INTRODUCTION
Short chain fatty acids (SCFAs), in particular butyric acid, play important roles in human intestinal health. They are the major source of energy for the colonic mucosal cells[1]. Maintaining a certain level of butyric acid production in the lumen can help to balance gut microbiota, regulate host immune response, and enhance intestinal mucosal barrier function. When butyrate is taken orally in food or as a medicine, it is digested and absorbed by the body before it reaches the colon, making it difficult for butyrate to perform its functions in the hindgut. Butyrate-producing bacteria are capable of fermenting undigested carbohydrates in the intestinal lumen, producing acidifying SCFAs such as butyric acid. Therefore, butyrate-producing bacteria may be used as probiotics with the goal of promoting gut health, and thus having a wide range of potential clinical applications[2]. This minireview focuses on recent research on butyrate-producing bacteria and their potential clinical applications, especially in disorders related to pediatrics.
BUTYRATE-PRODUCING BACTERIA AND THEIR MAIN PHYSIOLOGICAL FUNCTIONS
Butyrate-producing bacteria are not a coherent phylogenetic group but rather a group of commensal intestinal flora that can ferment carbohydrates and produce butyric acid[2,3]. Both lactic acid and acetic acid can be used as substrates in the biochemical synthesis of butyric acid[3]. The majority of Firmicutes are butyrate-producing bacteria. At the genus level, Ruminococcus, Clostridium, Eubacterium, and Coprococcus are common butyrate-producing bacteria. Clostridium butyricum (C. butyricum) is relatively common in the Clostridium genus[4]. Others include Faecalibacterium, Butyrivibrio, etc.,[5]. In the genus Eubacterium, Eubacterium Hallii (E. Hallii) and Eubacterium Rectale are among the most abundant butyrate-producing bacterial strains in human feces[6]. Actinomycetes, Bacteroidetes, Proteobacteria, Spirochetes also have been identified as potential butyrate-producing bacteria[2].
The butyrate-producing commensal bacteria are mainly anaerobes. The acidic environment generated by butyrate-producing bacteria during metabolism keeps a balanced microbiota and maintains a normal microecological environment in the intestinal tract. Therefore, butyrate-producing bacteria act as probiotics and play important roles in a variety of normal biological functions, such as maintaining the mucosal barrier, improving immunity, and facilitating nutrient digestion and absorption in animals[7]. Like other probiotics, butyrate-producing bacteria can ferment carbohydrates to produce SCFAs and synthesize folic acid, pyridoxol, vitamin B1 and other vitamins[8,9]. By using an in vitro model of the colonic mucosa barrier, Lewis et al[10] have shown that butyrate can ameliorate increased translocation of bacteria across metabolically stressed intestinal epithelia. With a similar model, we have shown previously that butyrate can enhance the intestinal barrier function by facilitating the assembly of tight junctions through the activation of AMP-activated protein kinase (AMPK) and have demonstrated that butyrate is important in the maintenance and regulation of the barrier function of the colonic epithelium[11]. Also, Wang et al[12] recently demonstrated that butyrate dynamically regulates intestinal homeostasis through regulation of synaptopodin, an actin-binding protein that is critical for barrier integrity and cell motility. Therefore, it is evident that production of butyrate in the intestinal lumen is vital for the maintenance of the intestinal mucosal barrier.
Butyrate is a potent histone deacetylase inhibitor, which can promote the proliferation and activation of regulatory T-cells (Treg cells) and thereby play an important role in the immune regulation[13,14]. Microbiota-derived butyrate can reduce the release of pro-inflammatory cytokines by regulating the activity of G protein-coupled receptors, NF-κB, JAK/STAT and other inflammation-related pathways, thereby inhibiting intestinal inflammation and maintaining intestinal immune balance[15]. In addition to the direct effects on the mucosal barrier, microbiota-derived butyrate can be absorbed and directly transmitted to mesenteric lymph nodes, into the lymphatic system, and then into the systemic circulation, affecting other organ systems. NF-kB pathway is involved in the expression of tumor necrosis factor (TNF), interleukin (IL)-1, IL-6 and other inflammation-related genes in the immune and inflammatory responses. The role of butyrate is to inhibit NF-κB from entering the nucleus. Without active NF-κB, the mRNA of pro-inflammatory factors cannot be transcribed and pro-inflammatory factors will not be expressed, resulting in inflammatory response inhibition[15]. Studies have shown that butyrate regulates the function of T cells in the induction of colitis by differentially regulating Th1 and Th17 cell differentiation, thus modulating the production of inflammatory cytokines[16,17]. Moreover, butyrate can inhibit the release of IL-12, TNF-α, IL-1β and nitric oxide in monocytes, up-regulate the expression of IL-10, and reduce the activity of NF-κB, thereby playing an anti-inflammatory role in other organ systems, such as the respiratory system[18]. In short, as the major source of energy for the colonic mucosa and as an important regulator of gene expression, inflammation, differentiation and apoptosis in host cells, microbiota-derived butyrate enhances the role of the intestinal mucosal immune barrier, modulates the systemic immune response, and thus prevents bacteria and their metabolites from entering the bloodstream and causing inflammation[19,20].
