Published online Jul 28, 2016. doi: 10.5320/wjr.v6.i2.49
Peer-review started: April 6, 2016
First decision: May 17, 2016
Revised: May 30, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: July 28, 2016
Processing time: 108 Days and 10.9 Hours
Mechanical ventilation (MV) is one of the lifesaving techniques applied to critically ill patients at bedside. However, some complications, such as ventilator-induced lung injury and ventilator-associated pneumonia, may occur in a patient undertaking MV and are often related to the duration of MV. Some written protocols have been proposed to reduce the risk of such complications, but they can be time consuming, leading to fluctuation in protocol implementation and compliance. Moreover, written instructions tend to be general and thus cannot cover all possible scenarios, resulting in variable interpretation of the protocol. To overcome these limiting factors, protocols have been computerized and there is convincing evidence in the literature showing that computerized protocols benefit management of the process and reduce the time a patient spends under MV. QuickWean is a computer-aided weaning protocol implemented on the Hamilton S1 ventilator (Hamilton Medical AG, Bonaduz, Switzerland), which guides the patient through the weaning process without requiring any intervention by the treating physician. The fully-automated ventilation mode is INTELLiVENT®-ASV (Hamilton Medical AG), which is set according to the patient’s respiratory mechanics, patient-ventilator interaction, peripheral oxygen saturation (SpO2) and pulmonary end-tidal carbon dioxide (PetCO2). The INTELLiVENT®-ASV mode sets automatically each minute to provide accurate ventilation, pressure support, fraction of inspired oxygen and positive end-expiratory pressure based on the patient’s needs. QuickWean can be pre-set to match the established weaning policy of an intensive care unit as well as being customized to a patient’s needs. It provides a progressive reduction of respiratory support, and guides the patient through the spontaneous breathing trial (SBT). At the end of the SBT, the ventilator re-starts the previous ventilation support and provides a report of the successful SBT. During all phases, PetCO2, SpO2 and all breathing parameters are monitored. This new automated weaning tool may improve the safety and effectiveness of an SBT, reducing the time spent in the process of weaning and providing a lower workload for the treating physician.
Core tip: Weaning from mechanical ventilation is a crucial point during respiratory therapy and most intensive care units have developed human-based protocols to wean the patient. Newer ventilators have implemented a computer-aided weaning protocol, and the QuickWean application may be the most complete because it can drive the patient automatically from total passivity to readiness to wean. A key feature of the full computer-driven process is the safeness of the procedure, ensured by the patient always being under control in terms of peripheral oxygen saturation, pulmonary end-tidal carbon dioxide and respiratory fatigue, and improving upon the discontinuous human-driven process.
- Citation: Belliato M. Automated weaning from mechanical ventilation. World J Respirol 2016; 6(2): 49-53
- URL: https://www.wjgnet.com/2218-6255/full/v6/i2/49.htm
- DOI: https://dx.doi.org/10.5320/wjr.v6.i2.49
Artificial mechanical ventilation (MV) is a lifesaving therapy, but some complications may occur that affect the outcome of critically ill patients. Most of these complications are related to the length of time that MV is applied; therefore, the development of strategies to reduce MV application time should be a priority in clinical and research settings. In 1994, Brochard et al[1] showed that the most important steps in the process of weaning a patient off of MV, and which help to prevent unnecessary prolongation of MV, are: Prompt recognition of readiness for both weaning and extubation. Since then, clinicians have determined that the complex process of withdrawal from MV may be affected by several factors, including pre-existing comorbidities. Patients may experience respiratory and cardiac failure associated with the increased work of breathing that accompanies reduction of ventilatory support[2,3]. Moreover, reintubation due to respiratory failure is related to increased mortality, as shown in a study comparing such patients to those who did not require reintubation[4].
Automated weaning systems appear to enable a more efficient weaning process than those that rely upon clinician-directed weaning protocols, as they provide greater adaptability of the ventilation support to each patient’s needs through continuous monitoring and real-time intervention[5-7]. A recent Cochrane systematic review and meta-analysis of 10 trials that compared the automated and non-automated weaning methods showed superiority of the automated methods in terms of weaning time, intensive care unit (ICU) length of stay (i.e., 7 and 21 d) and number of patients requiring MV[8]. Moreover, a systematic review of 21 trials on automated systems and a subsequent meta-analysis, regardless of spontaneous breathing trial (SBT) capability, by Rose et al[9] demonstrated that the automated systems provide less duration of weaning as well as of total duration of MV, as compared to the manual weaning methods. However, despite the evidence that has suggested effectiveness for improving efficiency of weaning, automated weaning systems are still largely underused in clinical practice.
