Published online Jan 9, 2022. doi: 10.5409/wjcp.v11.i1.48
Peer-review started: March 24, 2021
First decision: June 17, 2021
Revised: July 8, 2021
Accepted: December 2, 2021
Article in press: December 2, 2021
Published online: January 9, 2022
Processing time: 289 Days and 5.2 Hours
Children with attention-deficit/hyperactivity disorder (ADHD) often exhibit behaviour challenges and deficits in executive functions (EF). Psychostimulant medications [e.g., methylphenidate (MPH)] are commonly prescribed for children with ADHD and are considered effective in 70% of the cases. Furthermore, only a handful of studies have investigated the long-term impact of MPH medication on EF and behaviour.
To evaluate behaviour and EF challenges in children with ADHD who were involved in an MPH treatment trial across three-time points.
Thirty-seven children with ADHD completed a stimulant medication trial to study the short- and long-term impact of medication. Children with ADHD completed three neuropsychological assessments [Continuous Performance Test (CPT)-II, Digit Span Backwards and Spatial Span Backwards]. Parents of children with ADHD completed behaviour rating scales [Behaviour Rating Inventory of Executive Functioning (BRIEF) and Behaviour Assessment System for Children-Second Edition (BASC-2)]. Participants were evaluated at: (1) Baseline (no medication); and (2) Best-dose (BD; following four-week MPH treatment). Additionally, 18 participants returned for a long-term naturalistic follow up (FU; up to two years following BD).
Repeated measure analyses of variance found significant effects of time on two subscales of BRIEF and four subscales of BASC-2. Neuropsychological assess
Parents of children with ADHD reported improvements in EF and behaviours during the MPH trial but were not sustained at FU. Combining screening tools and neuropsychological assessments may be useful for monitoring medication responses.
Core Tip: Parents of children with attention-deficit/hyperactivity disorder reported improvements in executive function and behaviours during the methylphenidate trial, but these improvements did not sustain at the long-term follow up condition. Com
- Citation: Hai T, Duffy HA, Lemay JA, Lemay JF. Impact of stimulant medication on behaviour and executive functions in children with attention-deficit/hyperactivity disorder. World J Clin Pediatr 2022; 11(1): 48-60
- URL: https://www.wjgnet.com/2219-2808/full/v11/i1/48.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v11.i1.48
Deficits in executive function (EF) skills and behaviour challenges are commonly reported in children with attention-deficit/hyperactivity disorder (ADHD)[1,2]. ADHD, a neurodevelopmental disorder, is highly prevalent (5%-7%) in school-aged children[3,4]. Symptoms of ADHD typically include developmentally inappropriate levels of inattention, or impulsivity, and hyperactivity[5].
Children with ADHD often exhibit challenges associated with behaviour as well as EF[2]. In the literature, EF is an umbrella term that refers to a complex range of cognitive abilities, including working memory, goal-directed planning, impulse control, cognitive flexibility, and self-monitoring[6]. There is presently no consensus in the literature regarding the exact definition of EF, with upwards of 18 different available definitions included across studies[7]. Nevertheless, it is accepted that EF represents a family of top-down cognitive processes that are needed to make judgments and decisions and initiate purposeful behaviour[8]. As well, EF challenges are known to impact children with ADHD academically and behaviourally, as well as with their interpersonal relationships[2,9,10]. For instance, EF challenges can impact or affect performance at school, including task initiation, organizing thoughts to complete written assignments, using problem-solving skills to complete math calculations, switching from one task to another, and keeping track of task completion[11]. At home, EF challenges can manifest as trouble initiating or completing house chores, inflexibility to changing routines, or difficulty regulating and modulating emotions[12]. Socially, EF challenges may result in continual interruption of others or difficulty engaging in appropriate reciprocal conversation[9].
The measurement of EF in children with ADHD is generally done through either performance-based neuropsychological measures or behaviour rating scales. Both the performance-based measures and rating scales are considered to be reliable measures of EF[1]. However, the relationship between performance-based and behaviour ratings of EF is less clear, especially when evaluating whether they measure the same underlying construct. Furthermore, children with ADHD exhibit variable EF per
Currently, psychostimulant medications [e.g., methylphenidate (MPH)], along with behavioural interventions, are the most common treatment options for children with ADHD[14-17]. Stimulant medications are considered effective in about 70% of the cases[1,15], and the efficacy and safety of psychostimulants for the treatment of ADHD have been well documented[18]. Specifically, numerous research studies have consistently demonstrated that stimulants such as MPH improve executive and nonexecutive memory, reaction time, reaction time variability, and response inhibition in individuals with ADHD[18-20]. Short-term efficacy for pharmacological treatments is supported by all major evidence-based guidelines, including the Canadian ADHD Resource Alliance guidelines[15,16]. Conversely, findings related to the long-term impact of MPH, including the multimodal treatment of ADHD study (MTA), have been inconsistent with some studies finding sustained behavioural improvement following medication trials[21], while other studies failed to demonstrate long-term behavioural improvements[22,23].
