Published online Mar 9, 2022. doi: 10.5409/wjcp.v11.i2.173
Peer-review started: April 30, 2021
First decision: July 27, 2021
Revised: August 9, 2021
Accepted: January 5, 2022
Article in press: January 5, 2022
Published online: March 9, 2022
Processing time: 313 Days and 10.3 Hours
Type 1 diabetes (T1D) incidence varies substantially between countries/ territories, with most studies indicating increasing incidence. In Western Pacific region (WPR), reported rates are much lower than European-origin populations. In contrast, there are reports of substantial numbers of young people with type 2 diabetes (T2D). A deeper understanding of T1D and T2D in the WPR may illuminate factors important in pathogenesis of these conditions. Furthermore, with varying resources and funding for diabetes treatment in this region, there is a need to more clearly determine the current burden of disease and also any gaps in knowledge.
To compile and summarise published epidemiologic and phenotypic data on childhood diabetes in non-European populations in and from WPR.
Research articles were systematically searched from PubMed (MEDLINE), Embase, Cochrane library, and gray literature. Primary outcome measures were incidence and prevalence, with secondary measures including phenotypic descriptions of diabetes, including diabetes type categorization, presence of diabetic ketoacidosis (DKA) at onset, autoantibody positivity, C-peptide levels, and human leucocyte antigen phenotype. Extracted data were collected using a customized template. Three hundred and thirty relevant records were identified from 16 countries/territories, with analysis conducted on 265 (80.3%) records published from the year 2000.
T1D incidence ranged from < 1-7.3/100000 individuals/year, rates were highest in emigrant/ mixed populations and lowest in South-East Asia, with most countries/territories (71.4%) having no data since 1999. Incidence was increasing in all six countries/territories with data (annual increases 0.5%-14.2%, highest in China). Peak age-of-onset was 10-14 years, with a female case excess. Rate of DKA at onset varied from 19.3%-70%. Pancreatic autoantibodies at diagnosis were similar to European-origin populations, with glutamic acid decarboxylase-65 autoantibody frequency of 44.1%-64.5%, insulinoma-associated 2 autoantibody 43.5%-70.7%, and zinc transporter-8 autoantibody frequency 54.3% (one study). Fulminant T1D also occurs. T2D was not uncommon, with incidence in Japan and one Chinese study exceeding T1D rates. Monogenic forms also occurred in a number of countries.
T1D is less common, but generally has a classic phenotype. Some countries/ territories have rapidly increasing incidence. T2D is relatively common. Registries and studies are needed to fill many information gaps.
Core Tip: This systematic review found type 1 diabetes (T1D) incidence was generally low in countries/ territories in the Western Pacific region. However, incidence is rising in most countries where this has been studied. Many countries do not have data or data are quite old. Peak age-of-onset was in later childhood. Rates of diabetic ketoacidosis vary but can be quite high (up to 70%). Autoantibody status is generally like European-origin populations. Fulminant and slowly progressive forms of T1D also occur in the region. Of note, type 2 diabetes was sometimes more common in countries than T1D. Establishment of registers will facilitate incidence studies and also define prevalence and mortality, and assist in outcome assessment. Such data will inform quality of care improvements, health professional training, and assist advocacy.
- Citation: James S, Maniam J, Cheung PT, Urakami T, von Oettingen J, Likitmaskul S, Ogle G. Epidemiology and phenotypes of diabetes in children and adolescents in non-European-origin populations in or from Western Pacific region. World J Clin Pediatr 2022; 11(2): 173-195
- URL: https://www.wjgnet.com/2219-2808/full/v11/i2/173.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v11.i2.173
A diagnosis of diabetes is particularly challenging in young people. An estimated 1.1 million children and adolescents aged < 20 years are estimated to have type 1 diabetes (T1D) globally, with the number with type 2 diabetes (T2D) unknown[1]. Published information on diabetes in this age group is from European origin populations, and yet over half of the global burden is from non-European origin populations.
The commonest form of diabetes in this age group is T1D but other forms do occur[2]. T1D incidence and prevalence varies substantially between countries/territories, with most studies indicating that incidence is increasing at an average of 3%-4%[3], but this appears to be tailoring off in some high-income nations/territories[1].
Increasing incidence in countries/territories with previously low rates offer a chance to better understand the link between genetics and environment in T1D development, especially in countries/territories with little population admixture[1,4]. Additionally, some studies have shown differences in diabetes incidence among migrant populations relative to the native population, which gives further support to the role of environment in T1D causation[5,6].
In the Western Pacific region (WPR), early studies had reported T1D being very rare in young people[4,7], and subsequent reports have shown incidence rates much lower than most European-origin populations[4,8-10]. In contrast, there are reports of substantial numbers of young people with T2D in some countries in the WPR[11,12].
A deeper understanding of both the epidemiology and phenotypes/endotypes of T1D and T2D in non-European populations such as those in WPR may illuminate factors important in pathogenesis of these conditions. Furthermore, with varying resources and funding for diabetes treatment in this region, there is a need to more clearly determine the current burden of disease and also any gaps in knowledge in related epidemiology and phenotypes/endotypes[1].
The objective of this systematic review is to compile and summarise current published epidemiologic and phenotypic data on childhood diabetes in non-European populations in and from the Western Pacific. Primary outcome measures were incidence and prevalence of diabetes in people < 20 years of age. Secondary measures included diabetes type categorisation and phenotype/endotype features including presence of diabetic ketoacidosis (DKA) at diagnosis, pancreatic autoantibody positivity rates, C-peptide levels, and human leucocyte antigen (HLA) phenotypes.
Non-European populations in and recently emigrated from the WPR.
Any relevant published study conducted in one or more of the 37 countries/territories of the Western Pacific, as determined by the World Health Organization[13], extending from the Mongolian steppes in central Asia, east to the Pitcairn Islands in the Pacific Ocean and south to New Zealand. The included countries/territories were Australia, Brunei, Cambodia, Cook Islands, Democratic People’s Republic of Korea, Federated States of Micronesia, Fiji, Guam, Hong Kong, Indonesia, Japan, Kiribati, Laos, Macau, Malaysia, Marshall Islands, Mongolia, Myanmar, Nauru, New Caledonia, New Zealand, Niue, North Korea, Palau, Papua New Guinea, South Korea, Samoa, Singapore, Solomon Islands, Taiwan, Thailand, Timor-Leste, Philippines, Tonga, Tuvalu, Vanuatu and Vietnam. Studies on recent emigrant populations from these countries/territories to others were also included.
Publications were included if they focused on incidence, prevalence, diabetes type, clinical presentation (presence/rate of DKA), pancreatic autoantibody status, and HLA phenotype. Studies that did not include data on at least one of these factors were excluded.
Data from Australia and New Zealand exclusively were only included if children and adolescents < 20 years of age identified as being an Aboriginal and/or Torres Strait Islander, or Maori, respectively.
Studies were of any study design and in any language. There was no restriction on publication date or type.
Primary outcomes: Incidence and prevalence of T1D, T2D and other forms in children and youth < 20 years in and from the WPR.
Secondary outcomes: Phenotypic descriptions of childhood- and youth-onset diabetes, including diabetes type categorization, the presence of DKA at onset, autoantibody positivity, C-peptide levels, and HLA phenotype.
Research articles were systematically searched in the following databases: PubMed (MEDLINE), Embase, and the Cochrane library. The search terms below were developed for PubMed and then adapted for other databases. The MeSH terminologies include Diabetes Mellitus, Epidemiology, Diagnosis, Symptoms, and Clinical Chemistry. The search strategy was: (Diabetes Mellitus) AND (Epidemiology OR Diagnosis OR symptom OR antibod* OR autoantibod* OR Ketoacidosis OR clinical chemistry OR HLA) AND (Country) AND (child* OR adolesc*).
For Embase database, the search terminology for “Diabetes Mellitus” was replaced with “insulin dependent diabetes mellitus”.
To search the gray literature, we searched the following: (1) ProQuest Dissertations and Theses Global for theses; (2) Citation searching, including reference list searching and forward citation searching in Google Scholar, Scopus and Web of Science Core Collection; and (3) Hand-searched paediatric diabetes conference abstracts not indexed in the above databases: International Society for Pediatric and Adolescent Diabetes (ISPAD, available in Pediatric Diabetes); Pediatric Endocrine Society (PES, available in Hormone Research in Children); European Society for Pediatric Endocrinology (ESPE, available in Hormone Research in Children); Asia Pacific Paediatric Endocrine Society (APPES, abstracts available in member’s area).
For each database, the years searched included the earliest available online year of indexing up to December 2019.
The Covidence systematic review platform (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org) was used to assist with data management. Two independent reviewers reviewed the titles and abstract of the identified studies for relevance. The same reviewers independently reviewed the full text of these studies in a first screen to assess if they met inclusion and exclusion criteria. The reasons for excluding articles were recorded in Covidence. Any disagreements or queries were discussed until a consensus was reached. Thereafter, a final list of studies was produced.
The extracted data was collected using a customized template in Microsoft Excel (Microsoft, Redmond, United States). The extracted data included the following: Country/territory, city/region, type of study, year of publication, time period of study, diagnosis criteria used, T1D incidence and/or prevalence, T2D studies, other forms of diabetes, age range distribution, sex distribution, DKA at diagnosis, pancreatic autoantibody test results, and HLA phenotype. Additional information about the derivation of each value was collected to help qualify the data. Descriptive analyses were performed using Excel. A qualitative comparison of the results across the collected variables is the main focus of this review.
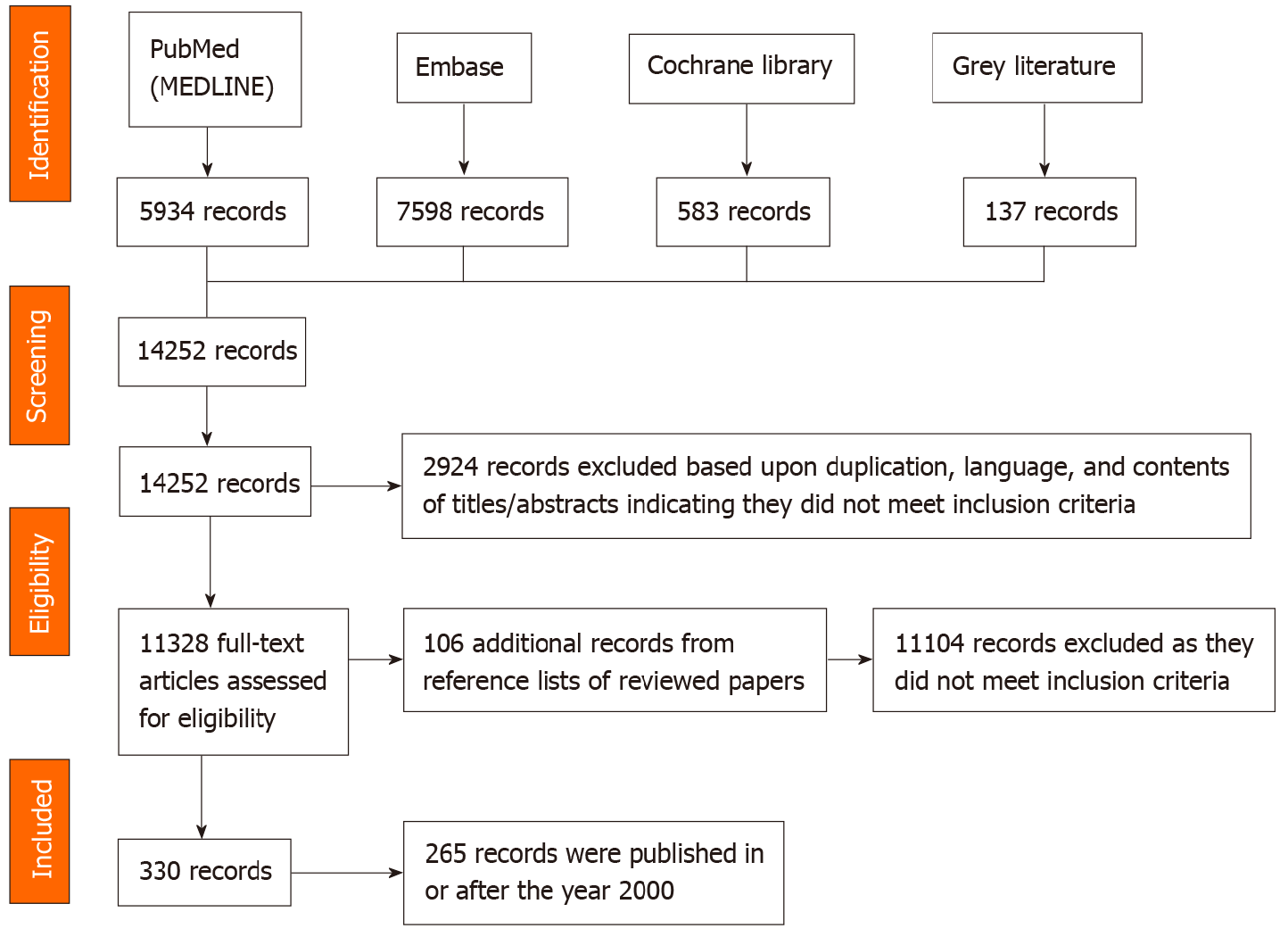
A total of 14252 records were identified, downloaded to EndNote version X9 and screened by reading titles and abstracts. Of these, 2924 records were excluded based upon duplication, language, and contents of titles/abstracts indicating they did not meet inclusion criteria. The remaining 11328 full-text articles were assessed for eligibility; their reference lists and citations were searched, and an additional 105 papers identified. Of these records, 11104 did not meet review inclusion criteria, leaving 330 relevant records. The search process and outcomes are summarised in Figure 1.
