it is essential and helpful to encourage positive lifestyle changes[41,42]. Emerging evidence suggests that individuals with prediabetes have pathophysiological changes in organs that are traditionally affected by diabetes, further validating it as a distinct disease entity[37].
For decades, the global prevalence of prediabetes in children was largely unknown. A recent systematic review and meta-analysis of 6630296 participants from 48 community-based pediatric studies found that the pooled prevalence of childhood prediabetes was 8.84% [95%CI (6.74, 10.95)] using a random-effects model. However, these data should be interpreted with caution given the heterogeneity of included studies, potential publication bias, and limited comparability based on different definitions and study designs[6]. Generally, the prevalence of prediabetes is substantially higher in the cohort targeting children with obesity. In an Italian study, the prevalence was 21.1%[43]. Our group demonstrated a prevalence of 15.4% in 879 Chinese pediatric patients from Hong Kong[44]. Another study conducted in German-speaking countries reported a prevalence of 11.9%[45]. Prevalence rate increases with age or deteriorating weight status[43,44].
Despite the large number of studies involving T2DM in children, little is known about the natural history of prediabetes, which is an intermediate stage along the continuum of normal glucose regulation to overt diabetes[10,46-48]. Using data from a cohort of White Canadian children with a parental history of obesity, Harnois-Leblanc et al[49] reported that 73% of children with prediabetes at baseline (8-10 years of age) reverted to normoglycemia by the end of adolescence. In contrast, only 53% of children with prediabetes detected at 10-12 years of age reverted to normoglycemia at 15-17 years of age. However, it should be noted that their complete cohort (with complete 7-year data covering the three evaluations) consisted of 350 children, including those with normal weight. Hence, the prevalence of dysglycemia was only 10% at baseline and first evaluation. Indeed, what is more alarming is that one in five children (21%) in their cohort, recruited based on parental history of obesity, developed prediabetes or diabetes over 7 years.
In another multiethnic, prospective observational study carried out in the United States, 526 adolescents with obesity completed two evaluations with a median follow-up of 2.9 years. Galderisi, Giannini[50] reported that 65% adolescents with dysglycemia at baseline (n = 162) reverted to normal glycemia. Notably, the remaining 27% showed persistent dysglycemia and 8% progressed to T2DM. One of the strengths of their study was confirmation of T2DM with a second oral glucose tolerance test to eliminate any reproducibility issue. Although it was an observational study, the standard of care during follow-up included dietary assessment and advice every 6 mo, suggestion to limit sugary drinks and screen time, and promotion of physically active lifestyle.
A recent search of PubMed/ MEDLINE and the Cochrane Library for articles published through May 3, 2021 by the United States Preventive Services Task Force (USPSTF) revealed few studies suggesting that 22% to 52% children and adolescents with prediabetes returned to normal glycemia without intervention over 6 mo to 2 years[51].
Understanding the natural history of disease is critical to recognizing and responding to preventive efforts. It offers a framework to conceptualize the illness and preventive strategies. In a strict sense, the natural history of a disease refers to the natural progression over time without any treatment or intervention. In modern medicine, this is constructed from multiple sources to form a composite clinical picture of underlying disease dynamics[41].
Outlining the real natural progression of prediabetes in children is of great interest to clinicians. However, children with prediabetes are mostly asymptomatic and cannot usually be identified. If they are screened due to obesity, health care providers are obligated to provide appropriate advice regarding dietary and lifestyle intervention. Even in a research setting, children and family are not blinded to their blood test results because it is not ethical to do so. Under such circumstances, they may exert substantial efforts to prevent further progression[34]. Simply informing participants of their abnormal results, even without intervention, can improve their dysglycemia[52]. This argument is supported by a retrospective cohort study in United States. Using data from the Children’s Hospital of Philadelphia Primary Care Network, Vajravelu et al[53] found a stable BMI Z-score trajectory in all adolescents screened for prediabetes comparing with unscreened individuals. The improvement was even more striking among youth testing positive for prediabetes, suggesting that screening may have an important role in motivating the youth to take appropriate measures to diminish the risk. Indeed, screening and education about prediabetes can improve follow-up rates[54]. Therefore, caution is necessary when interpretating and extrapolating clinical research findings. Also, participants included in the analysis of the “natural course” of disease are those attended follow-up, and with complete data available. It is well known that a large number of children and adolescents are lost to follow-up, even after they are diagnosed with T2DM[36,55,56]. It is inaccurate or misleading to assume that more than half of these dysglycemic children will revert to normal glycemia eventually.
Early detection and timely intervention of dysglycemia can delay or prevent microvascular complications in adults[48]. While screening for prediabetes and T2DM in adults is considered cost-effective, it is highly complicated in children[57-60]. The latest USPSTF concluded that evidence to recommend screening for prediabetes in asymptomatic children and adolescents is unavailable[61]. Explicitly, their position is neither for nor against screening prediabetes. Pediatricians and health care providers should continue to use their clinical judgement in deciding whether or not screening is warranted[61,62].