The most recent automated weaning tool available on the global market is QuickWean, which is implemented on the S1 ventilator (Hamilton Medical AG, Bonaduz, Switzerland) as a part of the INTELLiVENT®-ASV ventilation mode. The design of QuickWean aims to provide a good and effective automatic weaning procedure under completely safe conditions for the patient.
QuickWean is a tool used in the INTELLiVENT®-ASV ventilation mode and is available as a supplemental software component on the S1 ventilator (Hamilton Medical AG).
INTELLiVENT®-ASV is a fully automatic ventilation mode system, in which the ventilator selects the respiratory pattern of the patient in terms of guaranteed minute ventilation (%MinVol), positive end-expiratory pressure (PEEP), fraction of inspired oxygen (FiO2), tidal volume, I:E ratio and respiratory rate (for the passive patient) or inspiratory support (for the active patient); the automated guidance of these parameters allows the patient to reach the peripheral oxygen saturation (SpO2) and pulmonary end-tidal carbon dioxide (PetCO2) targets set by caregiver. The inputs are body weight (actual or predicted) and sex of the patient, and clinical targets of SpO2 and PetCO2; in addition, the inputs that are fundamental for computing and for the selection of adequate ventilator settings involve the respiratory mechanics of the patient (measured continuously, breath-by-breath) and the parameters of the Lung Protective Strategy rules (suggested by the ARDSnet tables in the studies published in 2000 and 2004[10,11]).
Recent publications have provided descriptions of the INTELLiVENT®-ASV system in use in clinical settings as a tool that has been integrated in routine practice of different ICUs[12-15]. The most important findings from these studies are that the fully-automated mode could be used extensively in all kinds of patients admitted to the ICU, suggesting that this system could improve the standard level of respiratory care while decreasing the workload of caregivers without any loss of patient safety.
QuickWean is suited for weaning of patients who are ventilated with the INTELLiVENT®-ASV mode. The philosophy behind its automated strategy is that the software allows for monitoring of readiness of the patient to be weaned. As such, it confirms the quality and the trend of the ventilator parameters that are used as indices for weaning, specifically by performing a “t-tube trial-like” period and alerting the clinician of the possibility for discontinuation from MV.
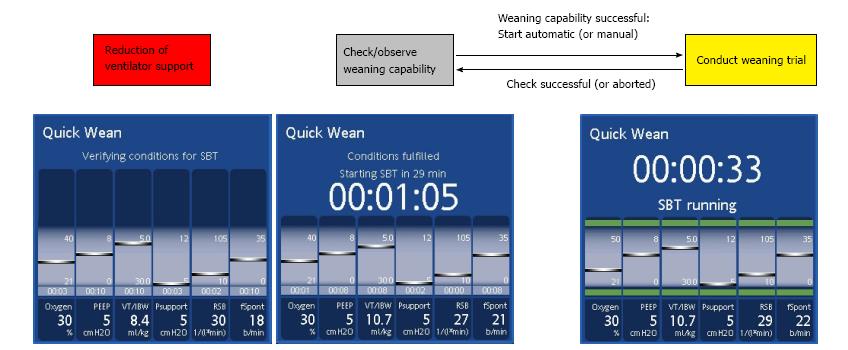
The timeline of the QuickWean process is shown in Figure 1. During the first phase, known as the “reduction phase”, the ventilator reduces respiratory support in terms of inspiratory support, PEEP and FiO2. If the patient’s clinical condition remains stable (i.e., SpO2, PetCO2 and respiratory rate), then the ventilator will start the second phase, known as the “observation phase”, in which it monitors the stability of the patient’s condition (i.e., SpO2, PetCO2 and respiratory rate remaining within the range of acceptability). These monitored data are shown as light blue bars inside the QuickWean box and equate to a set period. The third phase, known as the “SBT phase”, is the trial with very low inspiratory pressure (Pinsp). At the end of the SBT, the ventilator returns to the normal INTELLiVENT®-ASV mode, with clinical settings and full support for the patient if necessary. The user will see on the screen a suggestion for discontinuing ventilation, or an alert to denote if the SBT was aborted.
If, during any of the three phases, the respiratory or ventilator parameters go out of range, then the ventilator will immediately return to the normal INTELLiVENT®-ASV mode, with full support of Pinsp, respiratory rate, PEEP and FiO2.