POTENTIAL CLINICAL APPLICATIONS OF BUTYRATE-PRODUCING BACTERIA
Maintenance of the intestinal mucosal barrier
A monolayer of intestinal epithelial cells separates the body tissues from the dense communities of bacteria in the intestinal lumen. Therefore, maintenance of the mucosal epithelial barrier that prevents the invasion of host tissues by resident bacteria is vital for normal intestinal function. It is well known that the main energy source for the colonic epithelium is derived directly from the lumen rather than from blood. More than 90% of SCFAs produced in the intestinal lumen by bacterial fermentation are normally absorbed by intestinal epithelial cells. Lack of luminal SCFAs or the inability to oxidize butyrate leads to a nutritional deficiency of the colonic epithelium, causing mucosal atrophy in the short term and ‘nutritional colitis’ in the long term[1]. In patients with ulcerative colitis, the ability of the colonic epithelial cells to oxidize butyrate is weakened, so the energy obtained through oxidation is reduced; and thus the ability of butyrate to repair colonic mucosa is decreased[21]. The depletion of gut commensal flora by a prolonged course of broad spectrum of antibiotics can lead to more severe intestinal mucosal injury in a dextran sulfate sodium (DSS)-induced mouse colitis model[22]. Furthermore, reduced abundance of butyrate-producing commensal bacteria species has been found in the fecal microbial community in patients with inflammatory bowel disease (IBD)[23,24].
Probiotics have been advocated in clinical practice for prevention or treatment of intestinal mucosal injury associated with IBD or neonatal necrotizing enterocolitis (NEC)[25,26]. In children with IBD, a specific probiotic preparation (VSL#3) combined with Lactobacillus was shown to have a significant effect in achieving a clinical response[27]. A study in an animal model of DSS-induced colitis has shown that administration of C. butyricum, one of the butyrate-producing bacterial strains, can increase the luminal production of butyrate in the cecum and alleviate DSS-induced injury to colonic mucosa[28]. C. butyricum may induce intestinal macrophages to secrete IL-10, thereby inhibiting the occurrence of experimental colitis[29]. Geirnaer et al[30] used an in vitro system to examine the response of microbiota from patients with Crohn’s disease to the treatment with different combinations of butyrate-producing bacterial stains. They assessed the effects of butyrate-producing bacteria supplementation on short-chain fatty acid production, bacterial colonization of the mucus environment and intestinal epithelial barrier function. They demonstrated that treatments with butyrate-producing bacteria improved epithelial barrier integrity in vitro. More recently, Steppe et al[31] isolated and characterized the butyrate-producing strain Butyricicoccus pullicaecorum 25-3(T) and identified it as a potential probiotic for patients with IBD.
Regulation of intestinal immune response
The human intestine normally harbors billions of commensal bacteria. Intestinal epithelia cells actively sense those commensal bacteria and play an essential role in maintaining host-microbial homeostasis at the mucosal interface[19]. Commensal bacteria such as butyrate-producing bacteria can ferment undigested carbohydrates to produce small molecular metabolites such as lactic acid and SCFAs in the intestine, promote the proliferation of beneficial intestinal bacteria such as bifidobacterium, lactobacillus and fecal bacillus, and inhibit the growth of pathogenic bacteria such as Staphylococcus, Escherichia coli, Salmonella typhus and Clostridium difficile (C. difficile)[32,33]. Thus, butyrate-producing bacteria promote intestinal microecological balance and participate in the regulation of the production of amines, indole, hydrogen sulfide and other potential harmful substances. Therefore, they not only can improve intestinal digestive and absorptive capacity, but also play important roles in improving the body’s immunity and preventing infections[8].