Given that psychostimulant medications are commonly prescribed for children with ADHD[18], it is important to understand the developmental impact of these medi
The purpose of the present study was to investigate the short- and long-term (naturalistic FU) impact of stimulant medications in children with ADHD using both behaviour rating scales completed by parents and neuropsychological performance-based measures. The study aims to answer the following research questions:
(1) What are the changes in behaviour and EF as observed by parents of children with ADHD at baseline (BL; no medication) compared to best-dose (BD; MPH dose that was recommended by their primary care physician) condition (following a four-week trial of MPH treatment)?
(2) What are the changes in EF performance in children with ADHD at BL (no medication) compared to BD condition (following a four-week trial of MPH treat
(3) What are the changes in EF and behaviour at the long-term FU (6 mo to 2 years following long-acting MPH treatment trial) as observed by parents?
(4) What are the changes in EF performance at the long-term FU (6 mo to 2 years following long-acting MPH treatment trial)?
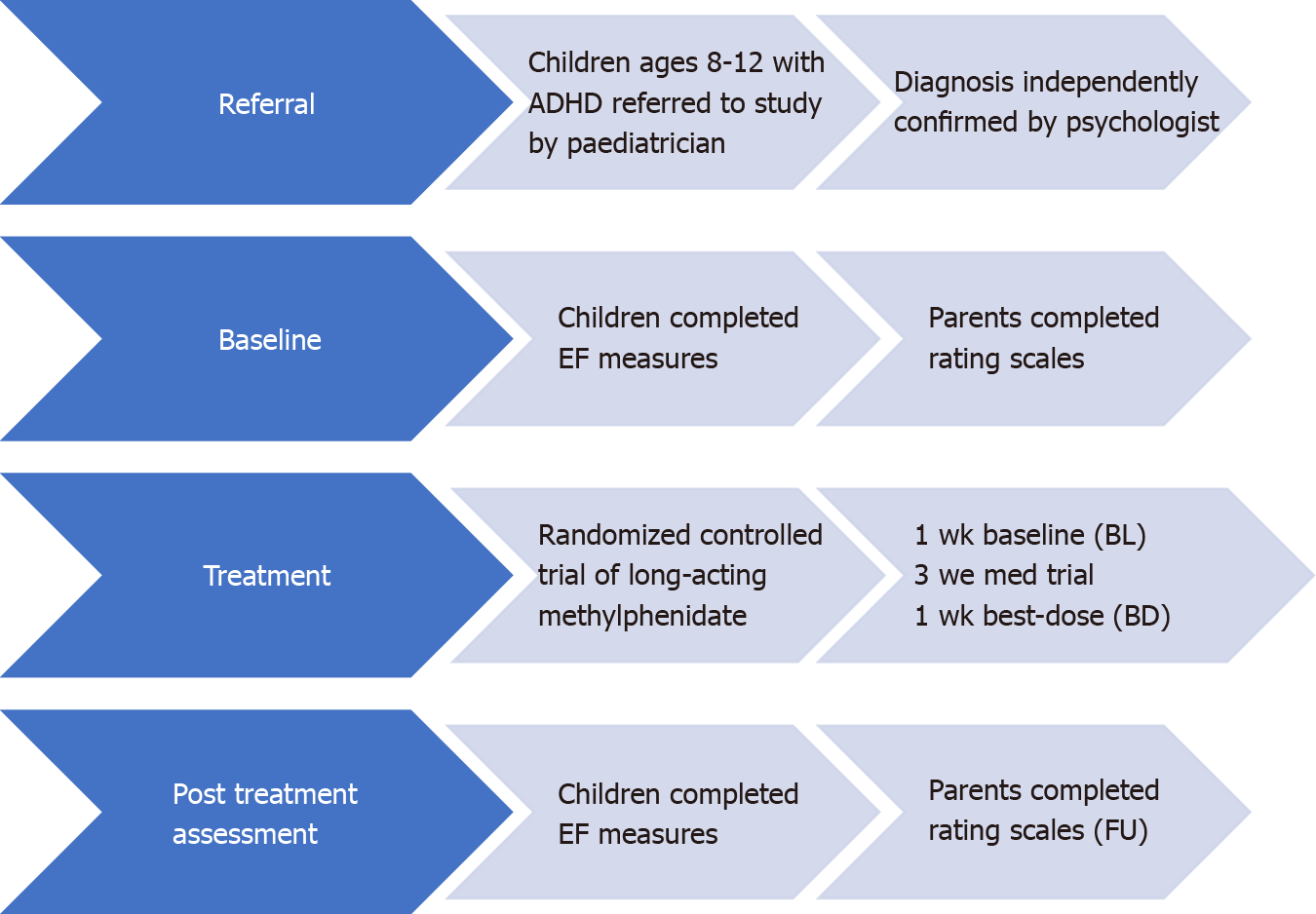
Children with ADHD: A total of 37 eligible participants with ADHD were included for analyses in the current study. Participants were excluded from the analyses if they did not return for the best-dose condition, were on medications at BL or did not meet the inclusion criteria. For the long-term naturalistic FU portion of the study, a total of 21 families elected to take part in the study.