In the case of weaning trial interruption, the ventilator will show which parameter was out of range and a reason for it (Figure 2). Moreover, the history of the weaning trial can be stored, allowing for further investigations that will help to optimize the therapeutic pathway.
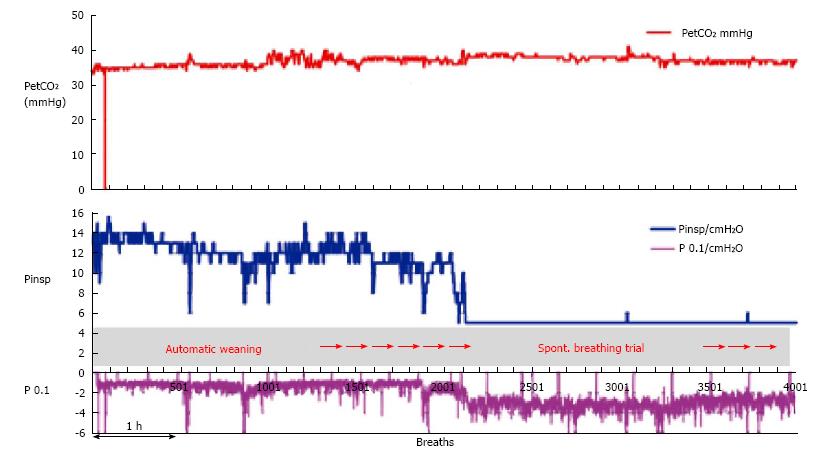
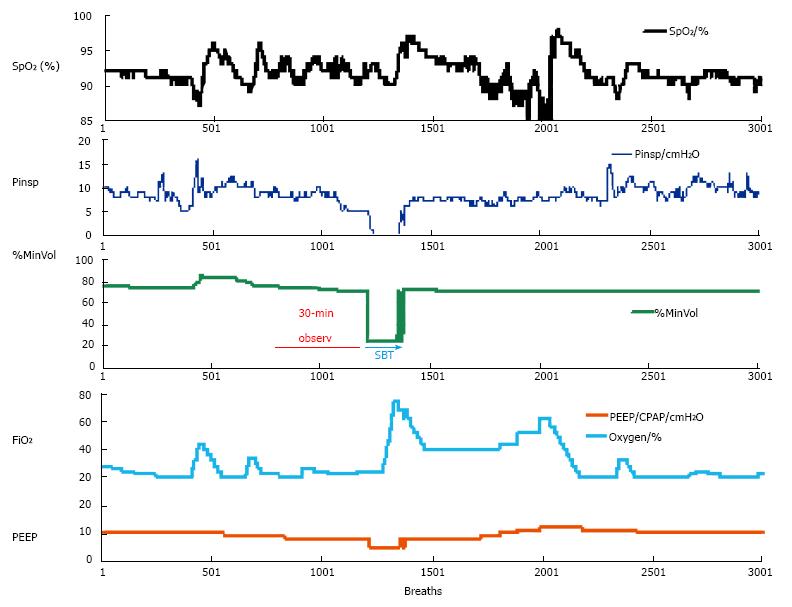
When a patient is ventilated in the INTELLiVENT®-ASV mode with low support (spontaneous breaths, minimum Pinsp, PEEP at 5 cmH2O and FiO2 at 30%), the automatic weaning process may be chosen. Figure 3 shows a patient on the automatic weaning modality, in which the ventilator starts progressive reduction of inspiratory support (Pinsp, corresponding to an increase of P0.1), which is used as a sign of activity improvement of the muscles. That phenomenon is considered as a “push to wean” for the patient, which is then automatically conducted by the machine while the patient remains with PetCO2 and SpO2 under tight control. If the respiratory rate or PetCO2 increase over the range that had been previously set as acceptable by the clinician, then the process is automatically stopped and the baseline settings are restored. Figure 4 shows a case of automatic SBT where the oxygenation was unstable and required a progressive increase in FiO2 over the set upper limit and which ultimately induced restoration of the full support mode; this case also required increasing PEEP and FiO2 to restore the baseline value of the patient’s SpO2. In general, immediately after stabilization of oxygenation, QuickWean restarts the process of reduction of FiO2 and PEEP in order to retry the attempt to wean the patient. Obviously, however, if the patient is not ready to be weaned then the process will be to self-restrain and allow for stabilization of the patient’s vital signals.