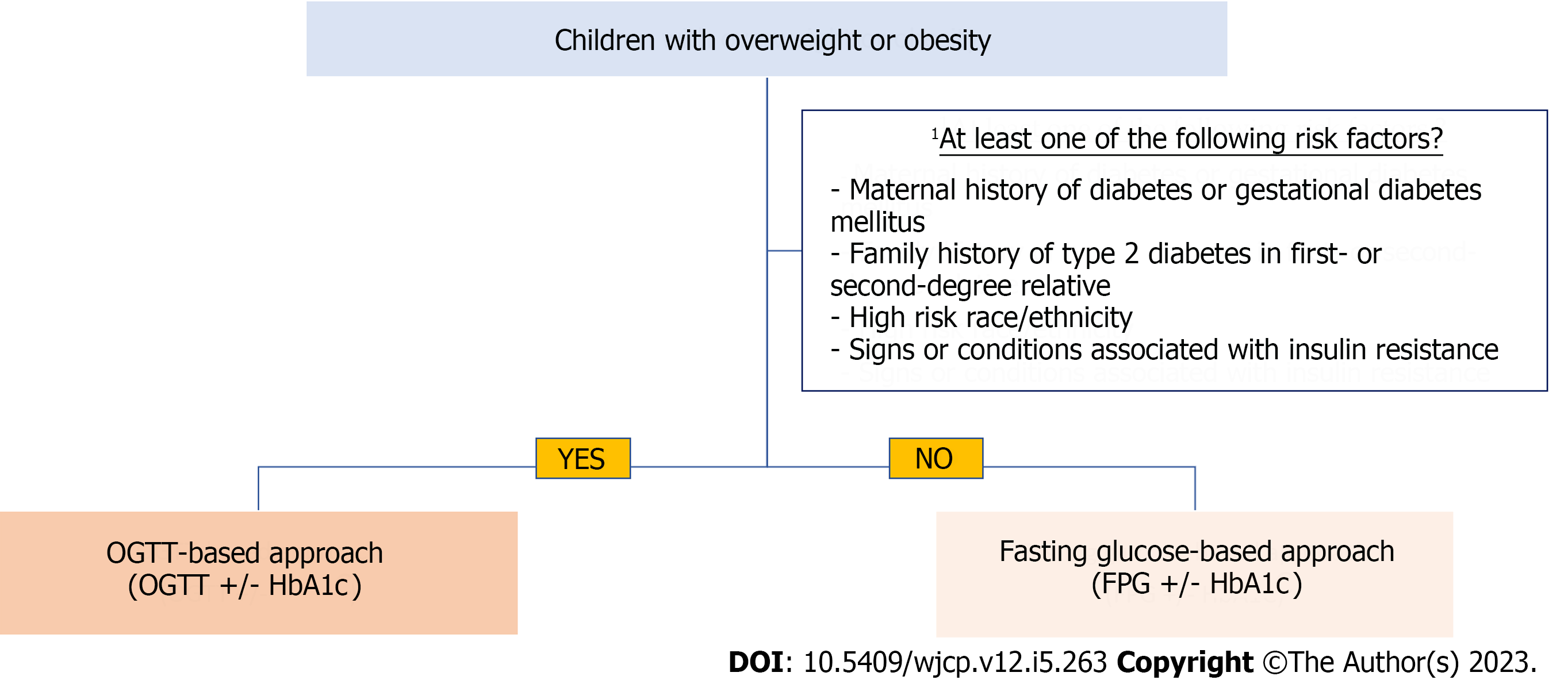
Going back to year 2000, a consensus group of expert representatives from American Academy of Pediatrics (AAP) and the American Diabetes Association (ADA) first recommended screening of asymptomatic youth carrying at least two risk factors[63,64]. In view of persistent surge in prevalence and incidence of prediabetes, the ADA expanded its recommendation to include youth with only one risk factor in 2018[65]. In the latest publication, ADA continuously recommends screening of high-risk children and adolescents. Screening should be carefully considered in children and youth with overweight or obesity who have at least one of the following risk factors: maternal diabetes or GDM; family history of T2DM; vulnerable race or ethnicity; and signs or conditions associated with insulin resistance. Screening should be started when they turn 10 years of age, or after the onset of puberty, whichever occurs first. Testing should be repeated in cases of deteriorating BMI or risk factor profiles, or at a minimum of 3-year intervals[11]. Currently, the International Society for Pediatric and Adolescent Diabetes (ISPAD) recommends risk-based screening, which is largely similar to the one recommended by ADA[48,66]. By adopting such strategy, it is hoped that early diagnosis can enable early interventions to slow down or prevent disease progression[57,67,68]. In a recent study, the prevalence of dysglycaemia was found to be 23% with individuals carrying only one risk factor referred for assessment in an academic center, suggesting that a single risk factor is sufficient to warrant screening[69].
Despite most authorities proposing risk-based screening, the optimal or the best strategy remains a matter of debate[43,62]. According to the latest ADA and ISPAD recommendations, fasting plasma glucose (FPG), 2-h plasma glucose level measured during oral glucose tolerance test (OGTT), and hemoglobin A1c (HbA1c) can be used to diagnose prediabetes and diabetes in childhood and adolescence[48,70]. Notably, studies reveal an overlap among the subgroups using different diagnostic tests and criteria. The three different tests cannot identify consistently the same group of individuals[43,71]. Indeed, it is now believed that each individual test may analyze different components of glucose metabolism[7,72]. This may complicate the understanding and comparison of this condition in different clinical studies[73]. The prevalence of prediabetes will differ largely if disparate test combinations are used[6,62], and the discussion is further complicated by the different impaired fasting glucose cutoffs adopted by international organizations[73-76]. Additionally, the cutoff thresholds used are derived and adopted from adult studies instead of longitudinal prospective studies involving children and adolescents. The suitability of these criteria will remain a matter of debate for years to come[48,62].