All participants had to have: (1) A confirmed diagnosis of ADHD through a standard-of-care health professional prior to study participation; (2) The healthcare professional overseeing their progress and a diagnosis of ADHD; (3) Parent ratings of child’s current ADHD behaviour ratings using the Behaviour Assessment System for Children-Second Edition (BASC-2)[26], to indicate the child currently meets DSM-5 ADHD criteria[5]; and (4) A cognitive screener reporting no intellectual disability (scaled score > 4) on both the vocabulary multiple choice and the matrix reasoning subtests from the Wechsler Intelligence Scale for Children-Fourth Edition Integrated (WISC-IV Integrated)[27]. The children were not involved in any behavioural in
Neuropsychological measures: Children with ADHD completed neuropsychological measures related to working memory and inhibition. Parents of children with ADHD completed two additional standardized behaviour rating scales (questionnaires).
Conners Continuous Performance Test: The Conners Continuous Performance Test (CPT-II) is a computerized task that requires sustained attention to visually presented stimuli[28]. The CPT-II is a 15-min task, with a total of 360 trials where respondents are presented with letters appearing on a computer screen at varying rates (i.e., 1-, 2-, or 4-second inter-stimulus intervals). Participants are required to press the spacebar whenever a "target" letter appears on the screen and refrain from responding (i.e., pressing the spacebar) whenever the non-target stimulus (i.e., letter "X") appears. The CPT-II provides an array of scores following task completion. For the purposes of this study, only the Omission and the Commission errors score was evaluated. Omission errors indicate the number of times the child missed the target item when it was presented. Commission errors represent errors where the child incorrectly pressed the spacebar in response to the non-target stimulus. The reliability coefficient for omission and commission errors were 0.85 and 0.83 respectively. Test-retest reliability for omission and commission errors were 0.48 and 0.65, suggestive of adequate consis
WISC-IV Integrated Digit Span Backwards: Digit span tasks are used to evaluate verbal working memory. The Digit Span Backwards task requires children to listen to orally presented numbers with spans increasing in length and repeating in reverse order[27]. The number of digits recalled correctly in the reverse order is used for scoring purposes. Participants were awarded one point if they correctly repeated the sequence in backward order and zero points for an incorrect or incomplete answer or no response. The overall Digit Span Backward reliability coefficient is 0.81 for the normative sample, suggestive of good internal consistency. Test-retest reliability for the Digit Span Backward subtest was 0.74, indicating adequate stability across time[30].
WISC-IV Integrated Spatial Span Backwards: Spatial Span tasks are used to assess visuospatial working memory and require participants to encode and immediately recall a series of presented stimuli mentally. The WISC-IV Integrated Spatial Span board consists of ten cubes attached in a random order to a whiteboard. During the Spatial Span task, examinees observed the examiner tapping a prearranged sequence of blocks on the board at a rate of one block per second. Participants were required to tap the blocks in the reverse order of that demonstrated by the examiner. Participants were awarded one point if they tapped the blocks in the correct backward order or zero points if they provided an incorrect order or no response. The overall Spatial Span Backward task reliability coefficient for the normative sample was found to be 0.81, suggestive of good internal consistency[31].
Parent questionnaires: Parents in the current study completed two behaviour rating scales.
The Behaviour Assessment System for Children (BASC-2) is a widely utilized, norm-referenced rating scale designed to assess emotional, behavioural, and adaptive functioning among children and adolescents[27]. The parent rating scale (PRS) provides T-scores (M = 50; SD = 10) for four broad composite scales [externalizing problems (EP), internalizing problems (IP), behavioural symptoms index (BSI), and adaptive skills (AS)]. For the EP, IP, and BSI composites and associated clinical scales, T-scores of 70 and above are considered clinically significant and suggest a high level of maladjustment. In contrast, lower scores within the adaptive domain denote more problematic behaviours; T-scores of 30 and below are considered clinically significant. Reliability coefficients of the BASC-2 rating scale range between 0.90 and 0.95 for the composite scores, suggestive of strong internal consistency. The BASC-2 PRS com
The Behaviour Rating Inventory of Executive Functioning (BRIEF) was used to assess parental perceptions of EF skills[28,32]. The BRIEF is a questionnaire for parents of school-aged children (ages 5 to 18) that is used to determine a range of EF skills at home and in the community. The BRIEF parent form consists of 86 items within eight theoretically and empirically derived clinical scales and three composite scores that measure different aspects of EF. The BRIEF parent rating scale has high internal consistency (0.80 to 0.98) and test-retest reliability (0.82)[26].