SCFAs promote intestinal peristalsis and reduce the duration of the presence of toxin in the intestinal tract. Among the SCFAs, butyrate is a potent mediator involved in the effects of gut microbiota on intestinal mucosal immune functions[34]. Butyrate can act as a ligand to activate specific G-protein-coupled receptors, activate intestinal mucosal immune activity, and enhance immunity[34]. Enhanced butyrate production by colonic butyrate-producing bacteria after diet manipulation is associated with increased levels of the anti-inflammatory cytokine IL-10 in mice[35]. Using intestinal mucosa biopsy tissues obtained from the patients with Crohn’s disease, Segain et al[15] have shown that butyrate can ameliorate the inflammatory response of isolated lamina propria cells and that of cultured peripheral blood mononuclear cells. NF-κB pathway is involved in the inhibition of immune cell activation[15].
Butyrate regulation of Toll-like receptor (TLR) expression in human colonic epithelial cells may be one of the key mechanisms mediating the cross talk and interplay between normal gut microbiota and a host’s innate and adaptive immune systems[36]. TLRs in intestinal epithelial cells and mucosal immune cells are pattern-recognition-receptors that are critical components of the symbiosis between the host and commensal microflora[37]. Therefore, bacterial production of butyrate plays a key role in maintaining intestinal homeostasis. Other factors such as antimicrobial peptides produced by commensal bacteria or the host may also be involved in the process[38-40]. More recently, a clinical study found that higher fecal SCFA concentrations were associated with the efficacy of immunotherapy in solid tumor cancer patients, indicating that gut microbiota might have wide-ranging impacts on host immune response[41].
Dysbiosis of intestinal microbiota and infection
Dysbiosis of intestinal microbiota may lead to so-called leaky gut and therefore microbial translocation, contributing to the development of infection. It is well accepted that an impaired interaction between intestinal microbiota and the host immune response can lead to an increased risk of infection caused by gram-negative bacteria or other pathogens[37,42]. It has been shown that reductions in mucosal butyrate from diminished colonic butyrate-producing bacteria contribute to HIV-associated mucosal pathogenesis[43]. SCFA uptake coupled with sodium absorption is one of the major mechanisms for salt and water uptake in the colon. The association between the depletion of intestinal microbiota and nosocomial diarrhea is well recognized. Normally abundant gut commensal organisms, including the butyrate-producing C2 to C4 anaerobic fermenters, are significantly depleted in the patients with C. difficile infection or C. difficile-negative nosocomial diarrhea[44]. Furthermore, dysfunction of the intestinal mucosal barrier and impaired mucosal immunity can lead to pathological translocation of intestinal bacteria or endotoxins, causing sepsis and multiple organ dysfunction syndrome in patients who experienced severe trauma, serious burn, major surgery or hemorrhagic shock[45]. Loss of the intestinal microbiota diversity and a subsequent loss of health-promoting SCFAs, such as butyrate, contribute to the dysregulated immune response and organ failure associated with sepsis[46].
Bacteriotherapeutic supplementation may restore normal gut microbiota. For example, using fecal microbiota transplantation (FMT) to restore butyrate-producing bacteria in the gut and therefore the normal host immune response has been tested in clinical practice for the treatment of diseases related to dysbiosis of the intestinal microbiota[47]. FMT has been very successful in the treatment of recurrent and refractory C. difficile infection[48]. FMT has also been trialed for aiding in the recovery of septic patients[49]. However, concerns for lethal complications associated with FMT prevent its use other than for quite restricted clinical indications. Oral administration of health-promoting next-generation probiotics to ameliorate dysbiotic microbiota may be a safe alternative[9]. As summarized by a recent systematic review and meta-analysis, administration of probiotic mixtures, not single-strain products, has a beneficial effect of reducing the incidence of late-onset sepsis in human milk-fed very low birth weight preterm infants[50].