In accordance with ADA, prediabetes should be viewed as risk factor for developing diabetes and cardiovascular disease. The risk starts below the lower end of the reference range and increases largely toward the higher end of the range, and is continuous[75]. Despite its reversibility in some children, prediabetes suggests that the beta-cell function is at its maximal capacity, predisposing to future failure[77].
HbA1c: According to ADA, an HbA1c value of 5.7%-6.4% has been used to define prediabetes in children[75]. It is an indicator of the average blood glucose concentration over the past three months. This test should be performed using a method that is certified by the National Glycohemoglobin Standardization Program to minimize bias[78]. It has the advantages of being stable at room temperature, without the need for fasting, and is associated with minimal day-to-day variations. Nonetheless, ethnic, racial, and age differences in levels of HbA1c exist. Medical conditions, such as anemia, hemoglobinopathies, malaria, and post transfusion, which affect red cell turnovers can affect its validity. Various medications and supplements may also interfere with the assay and alter the value[72,79,80]. It is also noteworthy that the use of HbA1c in children remains controversial[79,81]. The recommendation in adult population is based on epidemiological studies[75]. Some pediatric studies using adult cutoff underestimated the prevalence of diabetes and prediabetes[79,82]. In a study involving Caribbean and African-American children with obesity, investigators found that HbA1c alone, using adult cutoff value, is not a good differentiator of dysglycemia[83]. In recent studies from different countries and jurisdictions, various HbA1c values had been suggested[81,82,84]. This is not surprising as HbA1c is known to depend on age, race, and ethnicity. Further, in a large cohort of ethnically and racially diverse youth (n = 4603) who have normal weight and are otherwise healthy, researchers found that 2.2% have HbA1c values exceeding ADA cutoff, which prompted clinicians to apply and adopt the cutoff value cautiously[48,85]. In sum, the optimal operational HbA1c cutoff in children remains uncertain and requires further study.
FPG and OGTT: FPG has been included as a screening test for dysglycemia in a majority of guidelines regarding management of youth with obesity[86]. It requires a single blood test and is easily available in all laboratories. However, it requires fasting and the result is affected by illness, stress, and time of the day[62,87]. Besides, it is not capable of detecting impaired glucose tolerance (IGT), which is common in children with prediabetes.
OGTT has been considered the “gold standard” for many decades although it has disadvantages of fasting requirement, complicated testing logistics, and reproducibility issues[72,85,88,89]. Although not ideal, it is the only test to assess post-prandial hyperglycemia[77]. Clinically, some individuals may have hyperglycemia only if challenged with a glucose load[48]. If OGTT is not done, half of children with prediabetes were missed in a Korean study[81]. We also demonstrated that 73% of children with prediabetes or diabetes were left out in a large cohort of Chinese Hong Kong Children. IGT is related to insulin resistance in the muscle and defective insulin secretion[90]. This phenotype was found to be associated with a worse cardiometabolic profile[91-93] and a high risk of developing T2DM and cardiovascular disease[57,62,93]. Contrary to usual belief, OGTT was well tolerated in our cohort of children and adolescents with more than 99.8% success rate[44]. Accordingly, it is suggested as the preferred screening method by some experts[94].
Additional parameters, or morphological features, can be obtained during OGTT at the expense of multiple venipunctures. These include but not limited to 1-hour glucose concentration, glucose response curve, and time to glucose peak. They are being investigated as a tool for prediabetes risk stratification. Nevertheless, further research and longitudinal studies are needed before their clinical utility can be considered[62,94-96].
Alternative tests or approaches: Instead of using OGTT, various studies have attempted to use a combination of blood tests or parameters, in an attempt to detect prediabetes. Combining fasting glucose with homeostatic model assessment of insulin resistance (HOMA-IR) cutoff of 3.4, van der Aa, Fazeli Farsani[97] detected all cases of diabetes while missing 36% of IGT. Poon et al[98] derived a clinical pathway using family history, HbA1c, and alanine transaminase. They omitted 50% of OGTTs, but 18.3% of children with dysglycemia were overlooked. Alternative glycemic markers, such as 1,5-anhydroglucitol, glycated albumin, and fructosamine, have been studied as screening tools. However, relevant and meaningful cutoff values associated with long-term risk and complications are still under investigation in pediatric population[62,99]. With the advent of diabetes technology, continuous glucose monitoring (CGM) is more capable of capturing detailed information and parameters of glucose fluctuations. There has been a growing interest in applying CGM technology in non-diabetic individuals[100]. However, its use in predicting prediabetes is still exploratory and preliminary[101].
Even though some management algorithms are reported in the literature, there is no consensus on the optimal screening approach for prediabetes and diabetes in children with obesity[10]. Magge et al[80] proposed a management algorithm for screening of high-risk youth. However, it is based on the definition of high risk as two or more risk factors instead of the 2018 ADA recommendation of a single risk factor or more. Nonetheless, it is complicated for daily clinical use. In a recent review article, Garonzi et al[62] proposed a flowchart based on the strengths and weaknesses of different screening tests, suggesting screening of children and adolescents with overweight or obesity using FPG and HbA1c. In case of abnormal findings, OGTT was suggested. Likewise, OGTT was recommended for high-risk children (with one or more risk factors).