The 330 papers were from 16 WPR countries/territories (Table 1), with 204 (62.1%) papers from three countries/territories only. These were from China (n = 72), Japan (n = 94) and South Korea (n = 38). Two-hundred and sixty-five (80.3%) of the 330 studies were published in or after the year 2000. Table 1 summarises the number of papers for each variable and other characteristics of the included studies.
| Country/territory | Total records | |
| n | Proportion of total | |
| Australia | 10 | 3.8% |
| China | 67 | 25.3% |
| Fiji | 1 | 0.4% |
| Hong Kong, China | 6 | 2.3% |
| Indonesia | 5 | 1.9% |
| Japan | 66 | 24.9% |
| Malaysia | 5 | 1.9% |
| New Zealand | 6 | 2.3% |
| Papua New Guinea | 1 | 0.4% |
| Philippines | 2 | 0.8% |
| Singapore | 5 | 1.9% |
| South Korea | 35 | 13.2% |
| Taiwan, China | 20 | 7.5% |
| Thailand | 21 | 7.9% |
| Tonga | 1 | 0.4% |
| Vietnam | 11 | 4.2% |
| Multiple countries/territories | 3 | 1.1% |
Incidence: Table 2 summarises the 25 studies from ten WPR countries/territories that had information about T1D incidence with data from 2000 or afterwards. Six studies were from China, five from South Korea, two from Thailand and Taiwan, and one each from four other countries/territories. Most studies (n = 18) reported data for youth aged < 15 years, and only 16 had been published within the past decade.
| Ref. | Country/territory | Study period | Incidence/100000 | n (%) | Age range (yr) |
| Zhang et al[26], 2008 | Harbin, China | 1990-2000 | 0.7 (average) | 103 | < 15 |
| Gong et al[28], 20151 | Beijing, China | 1995-2010 | 1.72 | 485 | < 15 |
| Shen et al[29], 2002 | Shanghai, China | 1997-2000 | 1.6 | 103 | < 15 |
| Gong et al[30], 2004 | Beijing, China | 1997-2000 | 1.0 (annual): 1997 (0.76); 2000 (1.21) | 71 | < 15 |
| Zhao et al[31], 2014 | Shanghai, China | 1997-2011 | 3.12 (annual): 1997-2001 (1.5); 2007-2011 (5.5) | 622 | < 15 |
| Wu et al[32], 2016 | Zhejiang, China | 2007-2013 | 2.02 (annual): 2007 (1.2); 2013 (2.5) | 611 | < 20 |
| Ogle et al[14], 2016 | Fiji | 2001-2012 | 0.9 (overall): 2.1 (Indo-Fijian); 0.2 (Native-Fijian) | 28 | < 15 |
| Huen et al[154], 2009 | Hong Kong, China | 1997-2007 | 2.42 < 15 yr, 2.0 2 < 19 yr | 335 | < 19 |
| Tung et al[22], 2018 | Hong Kong, China | 2008-2017 | 2.4 (annual) | 498 | < 18 |
| Tung et al[34], 2020 | Hong Kong, China | 1997-2007; 2008-2017 | 2.12 (annual): 1997 (1.6); 2007 (2.3). 3.52 (annual): 2008 (4.0); 2017 (4.5) | 498 | < 18 |
| Pulungan[15], 2013 | Indonesia | 2010 | 0.033 | 825 | NS |
| Urakami et al[35], 2008 | Japan | 1974-2004 | 0.64 | 54 | < 15 |
| Onda et al[19], 2017 | Japan | 2005-2010 | 2.3 (annual): 2005 (2.17); 2010 (2.23) | 2326 | < 15 |
| Campbell-Stokes and Taylor[138], 2005 | New Zealand | 1999-2000 | 5.6 (Māori) | 22 | < 15 |
| Ogle et al[18], 2001 | Papua, New Guinea | 1996-2000 | 0.1 | 8 | < 15 |
| Lee[141], 2014 | South Korea | 1995-2000 and 2012 | 1995-2000 (1.4); 2012 (2.9) | 217 | < 15 |
| Lee et al[33], 2015 | South Korea | 2001-2010 | 2.0 (annual): 2001 (1.3); 2010 (2.7) | 239 | < 15 |
| Song et al[20], 2016 | South Korea | 2011-2013 | 3.3 | 2346 | < 20 |
| Kim et all[21], 2015 | South Korea | 1995-2000 and 2012-2014 | 1995-2000 (1.4); 2012-2014 (3.2) | 706 | < 15 |
| Hong et al[149], 2013 | South Korea | 2001-2010 | 2.0 (annual) | 239 | < 15 |
| Lin et al[23], 2014 | Taiwan, China | 1999-2010 | 4.6 (annual): 1999-2000 (3.6); 2009-2010 (5.9) | 1280 | < 15 |
| Lu et al[24], 2014 | Taiwan, China | 2003-2008 | 5.3 | 1306 | < 15 |
| Panamonta et al[16], 2011 | Thailand | 1996-2005 | 0.6 | 340 | < 15 |
| Patarakijvanich et al[17], 2008 | Thailand | 1997-2005 | 0.7 | 116 | < 15 |
| The Writing Group for SEARCH[25] | United States-Asian and Pacific Islander immigrants | 2002-2003 | 7.3 | 56 | < 20 |
Incidence ranged from < 1 to 7.3 per 100000 individuals per year. An incidence of < 1 per 100000 were reported in four countries: Fiji[14], Indonesia[15], Thailand[16,17] and Papua New Guinea[18]. However in Fiji, the rate in Indo-Fijians was 9.3 times higher than the rate in Native Fijians[14].
T1D rates of approximately two per 100000 were observed in Japan[19], three in South Korea[20,21], four in Hong Kong[22], five in Taiwan[23,24] and seven in mixed population immigrants in the United States[25]. In China, average T1D rates were variable, with rates ranging from 0.7 to 3.1[26-31].
Single-study data looking at changes in incidence rates over time was available from six countries/territories. In China, rates rose 7.4% per annum (pa) in Harbin from 1990-2000[26], 12.0% pa in Zhejiang from 2007-2013[32], 4.4% pa in Beijing from 1995-2010[28], and 14.2% pa in Shanghai from 1997-2011[31]. In South Korea, rates rose by 7.6% pa from 2001-2010[33]. In Hong Kong, data was presented from 2008-2017 in an abstract published in 2018[22], with full data published in 2020[34]. The annual increment between 2008-2017 was 3.5% pa, with authors noting that this was less than the increment of 4.3% in the period 1997-2007. In Taiwan, rates rose 8.7% every two years from 1999-2000 to 2009-2010[23]. In Thailand, incidence almost quadrupled between 1996 and 2005, however, the authors commented that increased diagnosis likely contributed to this[16]. However, in Japan, incidence barely changed from 2005-2010 with a 0.5% pa increase[19].
Subtypes of T1D: In Japan, three sub-groups of T1D have been identified: “abrupt-onset” form (65%), “slowly-progressive” form (30%) and “fulminant” form (5%)[35]. Childhood-onset slowly-progressive T1D is usually detected by urine-glucose screening at schools, or testing by chance, and has minimum symptoms of diabetes without showing ketosis. This type of diabetes is commonest in adolescent females, and has positive beta-cell associated antibodies in approximately 70% of the cases[35]. Fulminant T1D is more common in adults with T1D where it represents around 20% of Japanese cases[36], although in children, age-of-onset has been reported as biphasic with one peak < 5 years[37]. Aside from other Japanese reports[38-40], fulminant T1D has also been reported in China[41-43] and South Korea[44].
Prevalence: Five countries reported prevalence of T1D, with two papers from South Korea[20,45], and one each from Fiji[14], Japan[19] and Papua New Guinea[18]. There was a wide variation in rates, from South Korea with 52 (< 25 years)[45] and 21 (< 20 years)[20] per 100000, Japan 13.5 (< 15 years)[19], Fiji 5.9 (< 15 years)[14], and Papua New Guinea 0.28 (< 15 years)[18]. The Fiji study[14] reported rates by ethnicity, with T1D prevalence in Indo-Fijians being almost 10 times higher than the rate in native-Fijians (13.6 vs 1.4).
Age at diagnosis: Table 3 summarises the 20 studies from seven WPR countries that had information about either mean/median or peak age of diagnosis. Only eight studies reported peak age of diagnosis. One study from Japan[19] reported peak age of onset in girls at 10 years and boys at 13 years. The remaining nine papers reported five-year interval data with peak 10-14 years.
| Ref. | Country/territory | n | Mean ± SD/median (IQR) age of diagnosis (yr) | Age range (yr) | Peak age of diagnosis (yr) |
| Gong et al[28], 2015 | Beijing, China | 485 | NS | < 15 | 10-14 |
| Huo et al[155], 2018 | Beijing, China and Shantou, China | 515 | 11 (7-14) | < 21 | 10-14 |
| Weng et al[156], 2018 | China (13 areas)1 | 1239 | NS | < 15 | 10-14 |
| Huen et al[154], 2009 | Hong Kong, China | 335 (< 19); 293 (< 15) | NS | < 19 | 10-14 |
| Tung et al[22], 2018 | Hong Kong, China | 498 | 10.5 (± 4.2) | < 18 | NS |
| Onda et al[19], 2017 | Japan | 2326 | NS | < 15 | 13 (boys); 10 (girls) |
| Lee et al[157], 2006 | Singapore | 211 | 7.9 (± 4.0) | < 17 | NS |
| Kim et al[21], 2012 | South Korea | 110 | 10.6 (± 0.9) | < 18 | NS |
| Kim and Kim[158], 2012 | South Korea | 113 | 9.26 (± 0.99) | < 18 | NS |
| Hong et al[149], 2013 | South Korea | 239 | NS | < 15 | 10-14 |
| Lee et al[141], 2014 | South Korea | 217 | NS | < 15 | 10-14 |
| Kim et al[159], 2016 | South Korea | 706 | NS | < 15 | 10-14 |
| Lee et al[160], 2017 | South Korea | 361 | 8.9 (± 4.0) | < 20 | NS |
| Lo et al[46], 2004 | Taiwan, China | 165 | 7.3 (± 4.1) | < 18 | NS |
| Ting et al[61], 2007 | Taiwan, China | 304 | 7.9 (± 3.8) | < 20 | NS |
| Panamonta et al[161], 2000 | Thailand | 77 | NS | < 15 | 10-14 |
| Likitmaskul et al[79], 2006 | Thailand | 195 | 9.2 (± 2.5) | < 19 | NS |
| Patarakijvanich et al[17], 2008 | Thailand | 116 | NS | < 15 | 11-14 |
| Panamonta et al[16], 2011 | Thailand | 340 | NS | < 15 | 10-14 |
| Khwanhatai et al[162], 2018 | Thailand | 229 | 7.71 (± 3.3) | < 18 | NS |
Gender ratio: Twenty-two papers from eight countries reported new-onset T1D cases by gender (Table 4). Ten of these reported rates according to respective population sizes and the remaining 12 just presented numbers for each gender. There was a female excess in almost all studies, with the male:female ratio ranging from 0.58-1.13. The mean ratio across the 22 papers was 0.81.