The current study was part of a larger-scale project investigating the effect of medications on EF, academic, behavioural, and neuroimaging outcomes in children with ADHD. The larger study used a quasi-experimental, cross-sectional design with simple random sampling. ADHD participants were recruited through referrals from healthcare professionals in a Western Canadian city. The study research assistant conducted the ADHD screening measures to evaluate eligibility for the study before seeking informed consent for participating in the study. Parents completed the rating scales to ensure that their child met the eligibility criteria. If data from the parent behaviour rating scales did not indicate clinical range for attention and hyperactivity problems of at least 1.5 SDs above the norm for the child's age, the child and parent were thanked for their participation, and no further testing took place. Following receiving consent, the study research assistants completed additional screener assessment that included the two subtests from the WISC-IV Integrated intellectual screener. If the child was found to be intellectually deficient on the two WISC-Integrated screener measures (e.g., a scaled score of four or less, M = 10, SD = 3), the physician was notified, and the trial was terminated.
Participants completed assessments at three-time points, BL, post medication trial (BD) and at long-term naturalistic FU. All eligible participants were then scheduled for additional assessments.
BL: On the second testing session, eligible participants were scheduled to complete additional neuropsychological measures, and parents completed further questionnaires. The appointment lasted approximately 90 min. Participants and parents were thanked and compensated for their participation.
Post-treatment trial: Following taking medications for four weeks, participants returned to complete the same neuropsychological assessments completed at BL. Parents also completed rating scales.
FU: Parents of participants with ADHD, who were part of the initial medication trial, were invited to participate in an additional study component that included the completion of parent behaviour rating scales and neuropsychological testing. Families that participated in all components of the current study were evaluated at three separate time points: (1) BL: no medication; (2) BD: following a four-week trial of MPH treatment; and (3) Long-term naturalistic FU: 6 mo to 2 years following BD, see Figure 1.
The Statistical Package for the Social Sciences version 26 was used to conduct all analyses. A preliminary inspection of the data was performed for accuracy and examination of missing values and outliers before running any analyses. Additionally, the assumptions of normality and Mauchly’s Test of Sphericity were evaluated in order to conduct parametric data analyses[33].
Descriptive statistics such as mean and standard deviations were calculated. Repeated measures analyses of variance (RmANOVA) were conducted to evaluate changes in EF and behavioural challenges. Specifically, changes were measured between the BL and BD time points. Additionally, changes were measured between the BD and FU time points for participants participating in the long-term FU. Biological sex differences between boys and girls were also conducted across the different EF, behaviour, and adaptive skills ratings.
Table 1 presents the sample characteristics regarding their cognitive and behavioural screening measures.
| Variable | mean ± SD (n = 37) |
| Age | 10.11 ± 1.27 |
| Cognitive Tasks | |
| WISC-IV-I VC SS | 98.11 ± 11.69 |
| WISC-IV-I MR SS | 97.70 ± 12.89 |
| BASC-2 Attention Problem T-Score | 69.59 ± 6.31 |
| BASC-2 Hyperactivity T-Score | 71.73 ± 12.77 |
| WJ-III Reading | 90.49 ± 13.19 |
| WJ-III Math | 80.95 ± 13.86 |
| WJ-III Written Language | 87.03 ± 14.75 |
| Biological Sex | n (%) |
| Female | 16 (43.2) |
| Male | 21 (56.8) |
Table 2 summarizes the BASC-2 behavioural rating results. Analyses revealed a significant difference between BL and BD conditions, EP, F (1, 29) = 44.18, P ≤ 0.001, partial eta square = 0.60, IP, F (1, 29) = 19.98, P ≤ 0.001, partial eta square = 0.41, BSI, F (1, 29) = 83.04, P ≤ 0.001, partial eta square = 0.74, and AS scores, F (1, 29) range = 44.98, P ≤ 0.001, partial eta square = 0.61. Specifically, significant improvements across all behavioural indices (EP, IP, BSI) were observed in addition to a significant increase in adaptive skills between the BL and BD time points.