To further simplify and streamline the screening process, we suggest a fasting glucose-based approach for overweight and obese children. An OGTT-based approach is warranted in the presence of risk factors suggested by the ADA. HbA1c is considered optional in both approaches as there is no evidence-based operational cutoff value. Limited data support the use of HbA1c in children and adolescents. Figure 1 outlines the simplified framework, as a starting point, for laboratory assessment.
Presumably, early identification of children at risk enables practitioners to intervene and interrupt the progression toward diabetes[102]. In the absence of consensus regarding optimal management of children with prediabetes, lifestyle interventions are still the cornerstone in this population[7,28]. A balanced diet consisting of adequate fruits and vegetables, less sugar and processed foods is key. Home-cooked meals are preferred to dining out. Regular daily exercise with limitation of screen time should be reinforced. Innovative strategies for patient education should be explored so that knowledge can be translated into behavioral changes[8,103,104].
Currently, there is no United States Food and Drug Administration (FDA)-approved pharmacologic agent for prediabetes in children. Nevertheless, metformin has been used off-label in pediatric weight-management programs for children with prediabetes and insulin resistance. It is relatively well tolerated with gastrointestinal intolerability being the most common side effect[34,55]. Lactic acidosis is rare and can be monitored during treatment[34,105]. Proponents suggest metformin as a second-line management in those refractory to lifestyle interventions[34]. Liraglutide, a glucagon-like peptide-1 receptor agonist, was approved by the FDA in 2019 for use in childhood T2DM[106]. It may improve beta-cell mass and function and represents a potential treatment for prediabetes in future[56,107].
The recent USPSTF attempted to search for direct evidence supporting screening of asymptomatic children for prediabetes and T2DM to improve health outcomes. However, their commissioned review found insufficient evidence to assess any benefits or harms of screening, mainly, due to a lack of studies[51,60,61]. The lack of prospective long-term longitudinal data to inform evidence-based practice for disease prevention and complication avoidance is the real challenge and major gap in pediatric prediabetes research. Clinical trials of pharmaceutical agents face the challenge of inadequate number of participants[108]. Further, the use of different screening tests and cutoff values in studies has led to discrepant results in different race and ethnicity. A “one-size-fits-all” approach may not be the best, suggesting the need for further validation[72]. Additionally, randomized controlled trials are urgently needed to evaluate the effectiveness of various preventive and management options in prediabetes[109].
Prediabetes is increasingly common in childhood and frequently goes unnoticed. It remains a challenging entity facing child health practitioners. Traditionally, it is diagnosed using adult criteria, which may not be readily applicable for children. This extrapolation of adult data is problematic and results in controversial and questionable approaches to prognosis, diagnostic criteria, investigation strategies, and management. The latest AAP guideline alerts pediatricians and healthcare providers to be aware of the pros and cons of each test based on clinical context, patient preferences, and accessibility issues[110]. Apparently, until effective prevention measures for childhood obesity can be found, managing and reversing the growing crisis of diabetes and prediabetes is still a major challenge.
| 1. | Al-Agailat L, Littlejohn E. Emerging Pediatric Obesity Epidemic with the COVID-19 Pandemic as an Influence. Pediatr Ann. 2023;52:e48-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Browne NT, Snethen JA, Greenberg CS, Frenn M, Kilanowski JF, Gance-Cleveland B, Burke PJ, Lewandowski L. When Pandemics Collide: The Impact of COVID-19 on Childhood Obesity. J Pediatr Nurs. 2021;56:90-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 3. | Palermi S, Vecchiato M, Pennella S, Marasca A, Spinelli A, De Luca M, De Martino L, Fernando F, Sirico F, Biffi A. The Impact of the COVID-19 Pandemic on Childhood Obesity and Lifestyle-A Report from Italy. Pediatr Rep. 2022;14:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Xu XY, Leung AYM, Smith R, Wong JYH, Chau PH, Fong DYT. The relative risk of developing type 2 diabetes among individuals with prediabetes compared with individuals with normoglycaemia: Meta-analysis and meta-regression. J Adv Nurs. 2020;76:3329-3345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Perng W, Conway R, Mayer-Davis E, Dabelea D. Youth-Onset Type 2 Diabetes: The Epidemiology of an Awakening Epidemic. Diabetes Care. 2023;46:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (2)] |
| 6. | Han C, Song Q, Ren Y, Chen X, Jiang X, Hu D. Global prevalence of prediabetes in children and adolescents: A systematic review and meta-analysis. J Diabetes. 2022;14:434-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 7. | Serbis A, Giapros V, Kotanidou EP, Galli-Tsinopoulou A, Siomou E. Diagnosis, treatment and prevention of type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2021;12:344-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (12)] |
| 8. | Valaiyapathi B, Gower B, Ashraf AP. Pathophysiology of Type 2 Diabetes in Children and Adolescents. Curr Diabetes Rev. 2020;16:220-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Esquivel Zuniga R, DeBoer MD. Prediabetes in Adolescents: Prevalence, Management and Diabetes Prevention Strategies. Diabetes Metab Syndr Obes. 2021;14:4609-4619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Nwosu BU. The Progression of Prediabetes to Type 2 Diabetes in Children and Adolescents in the United States: Current Challenges and Solutions. Endocrines. 2022;3:545-551. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1609] [Article Influence: 536.3] [Reference Citation Analysis (70)] |