| Ref. | Country/territory | Ratio (M:F) | Age range (yr) |
| Xin et al[163], 2010 | Shenyang, China | 0.77 | < 15 |
| Gong et al[27], 2013 | Beijing, China | 0.581 (1995-2002); 0.741 (2003-2010) | < 15 |
| Zhao et al[31], 2014 | Shanghai, China | 0.971 | < 15 |
| Gong et al[28], 2015 | Beijing, China | 0.701 | < 15 |
| Wu et al[32], 2016 | Zhejiang, China | 0.781 | < 20 |
| Tao et al[164], 2017 | Kunming, China | 1.13 | < 15 |
| Huo et al[155], 2018 | Beijing, China and Shantou, China | 0.77 | < 21 |
| Weng et al[156], 2018 | China (13 areas)2 | 0.781 | < 15 |
| Huen et al[154], 2009 | Hong Kong, China | 0.76 | < 19 |
| Tung et al[22], 2018 | Hong Kong, China | 0.75 | < 18 |
| Onda et al[19], 2017 | Japan | 0.761 | < 15 |
| Lee et al[157], 2006 | Singapore | 0.77 | < 17 |
| Hong et al[149], 2012 | South Korea | 0.861 | < 15 |
| Lee et al[141], 2014 | South Korea | 0.841 | < 15 |
| Kim et al[159], 2016 | South Korea | 0.801 | < 15 |
| Song et al[20], 2016 | South Korea | 0.89 | < 20 |
| Lee et al[160], 2017 | South Korea | 0.86 | < 20 |
| Lo et al[46], 2004 | Taiwan, China | 0.70 | < 18 |
| Ting et al[61], 2007 | Taiwan, China | 0.94 | < 20 |
| Lu et al[24], 2014 | Taiwan, China | 0.781 | < 15 |
| Patarakujvanich et al[165], 2001 | Thailand | 1.0 | < 15 |
| Panamonta et al[16], 2011 | Thailand | 0.65 | < 15 |
DKA at diagnosis: Twenty papers from seven countries reported on the rate of DKA at onset (Table 5). The rates varied from 19.3% in one Taiwanese study[46] to 75.3% in one study from Malaysia[47]. Only three studies had rates below 33%.
| Ref. | Country/territory | % with DKA | n | Age range (yr) |
| Huen et al[154], 2009 | Hong Kong, China | 60.0 | 335 | < 19 |
| Tung et al[22], 2018 | Hong Kong, China | 41.0 | 498 | < 18 |
| Jalaludin and Harun[47], 2005 | Malaysia | 75.3 | 55 | < 13 |
| Fuziah et al[166], 2008 | Malaysia | 57.1 | 166 | < 20 |
| Gunn et al[167], 2017 | New Zealand | 28.7 (overall); 23.71; 34.32 | 381; 352 | < 15 |
| Lee et al[157], 2006 | Singapore | 53.0 | 211 | < 17 |
| Park et al[168], 2011 | South Korea | 55.0 | 23 | NS |
| Kim et al[158], 2012 | South Korea | 36.4 | 110 | < 18 |
| Kim et al[169], 2013 | South Korea | 32.0 | 100 | < 18 |
| Kim and Kim[170], 2014 | South Korea | 39.0 | 113 | < 18 |
| Kim et al[21], 2015 | South Korea | 39.7 | 706 | < 15 |
| Lee et al[160], 2017 | South Korea | 56.5 | 361 | < 13 |
| Lo et al[46], 2004 | Taiwan, China | 19.3 | 165 | < 17 |
| Ting et al[61], 2007 | Taiwan, China | 65.1 | 304 | < 19 |
| Tung et al[62], 2009 | Taiwan, China | 67.0 | 157 | < 19 |
| Chen et al[171], 2017 | Taiwan, China | 66.2 (overall): 87.0; 55.0 | 52; 94 | < 6; 6-18 |
| Likitmaskul et al[172], 2003 | Thailand | 55.0; 78.0 | 94; 28 | 6-18; < 15 |
| Patjamontri and Santiprabjob[173], 2012 | Thailand | 40.8 | 49 | < 15 |
| Jaruratanasirikul et al[80], 2017 | Thailand | 70.0 | 99 | < 15 |
| Trisorus et al[48], 2018 | Thailand | 63.0 | 81 | < 15 |
Autoantibodies at diagnosis: Table 6 lists the 15 studies from four countries that reported autoantibody testing. All studies had glutamic acid decarboxylase 65 autoantibody (GAD65) data, with average frequencies of 51.3% (China), 58.1% (Japan), 64.5% (Taiwan), and 62.7% (Thailand). The frequencies in South Korea, Phillipines and Singapore were 53.0%, 44.1%, and 41.5%, respectively. Nine studies reported on insulinoma-associated 2 autoantibody (IA-2), with average prevalence of 43.5% (China), 70.7% (Taiwan) and 54.9% (Thailand). However, rates for islet autoantibody (ICA) were variable, ranging from 4 to 68.8%. Only one study (from Thailand[48]) reported zinc transporter 8 autoantibody (ZnT8) results, with 54.3% of cases positive.
| Ref. | Country/territory | n | Age range (yr) | % positive for GAD65 | % positive for IA-2 | % positive for IAA | % positive for ZnT8A | % positive for ICA |
| Huang[174], 2004 | Guangdong, China | 34 | 7-12 | 44.1 | 35.3 | 17.6 | ||
| Li et al[175], 2008 | Changsha, China | 35; 51 | 0-9; 10-14 | 60.0; 64.7 | 62.8; 33.3 | |||
| Baoerhan and Maimaiti[176], 2015 | Urumqi, China. | 94 | < 15 | 45.0 | 62.0 | 76.0 | ||
| Urakami et al[35], 2008 | Japan | 48 | 6-15 | 70.8 | 68.8 | |||
| Iwabuchi et al[177], 2013 | Japan | 43 | Children | 44.0 | ||||
| Habu et al[134], 2013 | Japan | 48 | < 19 | 59.5 | 68.1 | |||
| Mabulac[178], 2013 | Philippines | 68 | Paediatric | 44.1 | ||||
| Lee et al[59], 2001 | Singapore | 41 | < 15 | 41.5 | 41.5 | |||
| Kim and Kim[170], 2014 | South Korea | 113 | < 18 | 53.0 | 26.0 | 4.0 | ||
| Chen et al[179], 2001 | Taiwan, China | 70 | < 17 | 54.3 | ||||
| Tung et al[62], 2009 | Taiwan, China | 157 | 12-18 | 73.0 | 76.0 | 21.0 | ||
| Cheng et al[146], 2018 | Taiwan, China | 750 | < 20 | 66.3 | 65.3 | 35.7 | ||
| Santiprabhob et al[180], 2007 | Thailand | 51 | < 15 | 63.0 | 61.0 | |||
| Patjamontri et al[173], 2012 | Thailand | 90 | < 20 | 50.0 | 58.0 | |||
| Trisorus et al[48], 2018 | Thailand | 81 | < 15 | 75.3 | 45.7 | 54.3 |
C-peptide at diagnosis: Nineteen studies (from China, India, Japan, South Korea, Singapore, Taiwan and Thailand)[37,41,44,46,48-62], reported C-peptide results. C-peptide levels were generally low, consistent with classic T1D. Kim et al[44] in South Korea found that C-peptide values were lower in fulminant versus autoimmune and idiopathic T1D. Lo et al[46] in Taiwan found that C-peptide levels were lower in subjects diagnosed younger. Finally, also in Taiwan, Ting et al[61] reported lower C-peptide levels in subjects who had DKA at diagnosis.
HLA status: Twelve studies reported HLA phenotype data, from China[49,63-67], Japan[68,69], South Korea[70,71], Taiwan[72] and Thailand[73]. Nine papers found an association between T1D and HLA-DRB1[49,63,67,69-72,74]. However, alleles contributing to T1D association differ among WPR countries. In China, several studies reported DRB1*0301[49,63,64] conferred the strongest risk for T1D, whereas in Japan, risk is conferred mainly from DRB1*0901 and *0802[69,74], with a contribution also from DRB1*0405[74] and *0404[69]. DRB1*0901 was strongly associated with early onset in preschool children in Japan with type 1A diabetes[68]. One study in a Japanese population reported that DRB1*0301 and *0302 were absent in T1D patients[74]. In South Korea, T1D risk was strongly associated with DRB1*0301,*0405 and *09012 alleles[70].
There were also significant findings for DQB1, with unique alleles contributing to T1D risk in various countries[49,65,66,69,73] and within different parts of China[49,66]. DQB1*0201 conferred the strongest risk and DQB1*0601 and *0602 were protective specifically amongst the Chinese Han population[66]. In Guangdong, T1D risk was linked with higher frequencies of DQB1*0303, *0401 and *0402 but DQB1*0301 was found to be protective[49]. DQB1*0601 and *0602 were associated with risk of type 1B in Japan[69]. In Thailand, higher frequencies of DQB1*0201,*0202 and *0302 were found in children with T1D.
There are also some reports of DQA alleles susceptible to T1D in China[49,64].
Incidence: Table 7 summarises the 14 studies from seven WPR countries that had information about T2D incidence. The studies from Australia and New Zealand on indigenous/regional origin populations, and also Asian/Pacific emigrants to the United States, showed high rates. The rates from four other countries/territories including China, Hong Kong, Japan and South Korea ranged from 0.43 to 2.63 per 100000 individuals. Rapid increases in incidence were seen in China[75] and Hong Kong[22], with data being published in 2021 showing a rate of 3.42[76]. In Fiji, the rate for Indo-Fijians was 20 times higher than the rate for Native Fijians[14]. The mixed population of Asian and Pacific Islanders emigrants to the United States recorded the highest T2D incidence rate (12.2 per 100000)[77].
| Ref. | Country/territory | Study period | n | Incidence/100000 | Age range (yr) |
| Craig et al[181], 2007 | Australia Torres Straits Islands | 2001-2006 | 23 | 12.7 | < 19 |
| Tran et al[182], 2014 | Australia, Torres Straits Islands | 2001-2008 | 31 | 20.7 | < 19 |
| Haynes et al[183], 2016 | Australia, Torres Straits Islands | 1990-2012 | 76 | 12.6 | < 17 |
| Wu et al[75], 2017 | Zhejiang, China | 2007-2013 | 392 | 1.73 (overall): 0.62 (2007); 3.62 (2013) | < 20 |
| Ogle et al[14], 2016 | Fiji | 2001-2012 | 13; 11; 1; 1 | 0.43 (overall): 1.171; 0.062; 0.703 | < 15 |
| Huen et al[154], 2009 | Hong Kong, China | 1997-2007 | 198 | 1.2 | < 19 |
| Tung et al[22], 2018; Tung et al[76], 2021 | Hong Kong, China | 2008-2017; 2008-2017 | 391; 391 | 1.9 (3.42) | < 18 |
| Urakami et al[184], 2005 | Japan | 1974-2002 | 232 | 2.63 (overall): 1.73 (< 1980); 2.76 (> 1981) | < 16 |
| Urakami et al[148], 2018 | Japan | 1975-2015 | 301 | 2.6 | < 16 |
| Campbell-Stokes and Taylor[138], 2005 | New Zealand | 1999-2000 | 74 | 1.78 | < 15 |
| Jefferies et al[185], 2012 | New Zealand | 1995-2007 | 434,5 | 3.4 | < 15 |
| Sjardin et al[186], 2018 | New Zealand | 1995-2015 | 344 | 3.3 (overall): 3.4 (1995-2007); 4.0 (2008-2015) | < 15 |
| 475 | 3.6 (overall): 3.4 (1995-2007); 4.0 (2008-2015) | ||||
| Hong et al[149], 2013 | South Korea | 2001-2010 | 89 | 0.76 | < 15 |
| Liu et al[77], 2009 | United States-Asian and Pacific Islander immigrants | 2002-2003 | 73 | 12.2 | < 15 |
Prevalence: Four countries reported population prevalence of T2D, with one paper each from China[11], Fiji[14], South Korea[45] and Taiwan[78]. There was a wide variation in rates, from South Korea with 249 per 100000 < 24 years[45], China 96.8 per 100000 < 18 years[11], Taiwan with 70 (males) and 80 (females) per 100000 (0-19 years)[78], and Fiji 2.4 per 100000 (< 15 years)[14]. The South Korea study[45] reported that between 2002 to 2013, T2D prevalence increased 2.35 fold; the 5-9 and 10-14 year age groups showed remarkable increases (2.59 and 2.54 fold respectively), although the age group 20-24 years had the highest prevalence. Similarly, the Taiwan study[78] reported a 33% increase from 2000 to 2008.
In Thailand, a multi-centre report in 2006 found that 18.6% of diabetes cases < 18 years were T2D[79]. A more recent report from Thailand showed clinic prevalence increasing from 10%-15% in 1995-2003 to 35%-40% in 2009-2014[80].
Monogenic causes: There are numerous reports of single gene defects causing diabetes in China, Japan, Vietnam, Thailand, Singapore, South Korea and Fiji. These include reports of gene mutations resulting in permanent and transient neonatal diabetes mellitus and diabetes with onset later in childhood.
Most reports were case studies[81-102]. Larger studies that conducted genetic testing on neonatal diabetes cases were undertaken in China[103] and Vietnam[104-106]. Cao et al[103] reported a total of 25 cases with neonatal period onset. 72.0% cases (n = 18) were permanent (five with KCNJ11 gene mutations, one ABCC8 mutation, two EIF2AK3, one each with INS, GLIS3 and SLC19A and seven without any known mutation) and seven cases (28%) with transient diabetes (two with ABCC8 mutation, one paternal UPD6q24, and four without mutations). In Vietnam, Craig et al[104] identified 13 neonatal cases that had gene mutations of KCNJ11 (n = 3), ABCC8 (n = 4), INS (n = 2) and uniparental disomy of chromosome 6q24 (n = 1) and three others without any mutations. Also in Vietnam, Can et al[105] genetically confirmed 16 neonatal cases with gene mutations of KCNJ11 (n = 6), ABCC8 (n = 5), INS (n = 2) and abnormality in chromosome 6q24 (n = 3). Finally, Ngoc et al[106] reported 38 cases (28 permanent and 10 transient) with monogenic diabetes, 31% with mutations of ABCC8, 29% KCNJ11, 16% INS, 16% chromosome 6q24, 3% FOXP3, 3% EIF2B1, and 2% EIF2AK3.