The automated weaning, therefore, plays the role of an expert user at the bedside, managing inspiratory support, PEEP and FiO2 continuously, 24 h/d and 7 d/wk. With the availability of such automated tools, it may be advisable to design and set a weaning policy of an ICU in the ventilator software that will suit individual patient needs within the range of acceptability and safeness of the respiratory parameters and SpO2 and PetCO2.
Automated weaning is a new promising option available to intensivists, respiratory therapists and pneumologists for weaning patients from MV. While this tool could improve the daily work routine in the ICU, it could also be particularly useful in reducing the time of intubation of patients, especially those who are post-surgery. Post-surgery patients represent a population that might benefit most from such a strategy, as continuous monitoring is an important aspect of recovery from anesthesia and restoration of spontaneous respiratory function. Certainly, further trials are needed to demonstrate the clinical impact of fully-automated weaning, not only in terms of length of ventilation but also considering morbidity and costs. It is also necessary to gain a detailed understanding of the impact of caregivers’ control of the system’s settings and of human judgments about adequacy of the weaning strategy, timing for starting and reliability of results.
| 1. | Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, Gasparetto A, Lemaire F. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994;150:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 535] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 2. | Jeganathan N, Kaplan CA, Balk RA. Ventilator Liberation for High-Risk-for-Failure Patients: Improving Value of the Spontaneous Breathing Trial. Respir Care. 2015;60:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Vagheggini G, Vlad EP, Mazzoleni S, Bortolotti U, Guarracino F, Ambrosino N. Outcomes for difficult-to-wean subjects after cardiac surgery. Respir Care. 2015;60:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Rothaar RC, Epstein SK. Extubation failure: magnitude of the problem, impact on outcomes, and prevention. Curr Opin Crit Care. 2003;9:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Dojat M, Harf A, Touchard D, Laforest M, Lemaire F, Brochard L. Evaluation of a knowledge-based system providing ventilatory management and decision for extubation. Am J Respir Crit Care Med. 1996;153:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Lellouche F, Brochard L. Advanced closed loops during mechanical ventilation (PAV, NAVA, ASV, SmartCare). Best Pract Res Clin Anaesthesiol. 2009;23:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Lellouche F, Bouchard PA, Simard S, L’Her E, Wysocki M. Evaluation of fully automated ventilation: a randomized controlled study in post-cardiac surgery patients. Intensive Care Med. 2013;39:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Burns KE, Lellouche F, Nisenbaum R, Lessard MR, Friedrich JO. Automated weaning and SBT systems versus non-automated weaning strategies for weaning time in invasively ventilated critically ill adults. Cochrane Database Syst Rev. 2014;9:CD008638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Rose L, Dainty KN, Jordan J, Blackwood B. Weaning from mechanical ventilation: a scoping review of qualitative studies. Am J Crit Care. 2014;23:e54-e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8487] [Cited by in RCA: 8455] [Article Influence: 325.2] [Reference Citation Analysis (3)] |
| 11. | Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327-336. [PubMed] |
| 12. | Bialais E, Wittebole X, Vignaux L, Roeseler J, Wysocki M, Meyer J, Reychler G, Novotni D, Sottiaux T, Laterre PF. Closed-loop ventilation mode (IntelliVent-ASV) in intensive care unit: a randomized trial of ventilation delivered. Minerva Anestesiol. 2016;82:657-668. [PubMed] |
| 13. | Arnal JM, Garnero A, Novonti D, Demory D, Ducros L, Berric A, Donati S, Corno G, Jaber S, Durand-Gasselin J. Feasibility study on full closed-loop control ventilation (IntelliVent-ASV™) in ICU patients with acute respiratory failure: a prospective observational comparative study. Crit Care. 2013;17:R196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Clavieras N, Wysocki M, Coisel Y, Galia F, Conseil M, Chanques G, Jung B, Arnal JM, Matecki S, Molinari N. Prospective randomized crossover study of a new closed-loop control system versus pressure support during weaning from mechanical ventilation. Anesthesiology. 2013;119:631-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Abutbul A, Sviri S, Zbedat W, Linton DM, van Heerden PV. A prospective comparison of the efficacy and safety of fully closed-loop control ventilation (INTELLiVENT®-ASV) with conventional ASV and SIMV modes. S Afr J Crit Care. 2014;30:28-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
P- Reviewer: Sugawara I, Wu S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