| 12. | Lee EY, Yoon KH. Epidemic obesity in children and adolescents: risk factors and prevention. Front Med. 2018;12:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 13. | The Lancet Diabetes Endocrinology. Childhood obesity: a growing pandemic. Lancet Diabetes Endocrinol. 2022;10:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Apperley LJ, Blackburn J, Erlandson-Parry K, Gait L, Laing P, Senniappan S. Childhood obesity: A review of current and future management options. Clin Endocrinol (Oxf). 2022;96:288-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Aris IM, Block JP. Childhood Obesity Interventions-Going Beyond the Individual. JAMA Pediatr. 2022;176:e214388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Vazquez CE, Cubbin C. Socioeconomic Status and Childhood Obesity: a Review of Literature from the Past Decade to Inform Intervention Research. Curr Obes Rep. 2020;9:562-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 17. | Van Lancker W, Parolin Z. COVID-19, school closures, and child poverty: a social crisis in the making. Lancet Public Health. 2020;5:e243-e244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 560] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 18. | Saliba K, Cuschieri S. Amidst the COVID-19 pandemic childhood obesity is still an epidemic-spotlight on obesity's multifactorial determinants. Health Sci Rev (Oxf). 2021;1:100006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Prosperi S, Chiarelli F. COVID-19 and diabetes in children. Ann Pediatr Endocrinol Metab. 2022;27:157-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 20. | Hauerslev M, Narang T, Gray N, Samuels TA, Bhutta ZA. Childhood obesity on the rise during COVID-19: A request for global leaders to change the trajectory. Obesity (Silver Spring). 2022;30:288-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Anderson LN, Yoshida-Montezuma Y, Dewart N, Jalil E, Khattar J, De Rubeis V, Carsley S, Griffith LE, Mbuagbaw L. Obesity and weight change during the COVID-19 pandemic in children and adults: A systematic review and meta-analysis. Obes Rev. 2023;24:e13550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 22. | Chang TH, Chen YC, Chen WY, Chen CY, Hsu WY, Chou Y, Chang YH. Weight Gain Associated with COVID-19 Lockdown in Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 23. | Dietz WH. The COVID-19 lckdown increased obesity disparities; will the increases in type 2 diabetes continue? Obesity (Silver Spring). 2023;31:699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 24. | Eneli I, Xu J, Pratt K. Change in weight category among youth early in the COVID-19 pandemic. Clin Obes. 2022;12:e12522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Marcus C, Danielsson P, Hagman E. Pediatric obesity-Long-term consequences and effect of weight loss. J Intern Med. 2022;292:870-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 26. | Ng HY. Acanthosis nigricans in obese adolescents: prevalence, impact, and management challenges. Adolesc Health Med Ther. 2017;8:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Ng HY, Young JH, Huen KF, Chan LT. Acanthosis nigricans in obese Chinese children. Hong Kong Med J. 2014;20:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Gunaratne N, Deplewski D. Metabolic Consequences of Pediatric Obesity: A Review of Pathophysiology, Screening, and Treatment. Pediatr Ann. 2023;52:e62-e67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and Management of Youth-Onset Type 2 Diabetes: A Position Statement by the American Diabetes Association. Diabetes Care. 2018;41:2648-2668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 30. | Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol. 2020;16:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 318] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 31. | Barrett T, Jalaludin MY, Turan S, Hafez M, Shehadeh N; Novo Nordisk Pediatric Type 2 Diabetes Global Expert Panel. Rapid progression of type 2 diabetes and related complications in children and young people-A literature review. Pediatr Diabetes. 2020;21:158-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Bjornstad P, Chao LC, Cree-Green M, Dart AB, King M, Looker HC, Magliano DJ, Nadeau KJ, Pinhas-Hamiel O, Shah AS, van Raalte DH, Pavkov ME, Nelson RG. Youth-onset type 2 diabetes mellitus: an urgent challenge. Nat Rev Nephrol. 2023;19:168-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 33. | Song SH. Prediabetes in youth: an opportunity to make a difference. Lancet Child Adolesc Health. 2018;2:693-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Hosey CM, Halpin K, Yan Y. Considering metformin as a second-line treatment for children and adolescents with prediabetes. J Pediatr Endocrinol Metab. 2022;35:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | TODAY Study Group; Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, Tesfaldet B, Tryggestad J, White NH, Zeitler P. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med. 2021;385:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 379] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 36. | Karavanaki K, Paschou SA, Tentolouris N, Karachaliou F, Soldatou A. Type 2 diabetes in children and adolescents: distinct characteristics and evidence-based management. Endocrine. 2022;78:280-295. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Rett K, Gottwald-Hostalek U. Understanding prediabetes: definition, prevalence, burden and treatment options for an emerging disease. Curr Med Res Opin. 2019;35:1529-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Hostalek U. Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol. 2019;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 253] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 39. | Mittal M, Jethwani P, Naik D, Garg MK. Non-medicalization of medical science: Rationalization for future. World J Methodol. 2022;12:402-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Brodersen J, Schwartz LM, Heneghan C, O'Sullivan JW, Aronson JK, Woloshin S. Overdiagnosis: what it is and what it isn't. BMJ Evid Based Med. 2018;23:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 41. | White F. Application of Disease Etiology and Natural History to Prevention in Primary Health Care: A Discourse. Med Princ Pract. 2020;29:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Kale MS, Korenstein D. Overdiagnosis in primary care: framing the problem and finding solutions. BMJ. 2018;362:k2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 43. | Pedicelli S, Fintini D, Ravà L, Inzaghi E, Deodati A, Spreghini MR, Bizzarri C, Mariani M, Cianfarani S, Cappa M, Manco M. Prevalence of prediabetes in children and adolescents by class of obesity. Pediatr Obes. 2022;17:e12900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Law VYH, Young JHM, Ng HY, Chan LTW. Abnormal glucose tolerance in children: oral glucose tolerance test is fit-for-purpose. Explor Med. 2023:235-245. [DOI] [Full Text] |
| 45. | Koutny F, Weghuber D, Bollow E, Greber-Platzer S, Hartmann K, Körner A, Reinehr T, Roebl M, Simic-Schleicher G, Wabitsch M, Widhalm K, Wiegand S, Holl RW. Prevalence of prediabetes and type 2 diabetes in children with obesity and increased transaminases in European German-speaking countries. Analysis of the APV initiative. Pediatr Obes. 2020;15:e12601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Echouffo-Tcheugui JB, Perreault L, Ji L, Dagogo-Jack S. Diagnosis and Management of Prediabetes: A Review. JAMA. 2023;329:1206-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 284] [Article Influence: 94.7] [Reference Citation Analysis (1)] |
| 47. | Echouffo-Tcheugui JB, Selvin E. Prediabetes and What It Means: The Epidemiological Evidence. Annu Rev Public Health. 2021;42:59-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 48. | Shah AS, Zeitler PS, Wong J, Pena AS, Wicklow B, Arslanian S, Chang N, Fu J, Dabadghao P, Pinhas-Hamiel O, Urakami T, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2022: Type 2 diabetes in children and adolescents. Pediatr Diabetes. 2022;23:872-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 49. | Harnois-Leblanc S, Van Hulst A, Ybarra M, Barnett TA, Mathieu MÈ, McGrath JJ, Tremblay A, Paradis G, Drapeau V, Sylvestre MP, Henderson M. Natural history and determinants of dysglycemia in Canadian children with parental obesity from ages 8-10 to 15-17 years: The QUALITY cohort. Pediatr Diabetes. 2022;23:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 50. | Galderisi A, Giannini C, Weiss R, Kim G, Shabanova V, Santoro N, Pierpont B, Savoye M, Caprio S. Trajectories of changes in glucose tolerance in a multiethnic cohort of obese youths: an observational prospective analysis. Lancet Child Adolesc Health. 2018;2:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Jonas DE, Vander Schaaf EB, Riley S, Allison BA, Middleton JC, Baker C, Ali R, Voisin CE, LeBlanc ES. Screening for Prediabetes and Type 2 Diabetes in Children and Adolescents: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2022;328:968-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Peña A, Olson ML, Hooker E, Ayers SL, Castro FG, Patrick DL, Corral L, Lish E, Knowler WC, Shaibi GQ. Effects of a Diabetes Prevention Program on Type 2 Diabetes Risk Factors and Quality of Life Among Latino Youths With Prediabetes: A Randomized Clinical Trial. JAMA Netw Open. 2022;5:e2231196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Vajravelu ME, Lee JM, Shah R, Shults J, Amaral S, Kelly A. Association between prediabetes diagnosis and body mass index trajectory of overweight and obese adolescents. Pediatr Diabetes. 2020;21:743-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Hannon TS. Promoting Prevention, Identification, and Treatment of Prediabetes and Type 2 Diabetes in Youth. Pediatrics. 2020;146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Nicolucci A, Maffeis C. The adolescent with obesity: what perspectives for treatment? Ital J Pediatr. 2022;48:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 56. | Lawson C, Ahmed SN, Brady C, Shoemaker AH. A Clinic-based Approach to Diagnosis and Management of Prediabetes in High-risk Children and Adolescents. J Endocr Soc. 2020;4:bvaa008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 57. | Iafusco D, Franceschi R, Maguolo A, Guercio Nuzio S, Crinò A, Delvecchio M, Iughetti L, Maffeis C, Calcaterra V, Manco M. From Metabolic Syndrome to Type 2 Diabetes in Youth. Children (Basel). 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 58. | US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, Kubik M, Li L, Ogedegbe G, Owens DK, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for Prediabetes and Type 2 Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 320] [Article Influence: 64.0] [Reference Citation Analysis (1)] |
| 59. | Schlesinger S, Neuenschwander M, Barbaresko J, Lang A, Maalmi H, Rathmann W, Roden M, Herder C. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies. Diabetologia. 2022;65:275-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 197] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 60. | Isganaitis E, Laffel L. Recommendations for Screening Children and Adolescents for Prediabetes and Type 2 Diabetes. JAMA. 2022;328:933-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Coker TR, Davidson KW, Davis EM, Donahue KE, Jaén CR, Kubik M, Li L, Ogedegbe G, Pbert L, Ruiz JM, Stevermer J, Tseng CW, Wong JB. Screening for Prediabetes and Type 2 Diabetes in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;328:963-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 62. | Garonzi C, Maguolo A, Maffeis C. Pros and Cons of Current Diagnostic Tools for Risk-Based Screening of Prediabetes and Type 2 Diabetes in Children and Adolescents with Overweight or Obesity. Horm Res Paediatr. 2023;96:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Wallace AS, Wang D, Shin JI, Selvin E. Screening and Diagnosis of Prediabetes and Diabetes in US Children and Adolescents. Pediatrics. 2020;146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 64. | Association AD. Type 2 Diabetes in Children and Adolescents. Pediatrics. 2000;105:671-680. [RCA] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 342] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 65. | American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2340] [Article Influence: 292.5] [Reference Citation Analysis (0)] |