Successful switching from insulin to sulfonylurea treatment was observed in cases with KCNJ11 V59M/C42R and ABCC8 mutations[82,83,88,102,107,108].
In addition, there are various reports of diabetes occurring as part of a known syndrome: DEND syndrome (developmental delay, epilepsy, and neonatal diabetes syndrome)[82,90,109], Wolfram syndrome[110-113], Prader-Willi syndrome[114], Wolcott-Rallison syndrome[81] and Kearns-Sayre syndrome[115].
There were reports of maturity-onset diabetes of the young (MODY) among children and adolescents < 20 years from China[116-122] and Japan[123-131], with this condition also seen in Hong Kong[120,132].
This systematic review examined all published information on diabetes in young people in and from the 37 countries/territories in the WPR, excluding European-origin populations. Three hundred and thirty papers were relevant for the review. The analysis demonstrates both differences and commonality compared to observations in European-origin populations.
T1D incidence is dependent on both genetic and environmental factors[2,133]. HLA haplotype variations are the main genetic driver, although some other genes also play significant roles[2,133]. The specific environmental factors are less well understood[2,134].
T1D is most common in European-origin and some Arab-origin populations, with annual incidences ranging from 13-60 per 100000 population < 15 years[1,135]. In contrast, this systematic review demonstrates that all published WPR rates are much lower, although data since 2000 are available for only ten countries as well as one migrant population. A review by Park[136] in 2006 proposed a lower incidence of high-risk HLA alleles as with respect to identical DR-DQ haplotypes, the association and transmission to diabetic offspring were similar for Asians and Caucasians.
Reported incidence is even lower in non-Chinese-origin South-East Asian and Pacific countries (Thailand, Indonesia, Papua New Guinea, and Fiji), than in Eastern Asian nations (China, Hong Kong, Japan, South Korea and Taiwan), although lack of ascertainment may underestimate the true incidence rate in Thailand, Indonesia and Papua New Guinea, as some cases may die at onset misdiagnosed with another condition[16,18,137]. However, it must be noted that in Fiji, incidence < 15 years was nine times higher in Indo-Fijians compared to Native Fijians[14] and the incidence in New Zealand Maori was 4.5 times lower than in European-origin children[138]. In addition, incidence is similarly low in Bangladesh which is adjacent to South East Asia[139].
The highest incidence seen was in South- and Western-Asian- and Pacific Island-origin children who had emigrated to the United States, although the rate remained less than a third of that in non-Hispanic white children[25]. Finally, in a study of all-age T1D incidence in Australia in 2013, incidence in the Aboriginal population was only 70% of that in the non-indigenous population[140], despite the extensive admixture between the two populations. Therefore, in these populations, changes in environment that could potentially increase incidence do not appear to fully overcome the impact of varying genetic susceptibility.
In the absence of large-scale immigration, genetic factors will remain essentially constant. Therefore, any changes in incidence will be due to changing environmental factors. Incidence in European-origin populations has increased by 3%-4% pa in many European-origin populations[135], although this is tailing off now in some countries[3]. There is some evidence that the rate of increase is higher in some lower-incidence countries[3].
The four studies from China[26,30-32] show that T1D incidence is rising quickly (from 4.4%-14.2% pa). South Korea[141] and Taiwan[23] also had high rates of increase at 7.6% and 8.7% pa respectively. However, the rate of rise was slowing in Hong Kong[22] and was virtually zero in Japan[19]. This may be due to evolving environmental factors which then approach a peak effect, as has been seen with slowing or peaking rates in some high-incidence countries[3].
Slowly-progressive diabetes that is clearly T1D is well described from Japan[35], and fulminant T1D (which occurs more in adults and in younger children) is well reported from Japan[36-40], China[41-43] and South Korea[44].
These distinct subtypes, as opposed to acute-onset T1D, do not have exact correlates in European-origin populations, although it is possible that to some extent these represent the more general heterogeneity of T1D, which is being increasingly recognised[142]. For instance, onset is more rapid in younger European-origin populations[143]. A study done in Western Asia and also in a European-origin population that used identical methodology to assess genotype, phenotype and endotype could help illuminate this and add to global knowledge of T1D pathogenesis.
Prevalence data from non-European origin populations in WPR are limited to five countries. Prevalence is dependent upon past incidence and mortality. We did not find any publications on mortality in these populations.
The age of onset of T1D cases, with peak age 10-14 years, is consistent with European-origin populations.
Nearly all studies found a female excess of cases. In high-incidence countries, T1D is slightly more common in males[2]. In contrast, as in this review, a female excess is more common in lower-incidence countries[144].
Pancreatic autoantibody rates varied across studies. This could be due to various factors including study group age, duration of diabetes at the time of measurement, assay variations, and diagnostic uncertainty. Most GAD-65 and IA2 autoantibody rates were consistent with European-origin data. Only one study in this review, from Thailand[48] measured ZnT8 autoantibodies. Positivity was high at 54.3% in new-onset cases, with 16% of all new-onset cases having ZnT8 but not GAD-65 or iA2 autoantibodies. A recent study from Japan found that ZnT8 positivity was most common < 10 years[145]. With respect to change in autoantibodies over time, Cheng et al[146] found that in Taiwan, the rate of GAD-65 and/or IA-2 autoantibodies were 89.4% in the first year after diagnosis but fell to 36.7% after nine years.
The rate of DKA at diagnosis in most studies was higher than in high-incidence countries[147]. Usher-Smith et al[147] found that lower-incidence countries generally had higher DKA rates, presumably due to decreased awareness. Less-resourced health systems also had higher DKA rates, and this factor may also be impacting rates in some WPR countries.
T1D HLA associations showed some variation compared to European-origin populations, with also some differences across the region.
Our review underscores the limited data on T2D in non-European youth from the WPR region, with six studies in indigenous populations conducted in Australia and New Zealand, and single studies from China, Hong Kong, Japan, South Korea and Fiji, as well as one study on emigrant Asian/Pacific youth to the United States. However, a clear finding is that T2D incidence exceeded the T1D rates in some countries, and unlike T1D were comparable to rates in European-origin populations. For instance, the incidence of T2D, detected by urine-glucose screening at schools in Tokyo, was higher compared with that of T1D (2.5-3.0/100000/year vs 2-2.5/100000/year, particularly among junior high school children aged 13-15 years (6.5/100000/year)[148]. On the other hand, the incidence of T2D in school children was increasing during 1975-1982, but there was decreased tendency in recent years. Lifestyle changes might contribute to improved incidence of T2D in Japanese school children. In contrast to this, the most recent data from South Korea[149], China[75] and Hong Kong[22,76] showed that incidence was increasing sharply.
While not addressed in detail in this review, several studies noted the phenotypic heterogeneity of T2D when compared to European-origin populations. While obesity or morbid obesity are a predominant feature in European origin youth with T2D, in Japan, for example, 10%-15% of youth with T2D are non-obese, with milder insulin resistance and substantial insulin secretion failure, in the absence of autoimmunity[148]. The genetic background likely plays a role[148], although more HLA and non-HLA genetic data are needed to further explore and support this hypothesis.
Overall, the high and, in some countries, increasing rates of T2D in the WPR region are concerning given their known and substantial risk of long-term complications and premature morbidity and mortality[150]. There is an urgent need for more and complete epidemiologic and phenotype data in youth with T2D from across the entire WPR in order to better understand and subsequently develop adequate and effective strategies that address T2D in youth as a public health concern.
Monogenic forms of diabetes were reported from various countries. Such disorders can present in the neonatal period or later in life. Genetic testing confirms diagnosis and helps identify selective forms responsive to alternate treatment: Most KCNJ11 and some ABCC8 mutations respond to oral sulphonylureas and so insulin can be discontinued, and also thiamine treatment is of benefit in SLC19A mutations (thiamine-resistant megaloblastic anaemia)[151]. In all monogenic cases, genetic counselling is indicated if desired by the family.
Given the population and number of countries in this region, many gaps in knowledge remain. A number of countries in WPR, including populous nations such as Indonesia, Philippines, and Vietnam, as well as a number of others, have no or minimal information published. Keeping this in mind as a major limitation, T1D with onset in childhood and adolescence is substantially less common in WPR than in European-origin populations, and incidence appears to be lower in South-East Asia than in Eastern Asia. The female preponderance differs from European-origin populations but is in line with the lower incidence rates. As incidence is rapidly increasing across the region, sex distribution will be informative to monitor. Age-of-onset, pancreatic autoantibody positivity rates and, at least across a large part of the WPR region HLA risk associations are similar to European-origin populations. Rates of DKA at onset are concerningly high across the region, consistent with published risk factors.
Data on youth-onset T2D are limited across WPR, with representations from only a handful of countries. Incidences are concerningly high and exceed those of T1D in some countries. Furthermore, rates are increasing.
Other forms of diabetes occur including various monogenic forms that also occur in European-origin and other populations.
Incidence studies are needed from all countries. A high ascertainment is needed, and it is preferable to have at least two overlapping data sources so a ‘capture-recapture’ method can be used[152]. Given the geographic size and ethnic diversity in some WPR countries, it is quite possible that T1D and T2D rates vary within countries as well. Establishment of registers will facilitate such incidence studies and also define prevalence and mortality, and assist in assessment of outcomes. These data will then inform quality of care improvements and health professional training, and assist in advocacy to improve provision of care by the respective government health system. An example of such a register is the “Thai Type 1 Diabetes and Diabetes Diagnosed Before Age 30 Years Registry, Care and Network”[153]. Table 8 gives recommendations for further research and interventions.
| No. | |
| 1 | Establishment registers of diabetes in young people in all countries, and, where necessary, in distinct geographic/ethnic regions within countries |
| 2 | Ongoing incidence, prevalence, and mortality studies for both T1D and T2D in all countries |
| 3 | Phenotype, endotype and genotype studies in youth with any type of diabetes |
| 4 | Education campaigns aimed at increasing awareness of the signs and symptoms of T1D and reducing rates of DKA at onset |
| 5 | Public health measures aimed at reducing incidence of T2D in young people |
| 6 | In-country/territory advocacy efforts, informed by updated and new epidemiological research, aimed at improving quality of care |
| 7 | Regional coordination and dissemination of data and research skills |
Type 1 diabetes (T1D) incidence varies, with most studies indicating increasing incidence. Reported rates are much lower in the Western Pacific region (WPR), than European-origin populations. Conversely, there are reports of substantial numbers of young people with type 2 diabetes (T2D).
A greater understanding of T1D and T2D in the WPR may highlight factors important in pathogenesis of these conditions. There is a need to determine the current burden of disease more clearly and also any gaps in knowledge, in view of varying funding and resources for diabetes treatment in this region.
To gather and summarise epidemiologic and phenotypic data on childhood diabetes in non-European populations in and from WPR.
A comprehensive systematic search of available literature was undertaken.
There are both differences and similarities compared to observations in European-origin populations. T1D was found to be less common, but generally has a classic phenotype. Some countries/territories have rapidly increasing incidence. T2D is relatively common. There are, however, many information gaps.
Given the population and number of countries in this region, many gaps in knowledge remain.