| Variable | BL T-score mean ± SD (n = 37) | BD T-score mean ± SD (n = 30) | BD-BL (P value) | FU T-score mean ± SD (n = 18) | FU-BD (P value) |
| Externalizing problems | 68.95 ± 13.20 | 54.83 ± 8.42 | P < 0.001 | 63.72 ± 10.26 | P = 0.003 |
| Internalizing problems | 62.86 ± 15.34 | 52.53 ± 13.0 | P < 0.001 | 60.89 ± 13.07 | P = 0.063 |
| Behaviour symptoms index | 72.32 ± 10.20 | 57.13 ± 7.73 | P < 0.001 | 68.06 ± 9.47 | P < 0.001 |
| Adaptive behaviours | 33.95 ± 8.92 | 40.57 ± 9.22 | P < 0.001 | 38.72 ± 8.79 | P = 0.124 |
Table 3 summarizes the BRIEF rating scale results. Similar to the BASC-2 scores, results from the BRIEF parent rating scale showed significant improvement from BL to BD condition, BRIEF behavioural regulation index [BRI; F (1, 30) = 90.48, P ≤ 0.001, partial eta square = 0.75) and metacognition index [MI; F (1, 30) = 94.38, P ≤ 0.001, partial eta square = 0.76).
| Variable | BL T-score mean ± SD | BD T-score mean ± SD | BD-BL | FU T-score mean ± SD | FU-BD |
| Behavioural regulation index | 72.43 ± 11.94 | 53.42 ± 8.78 | P < 0.001 | 68.28 ± 13.23 | P = 0.001 |
| Metacognition index | 74.76 ± 7.72 | 57.94 ± 8.48 | P < 0.001 | 72.06 ± 9.51 | P < 0.001 |
Results indicated significant differences in performance between BL and BD con
| Variable | BL T-score mean ± SD (n = 37) | BD T-score mean ± SD (n = 33) | BD-BL (P value) | FU T-score mean ± SD (n = 18) | FU-BD (P value) |
| CPT omission errors (T-Score) | 61.11 ± 15.76 | 52.88 ± 8.09 | P = 0.001 | 49.34 ± 5.90 | P = 0.10 |
| CPT commission errors (T-Score) | 53.59 ± 6.74 | 49.70 ± 11.75 | P = 0.097 | 49.69 ± 10.17 | P = 0.04 |
| Digit Span Backwards | 95.54 ± 11.04 | 99.19 ± 11.48 | P = 0.059 | 96.94 ± 15.54 | P = 0.055 |
| Spatial Span Backwards | 105.68 ± 12.42 | 108.23 ± 13.0 | P = 0.332 | 108.06 ± 12.96 | P = 0.782 |
Analyses revealed a significant effect of time on the EP, F (1, 16) = 12.73, P ≤ 0.01, partial eta square = 0.44, and BSI, F (1, 16) = 19.38, P ≤ 0.001, partial eta square = 0.55. Specifically, significant decrease in behaviour was observed by parents at FU time point (6 mo to 2 years after the MPH trial).
No significant difference was observed between BL and FU for the IP, F (1, 16) = 4.00, P ≥ 0.05, partial eta square = 0.20, and AS, F (1, 16) = 2.63, P ≥ 0.05, partial eta square = 0.14, suggesting no change in internalizing problems and adaptive skills were observed at the FU time.
No significant group differences were observed for any of the BASC-2 scales (EP, IP, BSI, AS) during the FU condition for individuals who were still taking medications compared to those who discontinued taking medications, F (4, 13) = 0.30, P ≥ 0.05. Lastly, no significant overall group differences emerged for any of the BASC-2 scales (EP, IP, BSI, AS) at the FU condition for biological sex, F (4, 13) = 2.35, P ≥ 0.05. However, when analyzing univariately, parents reported higher scores on the Internalizing Problems scale for females compared to males, F (1, 16) = 9.83, P ≤ 0.05).
The BRIEF parent ratings are presented in Table 3. The EF ratings completed by parents on the BRIEF revealed a significant effect over time: BRIEF BRI [F (1, 16) = 16.16, P ≤ 0.001, partial eta square = 0.50] and MI [F (1, 16) = 31/64, P ≤ 0.001, partial eta square = 0.66]. Specifically, results show an increase in symptom ratings between time points BD (BRI M = 54.47; MI M = 59.12) and FU time points (BRI M = 67.12; MI M = 71.71).
MANOVA was used to investigate the impact of medications on EF at the FU time point. Results indicated no significant differences between BRIEF ratings (BRI and MI) at FU condition between participants still taking medications compared to participants who had discontinued, F (2, 15) = 0.40, P ≥ 0.05. No significant overall biological sex differences between BRIEF ratings (BRI and MI) at FU condition were observed, F (2, 15) = 3.10, P ≥ 0.05. However, the univariate analyses indicated parents reporting higher BRIEF-MI ratings for males than for females, F (1, 16) = 6.10, P ≤ 0.05.