| 66. | Lee J, Kim JH. Endocrine comorbidities of pediatric obesity. Clin Exp Pediatr. 2021;64:619-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Savoye M, Caprio S, Dziura J, Camp A, Germain G, Summers C, Li F, Shaw M, Nowicka P, Kursawe R, Depourcq F, Kim G, Tamborlane WV. Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care. 2014;37:317-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 68. | Zimmermann E, Bjerregaard LG, Gamborg M, Vaag AA, Sørensen TIA, Baker JL. Childhood body mass index and development of type 2 diabetes throughout adult life-A large-scale danish cohort study. Obesity (Silver Spring). 2017;25:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 69. | Saleh M, Kim JY, March C, Gebara N, Arslanian S. Youth prediabetes and type 2 diabetes: Risk factors and prevalence of dysglycaemia. Pediatr Obes. 2022;17:e12841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | American Diabetes Association Professional Practice Committee. 14. Children and Adolescents: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S208-S231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 71. | Ehehalt S, Wiegand S, Körner A, Schweizer R, Liesenkötter KP, Partsch CJ, Blumenstock G, Spielau U, Denzer C, Ranke MB, Neu A, Binder G, Wabitsch M, Kiess W, Reinehr T. Diabetes screening in overweight and obese children and adolescents: choosing the right test. Eur J Pediatr. 2017;176:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 72. | Brar PC. Update on the current modalities used to screen high risk youth for prediabetes and/or type 2 diabetes mellitus. Ann Pediatr Endocrinol Metab. 2019;24:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Echouffo-Tcheugui JB, Kengne AP, Ali MK. Issues in Defining the Burden of Prediabetes Globally. Curr Diab Rep. 2018;18:105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Davidson MB. Historical review of the diagnosis of prediabetes/intermediate hyperglycemia: Case for the international criteria. Diabetes Res Clin Pract. 2022;185:109219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 75. | American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1614] [Article Influence: 403.5] [Reference Citation Analysis (1)] |
| 76. | Hagman E, Ek AE, Marcus C. Insulin function in obese children within the low and high ranges of impaired fasting glycemia. Pediatr Diabetes. 2019;20:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | Weiss R, Santoro N, Giannini C, Galderisi A, Umano GR, Caprio S. Prediabetes in youth - mechanisms and biomarkers. Lancet Child Adolesc Health. 2017;1:240-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 78. | Little RR, Rohlfing C, Sacks DB. The National Glycohemoglobin Standardization Program: Over 20 Years of Improving Hemoglobin A(1c) Measurement. Clin Chem. 2019;65:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 79. | Vajravelu ME, Lee JM. Identifying Prediabetes and Type 2 Diabetes in Asymptomatic Youth: Should HbA1c Be Used as a Diagnostic Approach? Curr Diab Rep. 2018;18:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Magge SN, Silverstein J, Elder D, Nadeau K, Hannon TS. Evaluation and Treatment of Prediabetes in Youth. J Pediatr. 2020;219:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 81. | Nam HK, Cho WK, Kim JH, Rhie YJ, Chung S, Lee KH, Suh BK. HbA1c Cutoff for Prediabetes and Diabetes Based on Oral Glucose Tolerance Test in Obese Children and Adolescents. J Korean Med Sci. 2018;33:e93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Di Bonito P, Licenziati MR, Corica D, Wasniewska M, Di Sessa A, Miraglia Del Giudice E, Morandi A, Maffeis C, Faienza MF, Mozzillo E, Calcaterra V, Franco F, Maltoni G, Valerio G. Which Is the Most Appropriate Cut-Off of HbA1c for Prediabetes Screening in Caucasian Youths with Overweight or Obesity? Int J Environ Res Public Health. 2023;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Khokhar A, Naraparaju G, Friedman M, Perez-Colon S, Umpaichitra V, Chin VL. Comparison of A1C to Oral Glucose Tolerance Test for the Diagnosis of Prediabetes in Overweight and Obese Youth. Clin Diabetes. 2017;35:133-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Batur T, Akbay Hİ, ÇOkluk E, EsendemİR A. Evaluation of the hemoglobin a1c test in detecting pediatric prediabetes. Journal of Contemporary Medicine. 2023;13:1-5. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 85. | Kelsey MM, Zeitler PS, Drews K, Chan CL. Normal Hemoglobin A1c Variability in Early Adolescence: Adult Criteria for Prediabetes Should Be Applied with Caution. J Pediatr. 2020;216:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Lim CYS, Foo YW, Tok CLX, Lim YY, Loke KY, Lee YS, Ng NBH. Screening for metabolic complications of childhood and adolescent obesity: A scoping review of national and international guidelines. Obes Rev. 2022;23:e13513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Troisi RJ, Cowie CC, Harris MI. Diurnal variation in fasting plasma glucose: implications for diagnosis of diabetes in patients examined in the afternoon. JAMA. 2000;284:3157-3159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93:4231-4237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 89. | Mcdonald GW, Fisher GF, Burnham C. Reproducibility of the oral glucose tolerance test. Diabetes. 