Registries and studies are needed to fill many information gaps. Establishment of registers will facilitate incidence studies and also define prevalence and mortality, and assist in outcome assessment. Such data will inform quality of care improvements, health professional training, and assist advocacy.
| 1. | International Diabetes Federation. IDF Diabetes Atlas. Ninth edition. International Diabetes Federation: Brussels. [cited 22 April 2021]. Available from: https://www.diabetesatlas.org/en/resources/. |
| 2. | Mayer-Davis EJ, Kahkoska AR, Jefferies C, Dabelea D, Balde N, Gong CX, Aschner P, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19 Suppl 27:7-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 392] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 3. | Tuomilehto J, Ogle GD, Lund-Blix NA, Stene LC. Update on Worldwide Trends in Occurrence of Childhood Type 1 Diabetes in 2020. Pediatr Endocrinol Rev. 2020;17:198-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 4. | Chan JC, Cho NH, Tajima N, Shaw J. Diabetes in the Western Pacific Region--past, present and future. Diabetes Res Clin Pract. 2014;103:244-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Oilinki T, Otonkoski T, Ilonen J, Knip M, Miettinen PJ. Prevalence and characteristics of diabetes among Somali children and adolescents living in Helsinki, Finland. Pediatr Diabetes. 2012;13:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Zung A, Elizur M, Weintrob N, Bistritzer T, Hanukoglu A, Zadik Z, Phillip M, Miller K, Koren I, Brautbar C, Israel S. Type 1 diabetes in Jewish Ethiopian immigrants in Israel: HLA class II immunogenetics and contribution of new environment. Hum Immunol. 2004;65:1463-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Tajima N, LaPorte RE, Hibi I, Kitagawa T, Fujita H, Drash AL. A comparison of the epidemiology of youth-onset insulin-dependent diabetes mellitus between Japan and the United States (Allegheny County, Pennsylvania). Diabetes Care. 1985;8 Suppl 1:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1511] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 10. | Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1146] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 11. | Fu JF, Liang L, Gong CX, Xiong F, Luo FH, Liu GL, Li P, Liu L, Xin Y, Yao H, Cui LW, Shi X, Yang Y, Chen LQ, Wei HY. Status and trends of diabetes in Chinese children: analysis of data from 14 medical centers. World J Pediatr. 2013;9:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L; SEARCH for Diabetes in Youth Study. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med. 2017;376:1419-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1093] [Article Influence: 121.4] [Reference Citation Analysis (0)] |
| 13. | World Health Organization. Western Pacific. [cited 20 January 2020]. Available from: https://www.who.int/westernpacific/about/where-we-work. |
| 14. | Ogle GD, Morrison MK, Silink M, Taito RS. Incidence and prevalence of diabetes in children aged <15 yr in Fiji, 2001-2012. Pediatr Diabetes. 2016;17:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Pulungan A. Increasing incidence of DM type 1 in Indonesia. 7th Asia Pacific Paediatric Endocrine Society Biennial Scientific Meeting (APPES) 2012 Indonesia, 2013. Int J Ped Endo. 2013;. |
| 16. | Panamonta O, Thamjaroen J, Panamonta M, Panamonta N, Suesirisawat C. The rising incidence of type 1 diabetes in the northeastern part of Thailand. J Med Assoc Thai. 2011;94:1447-1450. [PubMed] |
| 17. | Patarakijvanich N, Tunyapanit W, Kaewjungwad L. Rising of the incidence of diabetes mellitus type 1 in children of Southern Thailand. APPES Hormone Research 2008 South Korea. 2008. |
| 18. | Ogle GD, Lesley J, Sine P, McMaster P. Type 1 diabetes mellitus in children in Papua New Guinea. P N G Med J. 2001;44:96-100. [PubMed] |
| 19. | Onda Y, Sugihara S, Ogata T, Yokoya S, Yokoyama T, Tajima N; Type 1 Diabetes (T1D) Study Group. Incidence and prevalence of childhood-onset Type 1 diabetes in Japan: the T1D study. Diabet Med. 2017;34:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Song SO, Song YD, Nam JY, Park KH, Yoon JH, Son KM, Ko Y, Lim DH. Epidemiology of Type 1 Diabetes Mellitus in Korea through an Investigation of the National Registration Project of Type 1 Diabetes for the Reimbursement of Glucometer Strips with Additional Analyses Using Claims Data. Diabetes Metab J. 2016;40:35-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Kim J, Lee Y, Yang S. Incidence of type 1 diabetes among Korean children and adolescents in 2012-2013: Analysis of data from the nationwide registry of Korea. 54th Annual Meeting of the European Society for Paediatric Endocrinology, ESPE 2015 Spain. Horm Res Paediatr. 2015;84:190. |
| 22. | Tung J, Wong W, Wong S, Chung J, Ching-yin L, Chan P. The Hong Kong childhood diabetes registry 2008 to 2017. APPES 2018 Chang Mai Conference Abstract Book. 2018. [cited 12 January 21]. Available from: https://www.appes.org/members/meeting-archive/scientific-meetings/2018-chiang-mai-thailand/. |
| 23. | Lin WH, Wang MC, Wang WM, Yang DC, Lam CF, Roan JN, Li CY. Incidence of and mortality from Type I diabetes in Taiwan from 1999 through 2010: a nationwide cohort study. PLoS One. 2014;9:e86172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Lu CL, Shen HN, Chen HF, Li CY. Epidemiology of childhood Type 1 diabetes in Taiwan, 2003 to 2008. Diabet Med. 2014;31:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Writing Group for the SEARCH for Diabetes in Youth Study Group; Dabelea D, Bell RA, D'Agostino RB Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716-2724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 697] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 26. | Zhang H, Xia W, Yu Q, Wang B, Chen S, Wang Z, Love EJ. Increasing incidence of type 1 diabetes in children aged 0-14 years in Harbin, China (1990-2000). Prim Care Diabetes. 2008;2:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Gong C, Meng X, Saenger P, Wu D, Cao B, Wei L. Trends in the incidence of childhood type 1 diabetes mellitus in Beijing based on hospitalization data from 1995 to 2010. Horm Res Paediatr. 2013;80:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Gong C, Meng X, Jiang Y, Wang X, Cui H, Chen X. Trends in childhood type 1 diabetes mellitus incidence in Beijing from 1995 to 2010: a retrospective multicenter study based on hospitalization data. Diabetes Technol Ther. 2015;17:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Shen S, Chen Z, Zhi D, Zhao Z, Luo F. The epidemiology of type 1 diabetes mellitus in Shanghai children: a two decades retrospective. Abstracts of the 28th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Graz, Austria. J Pediatr Endocrinol Metab. 2002;15. |
| 30. | Gong CX, Zhu C, Yan C, Liang JP, Ni GC, Gao J, Li YC, Liu M, Peng XX, Yang Z. [Survey of type 1 diabetes incidence in children from 1997 to 2000 in Beijing area]. Zhonghua Er Ke Za Zhi. 2004;42:113-116. [PubMed] |
| 31. | Zhao Z, Sun C, Wang C, Li P, Wang W, Ye J, Gu X, Wang X, Shen S, Zhi D, Lu Z, Ye R, Cheng R, Xi L, Li X, Zheng Z, Zhang M, Luo F. Rapidly rising incidence of childhood type 1 diabetes in Chinese population: epidemiology in Shanghai during 1997-2011. Acta Diabetol. 2014;51:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Wu HB, Zhong JM, Hu RY, Wang H, Gong WW, Pan J, Fei FR, Wang M, Guo LH, Yang L, Yu M. Rapidly rising incidence of Type 1 diabetes in children and adolescents aged 0-19 years in Zhejiang, China, 2007 to 2013. Diabet Med. 2016;33:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Lee JH, Kim YM, Kwak MJ, Kim SY, Kim HJ, Cheon CK, Chung WY, Choi IJ, Hong SY, Chueh HW, Yoo JH. Incidence trends and associated factors of diabetes mellitus in Korean children and adolescents: a retrospective cohort study in Busan and Gyeongnam. Ann Pediatr Endocrinol Metab. 2015;20:206-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Tung JY, Kwan EY, But BW, Wong WH, Fu AC, Pang G, Tsang JW, Yau HC, Belaramani K, Wong LM, Wong SM, Lo P, Ng KL, Yeung WK, Chan KT, Chan AM, Wong SW, Tay MK, Chung J, Lee CY, Lam YY, Cheung PT. Increasing incidence of type 1 diabetes among Hong Kong children and adolescents: The Hong Kong Childhood Diabetes Registry 2008 to 2017. Pediatr Diabetes. 2020;21:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Urakami T, Suzuki J, Yoshida A, Saito H, Mugishima H. Incidence of children with slowly progressive form of type 1 diabetes detected by the urine glucose screening at schools in the Tokyo Metropolitan Area. Diabetes Res Clin Pract. 2008;80:473-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Imagawa A, Hanafusa T, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Shimizu I, Maruyama T, Makino H. Different contribution of class II HLA in fulminant and typical autoimmune type 1 diabetes mellitus. Diabetologia. 2005;48:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Shiga K, Urakami T, Suzuki J, Igarashi Y, Tajima H, Amemiya S, Sugihara S; Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT). Fulminant type 1 diabetes mellitus in Japanese children and adolescents: multi-institutional joint research of the Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes. Endocr J. 2018;65:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Tsutsumi C, Imagawa A, Ikegami H, Makino H, Kobayashi T, Hanafusa T; Japan Diabetes Society Committee on Type 1 Diabetes Mellitus Research. Class II HLA genotype in fulminant type 1 diabetes: A nationwide survey with reference to glutamic acid decarboxylase antibodies. J Diabetes Investig. 2012;3:62-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Miyamoto S, Asayama K, Sasaki N, Shiga K, Someya T, Yasusada K. Newly onset diabetic ketoacidotic children without elevation of HbA1c levels. Abstracts of the 29th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). St. Malo, France. J Pediatr Diabetes Endocrinol Metab. 2003;16:919-955. |
| 40. | Imagawa A, Hanafusa T, Iwahashi H, Uchigata Y, Kanatsuka A, Kawasaki E, Kobayashi T, Shimada A, Shimizu I, Maruyama T, Makino H. Uniformity in clinical and HLA-DR status regardless of age and gender within fulminant type 1 diabetes. Diabetes Res Clin Pract. 2008;82:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Luo S, Zhang Z, Li X, Yang L, Lin J, Yan X, Wang Z, Zheng C, Huang G, Zhou Z. Fulminant type 1 diabetes: a collaborative clinical cases investigation in China. Acta Diabetol. 2013;50:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Zheng C, Zhou Z, Yang L, Lin J, Huang G, Li X, Zhou W, Wang X, Liu Z. Fulminant type 1 diabetes mellitus exhibits distinct clinical and autoimmunity features from classical type 1 diabetes mellitus in Chinese. Diabetes Metab Res Rev. 2011;27:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Wang T, Xiao XH, Li WH, Yuan T, Sun XF, Wang H. Fulminant type 1 diabetes: report of two cases. Chin Med J (Engl). 2008;121:181-182. [PubMed] |
| 44. | Kim MS, Kim CJ, Ko CW, Hwang PH, Lee DY. Fulminant type 1 diabetes mellitus in Korean adolescents. J Pediatr Endocrinol Metab. 2011;24:679-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Chung S. Prevalence of diabetes among children and adolescents from 2002 to 2013 in Korea. 98th Annual Meeting and Expo of the Endocrine Society, ENDO 2016. United States. Endo Reviews, 2016: 37. |
| 46. | Lo FS, Yang MH, Chang LY, Ou YC, Van YH. Clinical features of type 1 diabetic children at initial diagnosis. Acta Paediatr Taiwan. 2004;45:218-223. [PubMed] |
| 47. | Jalaludin M, Harun F. Clinical presentation and frequency of diabetic ketoacidosis at first diagnosis of diabetes. Abstracts of the 31st Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Krakow, Poland. Pediatr Diabetes. 2005;6:1-75. |
| 48. | Trisorus C, Aroonparkmongkol S, Kongmanas HB, Sahakitrungruang T. Prevalence of islet autoantibodies in Thai juvenile-onset type 1 diabetes. Pediatr Int. 2018;60:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Li X, Huang C, Liu L. The distributions of HLA-DQ, DR alleles in type 1 diabetes children in Guangdong China. Abstracts of the 30th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Singapore. Pediatr Diabetes. 2004;5:1-66. |