RmANOVA analyses were conducted to investigate the difference in neuropsychological test performance across the BD and FU. Results indicated significant differences over time on the CPT omission errors, F (1, 19) = 5.58, P ≤ 0.05, partial eta square = 0.28). No significant difference over time on the CPT commission errors, F (1, 19) = 3.80, P ≥ 0.05, partial eta square = 0.17, Digit Span Backwards, F (1, 15) = 4.31, P ≥ 0.05, partial eta square = 0.22 and spatial span backwards, F (1, 17) = 0.12, P ≥ 0.05, partial eta square = 0.007) tasks. Furthermore, MANOVA was used to investigate the impact of medications on EF performance measures at the FU time point. Results indicated no significant difference at FU condition between participants still taking medications compared to participants who had discontinued, F (4, 13) = 1.24, P ≥ 0.05. No biological sex differences on neuropsychological test performances were observed at the FU condition, F (4, 13) = 1.08, P ≥ 0.05.
The purpose of this study was to evaluate the short- and long-term impact of psychostimulant medications on EF and behaviour across three-time points in children with ADHD who were involved in a medication treatment trial.
In terms of parent behaviour ratings, parents observed improved behaviour in children with ADHD following the medication trial across various internalizing, externalizing, and adaptive domains. This is consistent with previous studies investigating the efficacy of stimulants for children with ADHD[14]. However, this improvement in parent behaviour ratings did not sustain at the naturalistic long-term FU condition, thus indicating that children with ADHD continue to struggle with behaviour challenges in the adolescent years. These results are in contrast to two of the previous naturalistic long-term FU studies where the authors did not find any significant difference between post-test and FU time points, except for inattention[24,25]. The observed differences in results could be due to different FU timelines between the studies, with the current study’s FU condition ranging from 6 mo to 2 years after initial MPH treatment compared to a range of 4.5-8.0 years after treatment in the other studies. Previous studies also included combined treatment modalities, whereas the current study only implemented pharmacotherapy intervention. It is also important to mention that the current findings are consistent with Molina et al[22] findings from the MTA study, the largest medication study to date with children with ADHD. This shows that the long-term impact of stimulant medication is variable across individuals and is dependent on other mediating and moderating factors[34].
A number of additional factors could have contributed to the lack of sustained behavioural improvement as measured by parent behaviour ratings. It is conceivable that children become tolerant to medication over time, and thus the effectiveness of the medication declines. Moreover, it is also plausible that adherence to medication was better in the BD medication condition compared to the FU condition when the children were no longer part of the treatment trial. Additionally, other external variables could have impacted the perceived effect of medications as reported by parents; for example, parents could have noticed heightened sleep and/or appetite issues as well as increased emotional lability, which may lead to increased perceived behavioural challenges. As well, it is possible that as children develop and reach the early adolescent years, they require more support to manage increasing educational and social demands. Thus, effective curricula and targeted interventions would be beneficial to complement medication treatment. Consequently, it is important for clinicians and other healthcare professionals to be aware of continued challenges in behaviour in children with ADHD during adolescent years.
Similar to the behaviour ratings described above, parents also reported significant improvements in EF skills as measured by the BRIEF parent rating scale. These results are consistent with previous studies where increases in EF skills were witnessed by parents following medication treatment[35]. However, the reported improvements in EF skills did not sustain at the long-term FU condition.
While some of the study participants did not continue with their medication treatment, there were no significant differences in EF ratings between the medicated and non-medicated groups, suggesting that other potential variables may have impacted the perceived efficacy of the medication during the FU condition. It is possible that as children with ADHD develop during their adolescent years, their EF challenges increase. Therefore, adolescents with ADHD would likely benefit from additional interventions to supplement medications to support this increasing need.
Given the discrepancies reported in the literature between parent rating scale and performance-based measures[1], the impact of stimulant medication on neuropsychological test performance was also evaluated. Results showed improved performance following the medication trial on the CPT omission errors score. However, CPT commission errors did not change following the four-week medication trial. Similarly, performance on the two working memory tasks (Digit Span Backwards and Spatial Span Backwards) did not change following the medication trial.
At the long-term FU condition, performance on the CPT omission decreased, and the improvement shown after the medication trial did not sustain. There were no significant changes in performance on the CPT commission error and the two working memory tasks. It is possible that these differences in performance could be task specific as the CPT-II task requires sustained attention and concentration. By way of comparison, the digit span backwards and the spatial span backwards is a much shorter task. It is also possible that children with ADHD need additional interventions on top of medications as they enter their early adolescent years.