1965;14:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 127] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Tura A, Pacini G, Moro E, Vrbíková J, Bendlová B, Kautzky-Willer A. Sex- and age-related differences of metabolic parameters in impaired glucose metabolism and type 2 diabetes compared to normal glucose tolerance. Diabetes Res Clin Pract. 2018;146:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 91. | Di Bonito P, Pacifico L, Chiesa C, Valerio G, Miraglia Del Giudice E, Maffeis C, Morandi A, Invitti C, Licenziati MR, Loche S, Tornese G, Franco F, Manco M, Baroni MG; “CARdiometabolic risk factors in overweight and obese children in ITALY” (CARITALY) Study Group. Impaired fasting glucose and impaired glucose tolerance in children and adolescents with overweight/obesity. J Endocrinol Invest. 2017;40:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Di Bonito P, Licenziati MR, Corica D, Wasniewska MG, Di Sessa A, Del Giudice EM, Morandi A, Maffeis C, Faienza MF, Mozzillo E, Calcaterra V, Franco F, Maltoni G, Valerio G. Phenotypes of prediabetes and metabolic risk in Caucasian youths with overweight or obesity. J Endocrinol Invest. 2022;45:1719-1727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 93. | Li Y, Feng D, Esangbedo IC, Zhao Y, Han L, Zhu Y, Fu J, Li G, Wang D, Wang Y, Li M, Gao S, Willi SM. Insulin resistance, beta-cell function, adipokine profiles and cardiometabolic risk factors among Chinese youth with isolated impaired fasting glucose vs impaired glucose tolerance: the BCAMS study. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | De Sanctis V, Soliman A, Daar S, Tzoulis P, Di Maio S, Kattamis C. Oral glucose tolerance test: Ηow to maximize its diagnostic value in children and adolescents. Acta Biomed. 2022;93:e2022318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 95. | Kasturi K, Onuzuruike AU, Kunnam S, Shomaker LB, Yanovski JA, Chung ST. Two- vs one-hour glucose tolerance testing: Predicting prediabetes in adolescent girls with obesity. Pediatr Diabetes. 2019;20:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Di Bonito P, Valerio G, Licenziati MR, Corica D, Wasniewska M, Di Sessa A, Miraglia Del Giudice E, Morandi A, Maffeis C, Mozzillo E, Calcaterra V, Franco F, Maltoni G, Faienza MF. One-Hour Post-Load Plasma Glucose and Altered Glucometabolic Profile in Youths with Overweight or Obesity. Int J Environ Res Public Health. 2023;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | van der Aa MP, Fazeli Farsani S, Kromwijk LA, de Boer A, Knibbe CA, van der Vorst MM. How to screen obese children at risk for type 2 diabetes mellitus? Clin Pediatr (Phila). 2014;53:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 98. | Poon SW, Wong WH, Tsang AM, Poon GW, Tung JY. Who should return for an oral glucose tolerance test? A proposed clinical pathway based on retrospective analysis of 332 children. J Pediatr Endocrinol Metab. 2021;34:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 99. | Krhač M, Lovrenčić MV. Update on biomarkers of glycemic control. World J Diabetes. 2019;10:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 100. | Klonoff DC, Nguyen KT, Xu NY, Gutierrez A, Espinoza JC, Vidmar AP. Use of Continuous Glucose Monitors by People Without Diabetes: An Idea Whose Time Has Come? J Diabetes Sci Technol. 2022.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 101. | Gottfried S, Pontiggia L, Newberg A, Laynor G, Monti D. Continuous glucose monitoring metrics for earlier identification of pre-diabetes: protocol for a systematic review and meta-analysis. BMJ Open. 2022;12:e061756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 102. | Polidori N, Mainieri F, Chiarelli F, Mohn A, Giannini C. Early Insulin Resistance, Type 2 Diabetes, and Treatment Options in Childhood. Horm Res Paediatr. 2022;95:149-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 103. | Hinchliffe N, Capehorn MS, Bewick M, Feenie J. The Potential Role of Digital Health in Obesity Care. Adv Ther. 2022;39:4397-4412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 104. | Stephens TN, Joerin A, Rauws M, Werk LN. Feasibility of pediatric obesity and prediabetes treatment support through Tess, the AI behavioral coaching chatbot. Transl Behav Med. 2019;9:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 105. | Hostalek U, Gwilt M, Hildemann S. Therapeutic Use of Metformin in Prediabetes and Diabetes Prevention. Drugs. 2015;75:1071-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 106. | Bacha F. FDA approval of GLP-1 receptor agonist (liraglutide) for use in children. Lancet Child Adolesc Health. 2019;3:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 107. | Chadda KR, Cheng TS, Ong KK. GLP-1 agonists for obesity and type 2 diabetes in children: Systematic review and meta-analysis. Obes Rev. 2021;22:e13177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 108. | Nadeau KJ, Anderson BJ, Berg EG, Chiang JL, Chou H, Copeland KC, Hannon TS, Huang TT, Lynch JL, Powell J, Sellers E, Tamborlane WV, Zeitler P. Youth-Onset Type 2 Diabetes Consensus Report: Current Status, Challenges, and Priorities. Diabetes Care. 2016;39:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 109. | Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 587] [Article Influence: 73.4] [Reference Citation Analysis (1)] |
| 110. | Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, Avila Edwards KC, Eneli I, Hamre R, Joseph MM, Lunsford D, Mendonca E, Michalsky MP, Mirza N, Ochoa ER, Sharifi M, Staiano AE, Weedn AE, Flinn SK, Lindros J, Okechukwu K. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents With Obesity. Pediatrics. 2023;151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 637] [Article Influence: 212.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gaman MA, Romania; Pop TL, Romania S-Editor: Liu JH L-Editor: A P-Editor: Cai YX