| 50. | Sang Y, Yang W, Yan J, Wu Y. KCNJ11 gene mutation analysis on nine Chinese patients with type 1B diabetes diagnosed before 3 years of age. J Pediatr Endocrinol Metab. 2014;27:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Luo Y, He XX, Li LY. [Fulminant type 1 diabetes in a child]. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16:435-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Ren W, Xu H, Yan J, Yang D, Luo S, Zheng X. Differential clinical phenotypes between adult-onset and childhood-onset type 1 diabetes mellitus (T1DM) in a Chinese population. 76th Scientific Sessions of the American Diabetes Association (ADA). United States. Diabetes. 2016;65:A345. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Hu T, Cheng Y, Huang G, Li X, Zhou Z, Yang L. [Clinical features for hospitalized type 1 diabetic patients with different ages of onset]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2019;44:813-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Lubis S, Deliana M, Hakimi H. The obstacles in managing type 1 diabetes mellitus patients in H. Adam Malik Hospital, North Sumatera, Indonesia. 7th Asia Pacific Paediatric Endocrine Society Biennial Scientific Meeting (APPES) 2012. Indonesia. Int J Ped Endo. 2013;. |
| 55. | Urakami T, Suzuki J, Yoshida A, Saito H, Wada M, Takahashi S, Mugishima H. Autoimmune characteristics in Japanese children diagnosed with type 1 diabetes before 5 years of age. Pediatr Int. 2009;51:460-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Park Y, Lee H, Takino H, Abiru N, Kawasaki E, Eisenbarth GS. Evaluation of the efficacy of the combination of multiple autoantibodies to islet-specific antigens in Korean type 1 diabetic patients. Acta Diabetol. 2001;38:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Ahn CW, Kim HS, Nam JH, Song YD, Lim SK, Kim KR, Lee HC, Huh KB. Clinical characteristics, GAD antibody (GADA) and change of C-peptide in Korean young age of onset diabetic patients. Diabet Med. 2002;19:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Yu J, Lee S. Clinical features of childhood diabetes mellitus according to the classification focusing on autoantibody status. 98th Annual Meeting and Expo of the Endocrine Society, ENDO 2016. United States. Endo Reviews. 2016;372. |
| 59. | Lee YS, Ng WY, Thai AC, Lui KF, Loke KY. Prevalence of ICA and GAD antibodies at initial presentation of type 1 diabetes mellitus in Singapore children. J Pediatr Endocrinol Metab. 2001;14:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Lee S, Yu J. Clinical characteristics of slowly progressive autoimmune diabetes mellitus of youth in a single center. Abstracts for the 42nd Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD), 26-29 October 2016, Valencia, Spain. Pediatr Diabetes. 2016;17:1-129. |
| 61. | Ting WH, Huang CY, Lo FS, Hung CM, Chan CJ, Li HJ, Lin CH, Lee HC, Lee YJ. Clinical and laboratory characteristics of type 1 diabetes in children and adolescents: experience from a medical center. Acta Paediatr Taiwan. 2007;48:119-124. [PubMed] |
| 62. | Tung YC, Chen MH, Lee CT, Tsai WY. Beta-cell autoantibodies and their function in Taiwanese children with type 1 diabetes mellitus. J Formos Med Assoc. 2009;108:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Zhang H, Wang B, Zhao X, Sun J, Liang Z, Zhang D, Yang Z, Sun Y, Shen J. [The susceptible alleles on HLA-DRB 1 of type I diabetes in children in Harbin]. Zhonghua Liu Xing Bing Xue Za Zhi. 2000;21:267-269. [PubMed] |
| 64. | Sang Y, Yan C, Zhu C, Ni G. Relationship between HLA-DRB1 and DQ alleles and the genetic susceptibility to type 1 diabetes. Chin Med J (Engl). 2001;114:407-409. [PubMed] |
| 65. | Wang JP, Zhang C, Lin J, Yuan Y, Zhou HF, Huang G, Zhou M, Zhou ZG. [Relationship between autoantibodies and HLA-DQ genotypes in patients with type 1 diabetes mellitus]. Zhonghua Yi Xue Za Zhi. 2007;87:2380-2384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 66. | Liu CL, Yu YR, Liu H, Zhang XX, Zhao GZ. [The associations of HLA-DQB1 gene with onset age and autoantibodies in type 1 diabetes]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2004;21:368-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 67. | Chen BH, Chiang W, Yen JH, Chao MG. The influence of age and gender on HLA-DR in Chinese child-onset type 1 diabetes mellitus patients. Kaohsiung J Med Sci. 2000;16:393-399. [PubMed] |
| 68. | Sugihara S, Ogata T, Kawamura T, Urakami T, Takemoto K, Kikuchi N, Takubo N, Tsubouchi K, Horikawa R, Kobayashi K, Kasahara Y, Kikuchi T, Koike A, Mochizuki T, Minamitani K, Takaya R, Mochizuki H, Nishii A, Yokota I, Kizaki Z, Mori T, Shimura N, Mukai T, Matsuura N, Fujisawa T, Ihara K, Kosaka K, Kizu R, Takahashi T, Matsuo S, Hanaki K, Igarashi Y, Sasaki G, Soneda S, Teno S, Kanzaki S, Saji H, Tokunaga K, Amemiya S; Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT). HLA-class II and class I genotypes among Japanese children with Type 1A diabetes and their families. Pediatr Diabetes. 2012;13:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Sugihara S, Amemiya S, Ogata T, Kawamura T, Urakami T, Kikuchi N; The Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes. The first nationwide multicenter study on the HLADRB1, DQB1, DPB1 genotypes in Japanese children with type 1 diabetes and their families. Abstracts of the 36th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). 27-30 October 2010. Buenos Aires, Argentina. Pediatr Diabetes. 2010;11:1-120. |
| 70. | Yu J, Shin CH, Yang SW, Park MH, Eisenbarth GS. Analysis of children with type 1 diabetes in Korea: high prevalence of specific anti-islet autoantibodies, immunogenetic similarities to Western populations with "unique" haplotypes, and lack of discrimination by aspartic acid at position 57 of DQB. Clin Immunol. 2004;113:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Park Y, She JX, Wang CY, Lee H, Babu S, Erlich HA, Noble JA, Eisenbarth GS. Common susceptibility and transmission pattern of human leukocyte antigen DRB1-DQB1 haplotypes to Korean and Caucasian patients with type 1 diabetes. J Clin Endocrinol Metab. 2000;85:4538-4542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 72. | Tung YC, Fann CS, Chang CC, Chu CC, Yang WS, Hwu WL, Chen PL, Tsai WY. Comprehensive human leukocyte antigen genotyping of patients with type 1 diabetes mellitus in Taiwan. Pediatr Diabetes. 2018;19:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Thammarakcharoen T, Hirankarn N, Sahakitrungruang T, Thongmee T, Kuptawintu P, Kanoonthong S, Chongsrisawat V. Frequency of HLA-DQB1*0201/02 and DQB1*0302 alleles and tissue transglutaminase antibody seropositivity in children with type 1 diabetes mellitus. Asian Pac J Allergy Immunol. 2017;35:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Mochizuki M, Amemiya S, Kobayashi K, Ishihara T, Aya M, Kato K, Kasuga A, Nakazawa S. The association of Ala45Thr polymorphism in NeuroD with child-onset Type 1a diabetes in Japanese. Diabetes Res Clin Pract. 2002;55:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Wu H, Zhong J, Yu M, Wang H, Gong W, Pan J, Fei F, Wang M, Yang L, Hu R. Incidence and time trends of type 2 diabetes mellitus in youth aged 5-19 years: a population-based registry in Zhejiang, China, 2007 to 2013. BMC Pediatr. 2017;17:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Tung JY, Kwan EY, But BW, Wong WH, Fu AC, Pang G, Tsang JW, Yau HC, Belaramani K, Wong LM, Wong SM, Lo P, Ng KL, Yeung WK, Chan KT, Chan AM, Wong SW, Tay MK, Chung J, Lee CY, Lam YY, Cheung PT. Incidence and clinical characteristics of pediatric-onset type 2 diabetes in Hong Kong: The Hong Kong childhood diabetes registry 2008 to 2017. Pediatr Diabetes. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Liu LL, Yi JP, Beyer J, Mayer-Davis EJ, Dolan LM, Dabelea DM, Lawrence JM, Rodriguez BL, Marcovina SM, Waitzfelder BE, Fujimoto WY; SEARCH for Diabetes in Youth Study Group. Type 1 and Type 2 diabetes in Asian and Pacific Islander U.S. youth: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32 Suppl 2:S133-S140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Jiang YD, Chang CH, Tai TY, Chen JF, Chuang LM. Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000-2009 Nationwide Health Insurance database. J Formos Med Assoc. 2012;111:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 79. | Likitmaskul S, Wacharasindhu S, Rawdaree P, Ngarmukos C, Deerochanawong C, Suwanwalaikorn S, Chetthakul T, Bunnag P, Kosachunhanun N, Plengvidhaya N, Leelawatana R, Krittiyawong S, Benjasuratwong Y, Pratipanawatr T. Thailand diabetes registry project: type of diabetes, glycemic control and prevalence of microvascular complications in children and adolescents with diabetes. J Med Assoc Thai. 2006;89 Suppl 1:S10-S16. [PubMed] |
| 80. | Jaruratanasirikul S, Thammaratchuchai S, Sriplung H. Trends of childhood diabetes in Southern Thailand: 20-year experience in a tertiary medical center. World J Pediatr. 2017;13:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Feng DR, Meng Y, Zhao SM, Shi HP, Wang WC, Huang SZ. [Two novel EIF2AK3 mutations in a Chinese boy with Wolcott-Rallison syndrome]. Zhonghua Er Ke Za Zhi. 2011;49:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Sang Y, Ni G, Gu Y, Liu M. AV59M KCNJ11 gene mutation leading to intermediate DEND syndrome in a Chinese child. J Pediatr Endocrinol Metab. 2011;24:763-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 83. | Yang W, Wei H, Sang Y. KCNJ11 in-frame 15-bp deletion leading to glibenclamide-responsive neonatal diabetes mellitus in a Chinese child. J Pediatr Endocrinol Metab. 2013;26:743-746. [PubMed] |
| 84. | Huang K, Liang L, Fu JF, Dong GP. Permanent neonatal diabetes mellitus in China. BMC Pediatr. 2014;14:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Cao BY, Gong CX, Wu D, Li XQ. Permanent neonatal diabetes caused by abnormalities in chromosome 6q24. Diabet Med. 2017;34:1800-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Chen T, Zhang D, Bai Z, Wu S, Wu H, Xie R, Li Y, Wang F, Chen X, Sun H, Wang X, Chen L. Successful Treatment of Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar Status in an Infant with KCNJ11-Related Neonatal Diabetes Mellitus via Continuous Renal Replacement Therapy. Diabetes Ther. 2018;9:2179-2184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Yorifuji T, Nagashima K, Kurokawa K, Kawai M, Oishi M, Akazawa Y, Hosokawa M, Yamada Y, Inagaki N, Nakahata T. The C42R mutation in the Kir6.2 (KCNJ11) gene as a cause of transient neonatal diabetes, childhood diabetes, or later-onset, apparently type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005;90:3174-3178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Okuno M, Kuwabara R, Habu M, Yoshida A, Suzuki J, Yorifuji T, Urakami T, Takahashi S, Mugishima H. Successful treatment with oral glibenclamide in neonatal diabetes mellitus caused by KCNJ11 gene mutation. Abstracts of the 38th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). 10-13 October 2012. Istanbul, Turkey. Pediatr Diabetes. 2012;13:1-173. |
| 89. | Takagi M, Takeda R, Yagi H, Ariyasu D, Fukuzawa R, Hasegawa T. A case of transient neonatal diabetes due to a novel mutation in ABCC8. Clin Pediatr Endocrinol. 2016;25:139-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 90. | Cho JH, Kang E, Lee BH, Kim GH, Choi JH, Yoo HW. DEND Syndrome with Heterozygous KCNJ11 Mutation Successfully Treated with Sulfonylurea. J Korean Med Sci. 2017;32:1042-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Heo JW, Kim SW, Cho EH. Unsuccessful switch from insulin to sulfonylurea therapy in permanent neonatal diabetes mellitus due to an R201H mutation in the KCNJ11 gene: a case report. Diabetes Res Clin Pract. 2013;100:e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 92. | Santiprabhob J, Sawathiparnich P, Likitmaskul S, Chaichanwattanakul K, Nunloi S, Weerakulwattana L. Etiology and metabolic control of childhood and adolescent diabetes mellitus: an experience in Siriraj Hospital, Bangkok, Thailand. Abstracts for the 31st Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD), Krakow, Poland, 31 August-3 September, 2005. Pediatr Diabetes. 2005;6 Suppl 3:1-71. [PubMed] [DOI] [Full Text] |
| 93. | Mangla P, Tripathy M, Sudhanshu S, Joshi K. Neonatal diabetes: Some unique presentations. 9th Biennial Scientific Meeting of the Asia Pacific Paediatric Endocrine Society (APPES) and the 50th Annual Meeting of the Japanese Society for Pediatric Endocrinology (JSPE). Japan. Int J Ped Endo. 2017;15. |
| 94. | Ahn SY, Kim GH, Yoo HW. Successful sulfonylurea treatment in a patient with permanent neonatal diabetes mellitus with a novel KCNJ11 mutation. Korean J Pediatr. 2015;58:309-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Lo FS. Mutation screening of INS and KCNJ11 genes in Taiwanese children with type 1B diabetic onset before the age of 5 years. J Formos Med Assoc. 2018;117:734-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Lee JH, Tsai WY, Chou HC, Tung YC, Hsieh WS. Permanent neonatal diabetes mellitus manifesting as diabetic ketoacidosis. J Formos Med Assoc. 2003;102:883-886. [PubMed] |
| 97. | Jeerawongpanich K, De Franco E. Case report: Transient neonatal diabetes in a 31 weeks old Thai premature baby. 9th Biennial Scientific Meeting of the Asia Pacific Paediatric Endocrine Society (APPES) and the 50th Annual Meeting of the Japanese Society for Pediatric Endocrinology (JSPE). Japan. Int J Ped Endo. 2017;. |