While this study adds valuable information to the existing literature on ADHD, the observed results should still be evaluated in the context of some limitations. We included a naturalistic FU where it is possible for participants to follow other psychosocial treatments or stop treatment after the post-test, possibly causing differences between initial treatment conditions at FU. Another notable limitation of the current study was the sample size as not all participants enrolled in the medication trial returned for the naturalistic FU portion of the study. While this research included an appropriate sample size to obtain statistically significant findings, the sample size is still considered small. As such, future studies need to be conducted to replicate the results. The small sample size also did not allow investigation of differences between the different presentations of ADHD; as such, the varying presentation subtypes (i.e., inattentive and combined) were collapsed into one heterogeneous group. Another limitation that was not considered in this study is the changes in lifestyle habits of the children with ADHD. It is possible that changes in sleep, diet and appetite could have impacted the effect of the stimulant medication. Lastly, this study only included data from parents. It would have been beneficial to obtain teacher ratings as well, in order to understand the impact of medications at school.
The current study provided valuable information about the impact of stimulant medication on behaviour and EF in children with ADHD. Results showed im
Children with attention-deficit/hyperactivity disorder (ADHD) often exhibit be
The main topics investigated in the current study were to measure EF and behaviour challenges in children with ADHD using both parent rating scale and neuropsychological assessment measures.
The main objectives of the current study were to evaluate behaviour and EF challenges in children with ADHD who were involved in a MPH treatment trial. The participants were assessed across three-time points using both parent rating scale and neuropsychological assessment measures to understand the short-term and long-term natu
Thirty-seven children with ADHD completed a stimulant medication trial (MPH). Children with ADHD completed neuropsychological assessments assessing working memory (Digit Span Backwards and Spatial Span Backwards) and response inhibition (Continuous Performance Test-2). Parents of children with ADHD completed be
The results of the current study found significant effects over time on two subscales of BRIEF and four subscales of BASC-2 measures indicating impact on behaviour and EF according to parents. Neuropsychological assessments showed some improvement, but not on all tasks following the medication trial. These improvements did not sustain at FU, with increases in EF and behaviour challenges and a decline in performance on the CPT-II task being observed.
Parents of children with ADHD reported improvements in EF and behaviours during the MPH trial but were not sustained at FU. Neuropsychological assessment findings were not consistent with participants showing improvement on some response inhibition tasks but not on the working memory tasks. As a result, it is important to combine screening tools and neuropsychological assessments for monitoring medi
The current study provided information about the impact of stimulant medication on behaviour and EF in children with ADHD. Results showed improvement in EF skills and behaviour in children with ADHD following medication treatment. These improvements were reported by parents through standardized behaviour rating scales. Neuropsychological tests of response inhibition also showed improved per
The authors would like to thank the children and adolescents and their families for their participation. Thank you to Linda Beatty for her assistance in manuscript formatting.
| 1. | Toplak ME, Bucciarelli SM, Jain U, Tannock R. Executive functions: performance-based measures and the behavior rating inventory of executive function (BRIEF) in adolescents with attention deficit/hyperactivity disorder (ADHD). Child Neuropsychol. 2009;15:53-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 260] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2234] [Cited by in RCA: 2249] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 3. | Brault MC, Lacourse É. Prevalence of prescribed attention-deficit hyperactivity disorder medications and diagnosis among Canadian preschoolers and school-age children: 1994-2007. Can J Psychiatry. 2012;57:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43:434-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1344] [Cited by in RCA: 1164] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 5. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th edition). Am J Psychiatry. 2013;991. [RCA] [DOI] [Full Text] [Cited by in Crossref: 66101] [Cited by in RCA: 61237] [Article Influence: 3602.2] [Reference Citation Analysis (5)] |
| 6. | Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD). J Abnorm Child Psychol. 2001;29:541-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 377] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | Wasserman T, Wasserman LD. Toward an integrated model of executive functioning in children. Appl Neuropsychol Child. 2013;2:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Duff CT, Sulla EM. Measuring Executive Function in the Differential Diagnosis of Attention-Deficit/Hyperactivity Disorder: Does It Really Tell Us Anything? Appl Neuropsychol Child. 2015;4:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Kofler MJ, Rapport MD, Bolden J, Sarver DE, Raiker JS, Alderson RM. Working memory deficits and social problems in children with ADHD. J Abnorm Child Psychol. 2011;39:805-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, Morgan CL, Faraone SV. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J Consult Clin Psychol. 2004;72:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 486] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 11. | Graham S, Fishman EJ, Reid R, Hebert M. Writing Characteristics of Students with Attention Deficit Hyperactive Disorder: A Meta-Analysis. Learn Disabil Res Pract. 2016;31:75-89. [DOI] [Full Text] |