Role in obesity-associated metabolic disorders
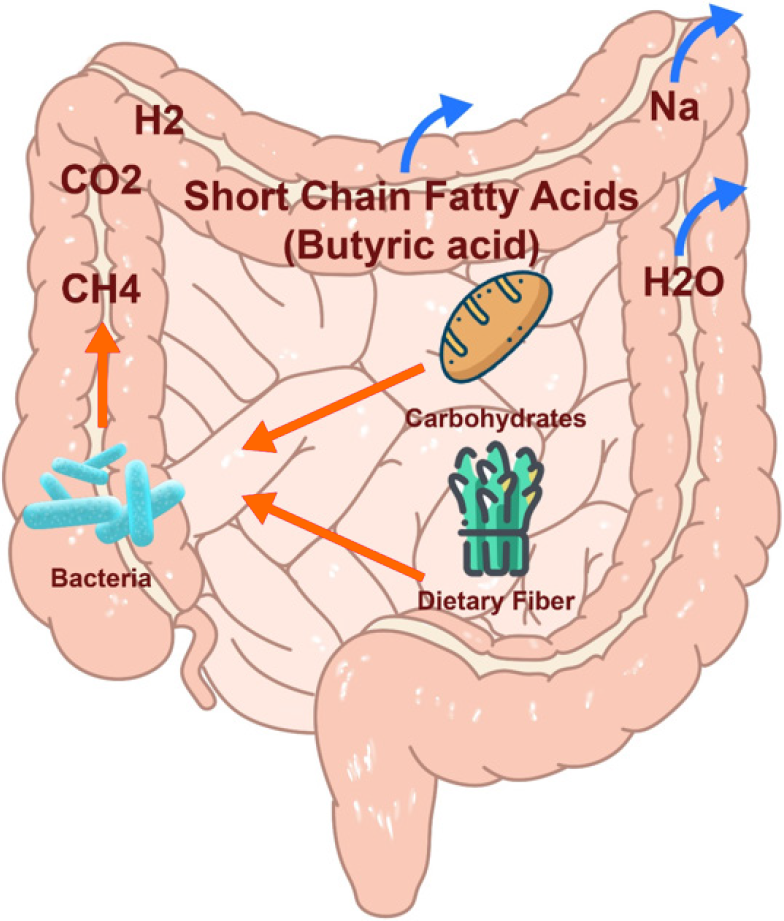
Diet can modulate and support the symbiotic microbial communities that colonize the digestive tract. Modulating gut microbiota with dietary approaches may improve health, and prevent or treat diseases related to intestinal dysbiosis[51]. Dietary prebiotics are a group of nutrients that are degraded by gut microbiota. It is defined as a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improving host health[52]. Most complex carbohydrates and plant polysaccharides ingested are metabolized by fermentation of commensal bacteria in the colon, which generate butyrate and other SCFAs (Figure 1). Consumption of a diet rich in fiber or prebiotic supplementation can boost the growth and metabolism of beneficial commensals in the colon, specifically targeting butyrate production[35,51].
Figure 1 Butyric acid production by bacterial fermentation.
Numerous studies have demonstrated the beneficial effects of a diet rich in fiber on obesity-associated metabolic syndrome. A fiber rich diet is beneficial in the prevention of obesity, improving insulin resistance, and control of abnormal blood lipid profile commonly seen in metabolic syndrome[53]. We previously have proposed that increased production of SCFAs as a result of colonic bacterial fermentation of dietary fiber might, in part, account for some of the beneficial effects of dietary fiber on the metabolic syndrome[53]. Indeed, while on a high-fat diet, supplementation of butyrate prevented development of insulin resistance and obesity in mice. Fasting blood glucose, fasting insulin, and insulin tolerance were all preserved in the treated mice. In the obese mice, supplementation of butyrate led to an increase in insulin sensitivity and a reduction in adiposity[54]. Oral administration of E. Hallii, a butyrate-producing bacterial stain, can improve insulin sensitivity and increase energy expenditure in diabetic db/db mice[55]. As a potential therapeutic strategy for obesity and metabolic syndrome, FMT has also been trialed in a few randomized controlled human studies with some mixed beneficial results[56]. Promotion of energy expenditure, induction of mitochondrial function by activation of AMPK, and serving as an agonist of free fatty acid receptors, may be some of the mechanisms underlying the beneficial effects of butyrate on the abnormalities characterizing the metabolic syndrome[54,57-59].
CONCLUSION
This minireview summarizes the potential clinical applications and possible underlying mechanisms of butyrate-producing bacteria in disorders related to pediatrics. As the major source of energy of the colonic mucosa and as an important regulator of gene expression, inflammation, differentiation and apoptosis in host cells, microbiota-derived butyrate enhances the role of the intestinal mucosal immune barrier, modulates the systemic immune response, and thus prevents bacteria and their metabolites from entering the bloodstream and causing inflammation. Butyrate regulation of energy metabolism may play a role in the beneficial effects of a high fiber diet on metabolic syndrome. Therefore, acting as probiotics, butyrate-producing bacteria play important roles in a variety of normal biological functions that include balancing gut microbiota, maintaining the mucosal barrier, modulating the host immune response, preventing infections, and regulating energy expenditure. Thus, butyrate-producing bacteria may have a potential therapeutic value in a wide range of clinical conditions associated with intestinal dysbiosis such as IBD, NEC, late-onset sepsis in premature infant, nosocomial diarrhea, and obesity-associated metabolic disorders.