| 98. | Ngoc C, Dung V, Thao N, Khanh N, Craig M, Hattersley A, NT H. Transient neonatal diabetes: A report of two cases. Abstracts of the 38th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). 10-13 October 2012. Istanbul, Turkey. Pediatr Diabetes. 2012;13:1-173. |
| 99. | Ngoc C, Dung V, Flanagan S. Neonatal diabetes in Wolcott-Rallison syndrome: A case report. Abstracts of the 39th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Gothenburg, Sweden. Pediatr Diabetes. 2013;14:1-162. |
| 100. | Ngoc C, Dung V, Thao B, Khanh N, Dat N, Craig M. Phenotype, genotype of neonatal diabetes mellitus due to insulin gene mutation. 8th Biennial Scientific Meeting of the Asia Pacific Paediatric Endocrine Society (APPES). 2014. Australia. Int J Ped Endo. 2015;12. |
| 101. | Can N, Vu D, Bui T, Nguyen K, Nguyen D, De Franco E. Molecular Characteristics in Vietnamese Patients with Neonatal Diabetic. 10th Joint Meeting of Paediatric Endocrinology, PES-APEG-APPES-ASPAE-CSPEM-ESPEJSPE- SLEP. United States. Horm Res Paediatr. 2017;88:465. |
| 102. | Takeda R, Takagi M, Miyai K, Shinohara H, Yagi H, Moritani M. A case of a Japanese patient with neonatal diabetes mellitus caused by a novel mutation in the ABCC8 gene and successfully controlled with oral glibenclamide. Clin Pediatr Endocrinol. 2015;244:191-193. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 103. | Cao B, Gong C, Di W, Lu C, Fang L. Genetic analysis and follow-up of 23 neonatal diabetes mellitus patients in China. ESPE Conference 2015 poster Spain. 2015. [cited 2 January 2021]. Available from: https://abstracts.eurospe.org/hrp/0084/hrpp2-248. |
| 104. | Craig M, Tran F, Vu D, Nguyen H, Bui T, Can N. Neonatal diabetes in Vietnam. Diabetes. 70th Scientific Sessions of the American Diabetes Association. Orlando, United States. [cited 12 January 2021]. Available from: https://professional.diabetes.org/search/site/Neonatal. |
| 105. | Can N, Vu D, Bui T, Nguyen K, Nguyen D, Nguyen H, Craig M, Ellard S. Molecular genetics in children with neonatal diabetes at Vietnam National Hospital of Pediatrics. Abstracts of the LWPES/ESPE 9th Joint Meeting Global Care in Paediatric Endocrinology, in collaboration with APEG, APPES, JSPE and SLEP. Italy. Horm Res Paediatr. 2013;80:1-489. |
| 106. | Ngoc C, Dung V, Thao B, Khanh N, Ellard S, Houghton J. Neonatal diabetes mellitus in Vietnam national children's hospital. ESPE Conference 2018 poster. Belgium. 2018. [cited 12 February 2021]. Available from: https://abstracts.eurospe.org/hrp/0089/hrpp3-p175.htm. |
| 107. | Li X, Liu L, Cheng J, Zhang W. Neonatal diabetes mellitus: A clinical analysis of 13 cases. Abstracts of the 36th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). 27-30 October 2010. Buenos Aires, Argentina. Pediatr Diabetes. 2010;11:1-120. |
| 108. | Yoon JS, Park KJ, Sohn YB, Lee HS, Hwang JS. Successful switching from insulin to sulfonylurea in a 3-month-old infant with diabetes due to p.G53D mutation in KCNJ11. Ann Pediatr Endocrinol Metab. 2018;23:154-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 109. | Choi J, Seo G, Oh A, Gu-Hwan K, Han-Wook Y. Frequency and etiologic spectrum of monogenic diabetes in pediatric diabetes in a single academic center. ESPE Conference 2018 poster Belgium. 2018. [cited 12 February 2021]. Available from: https://abstracts.eurospe.org/hrp/0089/hrpp2-p070. |
| 110. | Rochmah N, Faizi M. Diabetes mellitus, deafness in 2 years old child: Wolfram syndrome? Abstracts of the 39th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Gothenburg, Sweden. Pediatr Diabetes. 2013;14:1-162. |
| 111. | Liao Y, Ko Y. A rare genetic disorder in juvenile diabetes: Wolfram syndrome - case report. Hong Kong J Paediatr. 2015;203:172-175. |
| 112. | Lin CH, Lee YJ, Huang CY, Shieh JW, Lin HC, Wang AM, Shih BF. Wolfram (DIDMOAD) syndrome: report of two patients. J Pediatr Endocrinol Metab. 2004;17:1461-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 113. | Morikawa S, Nakamura A, Ishizu K. A novel heterozygous mutation of WFS1 gene in a Japanese infant of wolfram syndrome. Conference: 99th annual meeting of the endocrine society, ENDO 2017. United states. Endo Reviews. 2015;36. |
| 114. | Urakami T, Morimoto S, Kubota S, Owada M, Harada K, Nakagawa M. Pathogensis, prevention and treatment for diabetes melllitus in prader-willi syndrome. Abstracts of the 28th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Graz, Austria. Pediatr Diabetes. 2002;15. |
| 115. | Rahul Reddy C, Loke K, Lim Y, Goh S, Ho W. A rare case of Kearns Sayre syndrome with three co-existing endocrine complications in a child. Abstracts of the 44th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD), 11-14 October 2018, Hyderabad, India. Pediatr Diabetes. 2018;19:1-110. |
| 116. | Li X, Ting TH, Sheng H, Liang CL, Shao Y, Jiang M, Xu A, Lin Y, Liu L. Genetic and clinical characteristics of Chinese children with Glucokinase-maturity-onset diabetes of the young (GCK-MODY). BMC Pediatr. 2018;18:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Li X, Liu L, Liang C, Sheng H, Zhao X. [Maturity-onset diabetes of the young 2 with a novel mutation of glucokinase gene in a Chinese boy and the clinical follow-up]. Zhonghua Er Ke Za Zhi. 2014;52:867-871. [PubMed] |
| 118. | Xiuzhen L, Liu L, Sheng H, Liang C. Six Chinese children with MODY2 due to GCK gene mutations. Abstracts of the Joint Annual Conference of the International Society for Pediatric and Adolescent Diabetes and Australasian Paediatric Endocrine Group (ISPAD+APEG 2015), 7-10 October 2015, Brisbane, Australia. Pediatr Diabetes. 2015;16:1-101. |
| 119. | Zhang M, Zhou JJ, Cui W, Li Y, Yang P, Chen X, Sheng C, Li H, Qu S. Molecular and phenotypic characteristics of maturity-onset diabetes of the young compared with early onset type 2 diabetes in China. J Diabetes. 2015;7:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 120. | Lee L, Wong W, Yau H, Tsang W, Yuen Y. The importance of awaring monogenic diabetes in Chinese pediatric population-a case series. Abstracts for the 42nd Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD), Valencia, Spain. Pediatr Diabetes. 2016;17:1-129. |
| 121. | Deng M, Xiao X, Zhou L, Wang T. First Case Report of Maturity-Onset Diabetes of the Young Type 4 Pedigree in a Chinese Family. Front Endocrinol (Lausanne). 2019;10:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 122. | Ming-Qiang Z, Yang-Li D, Ke H, Wei W, Jun-Fen F, Chao-Chun Z, Guan-Ping D. Maturity onset diabetes of the young (MODY) in Chinese children: genes and clinical phenotypes. J Pediatr Endocrinol Metab. 2019;32:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 123. | Fujiwara M, Namba N, Miura K, Kitaoka T, Hirai H, Kondou H, Shimotsuji T, Numakura C, Ozono K. Detection and characterization of two novel mutations in the HNF4A gene in maturity-onset diabetes of the young type 1 in two Japanese families. Horm Res Paediatr. 2013;79:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 124. | Iwabuchi A, Kamoda T, Shinohara H, Sumazaki R. Japanese boy with maturity-onset diabetes of the young type 3 who developed diabetes at 19 months old. Pediatr Int. 2013;55:e32-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 125. | Yokota I, Moritani M, Sugihara S, Amemiya S; the Japanese Study Group of Insulin Therapy for Children and Adolescent Diabetes. Mutations of monogenic forms of diabetes, especially INS gene mutation, in Japanese children with type 1B diabetes. Abstracts of the LWPES/ESPE 9th Joint Meeting of Paediatric Endocrinology, in collaboration with APEG, APPES, JSPE and SLEP. Italy. Horm Res Paediatr. 2013;80:1-489. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 126. | Mine Y, Habu M, Suzuki J, Okuno M, Urakami T, Takahasi S. Clinical characteristics of 13 children with MODY. Abstracts of the Joint Annual Conference of the International Society for Pediatric and Adolescent Diabetes and Australasian Paediatric Endocrine Group (ISPAD+APEG), 7-10 October 2015, Brisbane, Australia. Pediatr Diabetes. 2015;16:1-101. |
| 127. | Ushijima K, Fukami M, Ayabe T, Okuno M, Narumi S, Ogata T. Next-generation sequencing-based screening of monogenic mutations in 43 Japanese children clinically diagnosed with type 1B diabetes. Abstracts of the 42nd Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD), 2016. Valencia, Spain. Pediatr Diabetes. 2016;17:165-176. |
| 128. | Horikawa Y, Enya M, Mabe H, Fukushima K, Takubo N, Ohashi M, Ikeda F, Hashimoto KI, Watada H, Takeda J. NEUROD1-deficient diabetes (MODY6): Identification of the first cases in Japanese and the clinical features. Pediatr Diabetes. 2018;19:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 129. | Konno H, Ohsugi K, Shiga K. Comparison of differences in clinical characters and courses between a girl with GCK-MODY and the normal sibling. Abstracts of the 44th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD), Hyderabad, India. Pediatr Diabetes. 2018;19:1-110. |
| 130. | Ushijima K, Kawamura T, Ogata T, Yokota I, Sugihara S, Narumi S. Functional characterization of a novel KLF11 mutation identified in a family with autoantibody-negative type 1 diabetes. Abstracts of the 57th Annual Meeting of the European Society for Paediatric Endocrinology (ESPE), 2018. Greece. Horm Res Paediatr. 2018;90:84-85. |
| 131. | Nam H, Baek J, Rhie Y. Characteristics according to autoantibodies and C-peptide level in children and adolescents with diabetes mellitus. 96th Annual Meeting and Expo of the Endocrine Society, ENDO 2014. United States. Endo Reviews. 2014;35. |
| 132. | Wong W. Early identification of monogenic diabetes: Implications on medical treatment and genetic counselling for an adolescent girl with MODY3. Abstracts of the 8th Biennial Scientific Meeting of the Asia Pacific Paediatric Endocrine Society (APPES) 2014. Australia. Int J Ped Endo. 2015;. |
| 133. | Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, Jacobsen LM, Schatz DA, Lernmark Å. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3:17016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 864] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 134. | Habu M, Kuwabara R, Okuno M, Suzuki J, Urakami T. Prevalences of antibodies to IA-2 and GAD at the time of diagnosis in children with type1 diabetes. 39th Annual Conference of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Gothenburg, Sweden. Pediatr Diabetes. 2013;14:1-162. |
| 135. | Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, Rami-Merhar B, Soltesz G, Svensson J, Parslow RC, Castell C, Schoenle EJ, Bingley PJ, Dahlquist G, Jarosz-Chobot PK, Marčiulionytė D, Roche EF, Rothe U, Bratina N, Ionescu-Tirgoviste C, Weets I, Kocova M, Cherubini V, Rojnic Putarek N, deBeaufort CE, Samardzic M, Green A. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. 2019;62:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 346] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 136. | Park Y. Why is type 1 diabetes uncommon in Asia? Ann N Y Acad Sci. 2006;1079:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 137. | Ogle GD, von Oettingen JE, Middlehurst AC, Hanas R, Orchard TJ. Levels of type 1 diabetes care in children and adolescents for countries at varying resource levels. Pediatr Diabetes. 2019;20:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 138. | Campbell-Stokes PL, Taylor BJ; New Zealand Children's Diabetes Working Group. Prospective incidence study of diabetes mellitus in New Zealand children aged 0 to 14 years. Diabetologia. 2005;48:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 139. | Zabeen B, Maniam J, Balsa AMM, Tayyeb S, Huda K, Azad K, Ogle GD. Incidence of diabetes in children and adolescents in Dhaka, Bangladesh. J Pediatr Endocrinol Metab. 2021;34:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 140. | Australian Institute of Health and Welfare. Incidence of Type 1 Diabetes in Australia 2000-2013. [cited 14 April 2021]. Available from: https://www.aihw.gov.au/reports/diabetes/incidence-type-1-diabetes-australia-2000-2013/contents/table-of-contents. |
| 141. | Lee S. Increasing incidence of type 1 diabetes mellitus among Korean children and adolescents in 2012: Analysis of data from the nationwide registry of Korea. 96th Annual Meeting and Expo of the Endocrine Society (ENDO). Chicago, United States. Endo Reviews. 2014;35. |