| 12. | Mash EJ, Barkley RA. Child psychopathology. 3rd ed. New York, NY, US: The Guilford Press, 2014: 1010. |
| 13. | Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A. 2012;109:6769-6774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 366] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 14. | A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2382] [Cited by in RCA: 1986] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 15. | Canadian Attention Deficit Hyperactive Disorder Research Association. CADDRA Guidelines 4th ed (2018) [Internet]. 2018; 91: 399-404 [cited 27 September 2020]. Available from: www.caddra.ca. |
| 16. | Wolraich ML, Hagan JF Jr, Allan C, Chan E, Davison D, Earls M, Evans SW, Flinn SK, Froehlich T, Frost J, Holbrook JR, Lehmann CU, Lessin HR, Okechukwu K, Pierce KL, Winner JD, Zurhellen W; Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactive Disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144:e20192528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 831] [Article Influence: 118.7] [Reference Citation Analysis (0)] |
| 17. | Van der Oord S, Prins PJ, Oosterlaan J, Emmelkamp PM. Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: a meta-analysis. Clin Psychol Rev. 2008;28:783-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, Atkinson LZ, Tessari L, Banaschewski T, Coghill D, Hollis C, Simonoff E, Zuddas A, Barbui C, Purgato M, Steinhausen HC, Shokraneh F, Xia J, Cipriani A. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5:727-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 923] [Cited by in RCA: 830] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 19. | Coghill D, Banaschewski T, Lecendreux M, Soutullo C, Johnson M, Zuddas A, Anderson C, Civil R, Higgins N, Lyne A, Squires L. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23:1208-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Chamberlain SR, Robbins TW, Winder-Rhodes S, Müller U, Sahakian BJ, Blackwell AD, Barnett JH. Translational approaches to frontostriatal dysfunction in attention-deficit/hyperactivity disorder using a computerized neuropsychological battery. Biol Psychiatry. 2011;69:1192-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Epstein JN, Hoza B, Hechtman L, Abikoff HB, Elliott GR, Greenhill LL, Newcorn JH, Wells KC, Wigal T, Gibbons RD, Hur K, Houck PR; MTA Cooperative Group. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 873] [Cited by in RCA: 730] [Article Influence: 42.9] [Reference Citation Analysis (1)] |
| 23. | Abikoff H, Nissley-Tsiopinis J, Gallagher R, Zambenedetti M, Seyffert M, Boorady R, McCarthy J. Effects of MPH-OROS on the organizational, time management, and planning behaviors of children with ADHD. J Am Acad Child Adolesc Psychiatry. 2009;48:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | van der Oord S, Prins PJ, Oosterlaan J, Emmelkamp PM. The adolescent outcome of children with attention deficit hyperactivity disorder treated with methylphenidate or methylphenidate combined with multimodal behaviour therapy: results of a naturalistic follow-up study. Clin Psychol Psychother. 2012;19:270-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Döpfner M, Ise E, Breuer D, Rademacher C, Metternich-Kaizman TW, Schürmann S. Long-Term Course After Adaptive Multimodal Treatment for Children With ADHD: An 8-Year Follow-Up. J Atten Disord. 2020;24:145-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Reynolds CR, Kamphaus RW. Behavior Assessment System for Children (BASC). 2nd ed. Circle Pines, MN: American Guidance Service, 2004. |
| 27. | Kaplan E, Fein D, Maerlander A, Morris R, Kramer J. Wechsler intelligence scale for children, fourth edition (Integrated). 4th ed. San Antonio, TX: The Psychological Corporation; 2004. |
| 28. | Conners K. Conners Continuous Performance Test II Pearson Assessment. 2004. |
| 29. | Soreni N, Crosbie J, Ickowicz A, Schachar R. Stop signal and Conners' continuous performance tasks: test--retest reliability of two inhibition measures in ADHD children. J Atten Disord. 2009;13:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Wechsler D, Kaplan E, Fein D, Kramer J, Morris R, Delis D, Maerlender A. Wechsler intelligence scale for children, fourth edition, integrated. San Antonio, TX: The Psychological Corporation, 2004. |
| 31. | Watkins MW, Dombrowski SC, Canivez GL. Reliability and factorial validity of the Canadian Wechsler Intelligence Scale for Children–Fifth Edition. Int J Sch Educ Psychol. 2018;6:252-265. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Gioia GA, Isquith PK, Guy SC, Kenworthy L, Baron IS. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6:235-238. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1141] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 33. | Tabachnick BG, Fidell LS. Using Multivariate Statistics, 6th ed. Toronto, ON, Canada: Pearson Education, 2013: 1–1018. |
| 34. | Hinshaw SP. Moderators and mediators of treatment outcome for youth with ADHD: understanding for whom and how interventions work. Ambul Pediatr. 2007;7:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Findling RL, Adeyi B, Dirks B, Babcock T, Scheckner B, Lasser R, DeLeon A, Ginsberg LD. Parent-reported executive function behaviors and clinician ratings of attention-deficit/hyperactivity disorder symptoms in children treated with lisdexamfetamine dimesylate. J Child Adolesc Psychopharmacol. 2013;23:28-35. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nassar G, Pillar G S-Editor: Zhang H L-Editor: A P-Editor: Zhang H