| 142. | Battaglia M, Ahmed S, Anderson MS, Atkinson MA, Becker D, Bingley PJ, Bosi E, Brusko TM, DiMeglio LA, Evans-Molina C, Gitelman SE, Greenbaum CJ, Gottlieb PA, Herold KC, Hessner MJ, Knip M, Jacobsen L, Krischer JP, Long SA, Lundgren M, McKinney EF, Morgan NG, Oram RA, Pastinen T, Peters MC, Petrelli A, Qian X, Redondo MJ, Roep BO, Schatz D, Skibinski D, Peakman M. Introducing the Endotype Concept to Address the Challenge of Disease Heterogeneity in Type 1 Diabetes. Diabetes Care. 2020;43:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 143. | Couper JJ, Haller MJ, Greenbaum CJ, Ziegler AG, Wherrett DK, Knip M, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2018: Stages of type 1 diabetes in children and adolescents. Pediatr Diabetes. 2018;19 Suppl 27:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 144. | Karvonen M, Pitkäniemi M, Pitkäniemi J, Kohtamäki K, Tajima N, Tuomilehto J. World Health Organization. DIAMOND Project Group. Sex difference in the incidence of insulin-dependent diabetes mellitus: an analysis of the recent epidemiological data. Diabetes Metab Rev. 1997;13:275-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 145. | Kawasaki E, Oikawa Y, Okada A, Kanatsuna N, Kawamura T, Kikuchi T, Terasaki J, Miura J, Ito Y, Hanafusa T. Zinc transporter 8 autoantibodies complement glutamic acid decarboxylase and insulinoma-associated antigen-2 autoantibodies in the identification and characterization of Japanese type 1 diabetes. J Diabetes Investig. 2020;11:1181-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 146. | Cheng BW, Lo FS, Wang AM, Hung CM, Huang CY, Ting WH, Yang MO, Lin CH, Chen CC, Lin CL, Wu YL, Lee YJ. Autoantibodies against islet cell antigens in children with type 1 diabetes mellitus. Oncotarget. 2018;9:16275-16283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 147. | Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia. 2012;55:2878-2894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 148. | Urakami T, Miyata M, Yoshida K, Mine Y, Kuwabara R, Aoki M, Suzuki J. Changes in annual incidence of school children with type 2 diabetes in the Tokyo Metropolitan Area during 1975-2015. Pediatr Diabetes. 2018;19:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 149. | Hong S, Kim H, Lee J, Yoo J. Epidemiologic characteristics of diabetes in children aged 0–14 years in Busan and Gyeonnam Province, Korea (2001–2010). APPES International journal of Pediatric Endocrinology 2012: Abstracts from the 7th Meeting, Indonesia. Int J Ped Endo. 2013;13. [DOI] [Full Text] |
| 150. | Zeitler P, Arslanian S, Fu J, Pinhas-Hamiel O, Reinehr T, Tandon N, Urakami T, Wong J, Maahs DM. ISPAD Clinical Practice Consensus Guidelines 2018: Type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19 Suppl 27:28-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 151. | Hattersley AT, Greeley SAW, Polak M, Rubio-Cabezas O, Njølstad PR, Mlynarski W, Castano L, Carlsson A, Raile K, Chi DV, Ellard S, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2018: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2018;19 Suppl 27:47-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 152. | International Diabetes Federation. International Diabetes Federation’s diabetes epidemiology guide. [cited 29 April 2021]. Available from: https://www.idf.org/our-activities/epidemiology-research/idf-guide-for-diabetes-epidemiology-studies.html. |
| 153. | Dejkhamron P, Santiprabhob J, Likitmaskul S, Deerochanawong C, Rawdaree P, Tharavanij T, Reutrakul S, Kongkanka C, Suprasongsin C, Numbenjapon N, Sahakitrungruang T, Lertwattanarak R, Engkakul P, Sriwijitkamol A, Korwutthikulrangsri M, Leelawattana R, Phimphilai M, Potisat S, Khananuraksa P, Nopmaneejumruslers C, Nitiyanant W; Thai Type 1 Diabetes and Diabetes Diagnosed Before Age 30 Years Registry, Care, and Network (T1DDAR CN). Type 1 diabetes management and outcomes: A multicenter study in Thailand. J Diabetes Investig. 2021;12:516-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 154. | Huen K, Low L, Cheung P, Wong G, But W, Kwan E. An update on the epidemiology of childhood diabetes in Hong Kong. Hong Kong J Paediatr. 2009;4:252-259. |
| 155. | Huo L, Ji L, Deng W, Shaw JE, Zhang P, Zhao F, McGuire HC, Kissimova-Skarbek K, Whiting D. Age distribution and metabolic disorders in people with Type 1 diabetes in Beijing and Shantou, China: a cross-sectional study. Diabet Med. 2018;35:721-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 156. | Weng J, Zhou Z, Guo L, Zhu D, Ji L, Luo X, Mu Y, Jia W; T1D China Study Group. Incidence of type 1 diabetes in China, 2010-13: population based study. BMJ. 2018;360:j5295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 157. | Lee W, Oh B, Lim S, Lim P, Tan W, Yap K. Changes in the epidemiology of childhood and adolescent diabetes in Singapore. Abstracts of the 32nd Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Cambridge, United Kingdom. Pediatr Diabetes. 2006;7:71-93. |
| 158. | Kim S, Kim E. Clinical characteristics and laboratory findings of children and adolescents with diabetes mellitus. Horm Res Paed. 2012;78:263. |
| 159. | Kim JH, Lee CG, Lee YA, Yang SW, Shin CH. Increasing incidence of type 1 diabetes among Korean children and adolescents: analysis of data from a nationwide registry in Korea. Pediatr Diabetes. 2016;17:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 160. | Lee HJ, Yu HW, Jung HW, Lee YA, Kim JH, Chung HR, Yoo J, Kim E, Yu J, Shin CH, Yang SW, Lee SY. Factors Associated with the Presence and Severity of Diabetic Ketoacidosis at Diagnosis of Type 1 Diabetes in Korean Children and Adolescents. J Korean Med Sci. 2017;32:303-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 161. | Panamonta O, Laopaiboon M, Tuchinda C. Incidence of childhood type 1 (insulin dependent) diabetes mellitus in northeastern Thailand. J Med Assoc Thai. 2000;83:821-824. |
| 162. | Khwanhatai K, Charoentawornpanich P, Pornpimol K, Narkdontri T, Tangjittipokin W, Preechasuk L. Sirraj pediatric diabetes registry: A tertiary care experience in Thailand. APPES 2018 Chang Mai Conference Abstract Book. 2018. [cited 12 January 21]. Available from: https://www.appes.org/members/meeting-archive/scientific-meetings/2018-chiang-mai-thailand/. |
| 163. | Xin Y, Yang M, Chen XJ, Tong YJ, Zhang LH. Clinical features at the onset of childhood type 1 diabetes mellitus in Shenyang, China. J Paediatr Child Health. 2010;46:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 164. | Tao N, Wang AP, Sun MY, Zhang HH, Chen YQ. [An investigation of ketoacidosis in children with newly diagnosed type 1 diabetes]. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:1066-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 165. | Patarakujvanich N, Tuchinda C. Incidence of diabetes mellitus type 1 in children of southern Thailand. J Med Assoc Thai. 2001;84:1071-1074. [PubMed] |
| 166. | Fuziah MZ, Hong JY, Zanariah H, Harun F, Chan SP, Rokiah P, Wu LL, Rahmah R, Jamaiyah H, Geeta A, Chen WS, Adam B. A national database on children and adolescent with diabetes (e-DiCARE): results from April 2006 to June 2007. Med J Malaysia. 2008;63 Suppl C:37-40. [PubMed] |
| 167. | Gunn ER, Albert BB, Hofman PL, Cutfield WS, Gunn AJ, Jefferies CA; Starbase Diabetes Working Group, Paediatric Diabetes Service, Starship Children's Hospital, Auckland, New Zealand. Pathways to reduce diabetic ketoacidosis with new onset type 1 diabetes: Evidence from a regional pediatric diabetes center: Auckland, New Zealand, 2010 to 2014. Pediatr Diabetes. 2017;18:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 168. | Park J, Oh J, Seong I. Autoantibody positivity and clinical characteristics of childhood diabetes. Abstracts of the 50th Annual Meeting of the European Society for Paediatric Endocrinology (ESPE). Glasgow, Scotland, United Kingdom. September 25-28, 2011. Horm Res Paediatr. 2011;76 Suppl 2:1-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 169. | Kim S, Kim E, Hwang J. Clinical characteristics and laboratory findings of children and adolescents with diabetes. Int J Ped Endo. 2013;15. [DOI] [Full Text] |
| 170. | Kim S, Kim E. Clinical characteristics and laboratory findings of children and adolescents with diabetes mellitus and obesity. Abstracts of the 40th Annual Conference of the International Society for Pediatric and Adolescent Diabetes (ISPAD), 3-6 September 2014, Toronto, Canada. Pediatr Diabetes. 2014;15:1-137. |
| 171. | Chen YC, Tung YC, Liu SY, Lee CT, Tsai WY. Clinical characteristics of type 1 diabetes mellitus in Taiwanese children aged younger than 6 years: A single-center experience. J Formos Med Assoc. 2017;116:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 172. | Likitmaskul S, Kiattisathavee P, Chaichanwatanakul K, Punnakanta L, Angsusingha K, Tuchinda C. Increasing prevalence of type 2 diabetes mellitus in Thai children and adolescents associated with increasing prevalence of obesity. J Pediatr Endocrinol Metab. 2003;16:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 173. | Patjamontri S, Santiprabhob J, Likitmaskul S. Diabetes mellitus among children and adolescents at Siriraj Hospital etiologies, clinical characteristics, glycemic control, and complications. Abstracts of the 38th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). 10-13 October 2012. Istanbul, Turkey. Pediatr Diabetes. Pediatr Diabetes. 2012;13:1-173. |
| 174. | Huang L. The frequency of autoantibodies positive (IAA, ICA, GADA) in type 1 diabetes children in Guangdong China. Abstracts of the 30th annual meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Singapore. Pediatr Diabetes. 2004;5:18. |
| 175. | Li H, Huang Q, Zhang S. The clinical significance of tyrosine phosphatase 2β antibody detection in patients with type 1 diabetes. Zhonghua Yixue Zazhi. 2008;14:939-942. |
| 176. | Baoerhan R, Maimaiti M. [Risk factors for type 1 diabetes among Uyghur children in Xinjiang, China]. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 177. | Iwabuchi A, Kamoda T, Tamai K, Shinohara H, Izumi I, Hirano T. Serum dipeptidyl peptidase 4 activity in children with type 1 diabetes mellitus indicates insulin insensitivity. Abstracts from the 9th Biennial Scientific Meeting of the Asia Pacific Paediatric Endocrine Society (APPES) and the 50th Annual Meeting of the Japanese Society for Pediatric Endocrinology (JSPE), Japan. Int J Pediatr Endocrinol. 2017;15. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 178. | Mabulac M. Frequency of glutamic acid dehydrogenaseantibodies among pediatric Filipino type 1 diabetes mellitus. Abstracts of the 39th Annual Meeting of the International Society for Pediatric and Adolescent Diabetes (ISPAD). Gothenburg, Sweden. Pediatr Diabetes. 2013;14:1-162. |
| 179. | Chen BH, Chung SB, Chiang W, Chao MC. GAD65 antibody prevalence and association with thyroid antibodies, HLA-DR in Chinese children with type 1 diabetes mellitus. Diabetes Res Clin Pract. 2001;54:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 180. | Santiprabhob J, Weerakulwattana P, Nunloi S, Kiattisakthavee P, Wongarn R, Wekawanich J, Nakavachara P, Chaichanwattanakul K, Likitmaskul S. Etiology and glycemic control among Thai children and adolescents with diabetes mellitus. J Med Assoc Thai. 2007;90:1608-1615. [PubMed] |
| 181. | Craig ME, Femia G, Broyda V, Lloyd M, Howard NJ. Type 2 diabetes in Indigenous and non-Indigenous children and adolescents in New South Wales. Med J Aust. 2007;186:497-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 182. | Tran F, Stone M, Huang CY, Lloyd M, Woodhead HJ, Elliott KD, Crock PA, Howard NJ, Craig ME. Population-based incidence of diabetes in Australian youth aged 10-18 yr: increase in type 1 diabetes but not type 2 diabetes. Pediatr Diabetes. 2014;15:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 183. | Haynes A, Kalic R, Cooper M, Hewitt JK, Davis EA. Increasing incidence of type 2 diabetes in Indigenous and non-Indigenous children in Western Australia, 1990-2012. Med J Aust. 2016;204:303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 184. | Urakami T, Kubota S, Nitadori Y, Harada K, Owada M, Kitagawa T. Annual incidence and clinical characteristics of type 2 diabetes in children as detected by urine glucose screening in the Tokyo metropolitan area. Diabetes Care. 2005;28:1876-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 185. | Jefferies C, Carter P, Reed PW, Cutfield W, Mouat F, Hofman PL, Gunn AJ. The incidence, clinical features, and treatment of type 2 diabetes in children <15 yr in a population-based cohort from Auckland, New Zealand, 1995–2007. Pediatr Diabetes. 2012;13:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 186. | Sjardin N, Reed P, Albert B, Mouat F, Carter PJ, Hofman P, Cutfield W, Gunn A, Jefferies C. Increasing incidence of type 2 diabetes in New Zealand children <15 years of age in a regional-based diabetes service, Auckland, New Zealand. J Paediatr Child Health. 2018;54:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yao K S-Editor: Fan JR L-Editor: A P-Editor: Fan JR