Published online Mar 28, 2016. doi: 10.5320/wjr.v6.i1.42
Peer-review started: September 4, 2015
First decision: November 27, 2015
Revised: January 22, 2016
Accepted: February 14, 2016
Article in press: February 16, 2016
Published online: March 28, 2016
Processing time: 210 Days and 0.3 Hours
AIM: To research the natural course of idiopathic pulmonary fibrosis (IPF) with advanced non-small cell lung cancer (NSCLC) and the association between acute exacerbation (AE) of IPF and chemotherapy (CT).
METHODS: From May 2007 through April 2011, 17 CT naive patients with IPF and advanced NSCLC were enrolled. Patients were classified into best supportive care (BSC) group or CT group based on the patient’s preference. Patients in the CT group received carboplatin (CBDCA) (AUC 5-6) plus paclitaxel (PTX) (175-200 mg/m2) on day 1 of each 21-d cycle as first-line therapy.
RESULTS: All patients but one chose the CT group. In the CT group, the objective response rate was 38%. The most frequent toxicity ≥ grade 3 was neutropenia (88%). Two patients (12.5%) developed AE-IPF. The median progression-free survival, the median survival time and the 1-year survival rate were 4.1 mo, 8.7 mo and 35%, respectively. Second-line CT-related AE and CT-unrelated AE occurred in 2 and 3 patients (1: BSC group; 2: CT group), respectively. Seven (41%) of all patients developed AE-IPF throughout the clinical course, and 6 of 7 patients with AE-IPF died within one month.
CONCLUSION: The incidence of AE-IPF was higher among IPF patients with advanced NSCLC than among those without NSCLC. CBDCA plus PTX regimen was tolerable and effective. However, AE-IPF has a fatal toxicity with or without CT in IPF patients with advanced NSCLC.
Core tip: Acute exacerbation (AE) of idiopathic pulmonary fibrosis (IPF) has been generally recognized. Little is known, however, about the natural history of IPF and the frequency of AE-IPF with advanced non-small cell lung cancer (NSCLC). We conducted a prospective observational study of IPF with advanced NSCLC for each group of patients receiving chemotherapy or the best supportive care according to the patient’s preference for the purpose of excluding a potential selection bias by the treating physicians.
- Citation: Ebi N, Tokunaga S, Itoh K, Okamoto I, Edakuni N, Fujii S, Watanabe K, Hayashi S, Maeyama T, Nakanishi Y. Multicenter cooperative observational study of idiopathic pulmonary fibrosis with non-small cell lung cancer. World J Respirol 2016; 6(1): 42-48
- URL: https://www.wjgnet.com/2218-6255/full/v6/i1/42.htm
- DOI: https://dx.doi.org/10.5320/wjr.v6.i1.42
Idiopathic pulmonary fibrosis (IPF) is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause by progressive worsening of dyspnea and lung function and is associated with a poor prognosis[1]. The association of IPF and lung cancer is well recognized and IPF patients have a higher incidence of lung cancer than the general population, with relative risks of 7 to 14 being reported[2,3]. According to recent observations, acute exacerbation (AE) of IPF has increased in some patients with IPF and occurs in approximately 5%-15% of patients with IPF annually[4-6]. AE-IPF often results in respiratory failure and has a fatal toxicity. The etiology of AE-IPF is unknown, however, chemotherapy (CT) agents are considered to be one of various factors associated with it. There have been only a few retrospective reports demonstrating that patients with lung cancer and IPF have a high risk of developing AE after CT. However, it is unknown how often AE-IPF happens throughout the natural course of IPF with advanced NSCLC and how much the frequency of AE-IPF increases due to CT. Therefore, we conducted a prospective observational study to research the clinical course of IPF with advanced NSCLC and the association between AE-IPF and CT.
Patients with histologically and/or cytologically confirmed NSCLC and histologically or clinically diagnosed IPF were eligible for participation in the study. Each patient had to meet the following criteria: Inoperable clinical stage III or IV, no prior CT, and/or radiotherapy for the primary site, age 20-74 years, Eastern Cooperative Oncology Group performance status (PS) of 0 or 1, estimated life expectancy > 3 mo, adequate organ functions and partial pressure of arterial oxygen (PaO2) > 60 mmHg. Main exclusion criteria included active concomitant malignancy, symptomatic brain metastasis, heart failure, uncontrolled diabetes mellitus, active infection, and a past history of drug allergy including hypersensitivity for polysorbate 80. The diagnosis of IPF was based on the histologic appearance of usual interstitial pneumonia (UIP) on surgical lung biopsy[1]. In the absence of surgical biopsy, the diagnosis of IPF was made according to the radiologic pattern on high-resolution computed tomography (HRCT) such as predominantly peripheral, subpleural, bibasal reticular abnormalities with honeycomb cysts and other clinical data. Patients with unstable IPF, oxygen inhalation or immunosuppressive drugs such as steroids were excluded. Patients who did not meet a % vital capacity (VC) < 60% of the predicted value, % diffusing capacity for carbon monoxide (DLCO) < 40% of the predicted value or desaturation < 88% during the 6-min walk test (6MWT) as poor prognostic factors[7-9] of patients with IPF were included. The diagnostic criteria for AE-IPF were as follows[10,11]: (1) exacerbation of dyspnea within 1 mo; (2) newly developed diffuse pulmonary opacities on chest CT and/or a chest X-ray; (3) a decrease in PaO2 of more than 10 mmHg under similar conditions; and (4) the absence of heart failure or infectious lung diseases. For the purpose of making the diagnosis of AE-IPF fairly certain, we excluded bacterial pneumonia, pulmonary embolism, and heart failure by physical examination, laboratory and culture findings, or echocardiography as necessary. When the diagnosis of AE-IPF was made, steroid pulse therapy and/or sivelestat sodium were actively administered. In this study, AE related to CT was defined as AE which occurred within three months after final CT. The diagnosis of IPF and AE-IPF in this study was confirmed centrally by three independent respirologists.
This study was performed in accordance with the principles of the Declaration of Helsinki and the good clinical practice guidelines. Written informed consent was obtained from all patients before study entry. This study was approved by our institutional review board and trial document approval was obtained from each participating institution. This study was registered with the UMIN Clinical Trials Registry (ID: UMIN000015929).
Patients were classified into best supportive care (BSC) group or CT group based on the patient’s preference. Patients in the CT group received carboplatin (CBDCA) (AUC 5-6) plus paclitaxel (PTX) (175-200 mg/m2) every 3 wk up to 6 cycles as first-line therapy unless there was a progression of the disease, an appearance of intolerable toxicity, or a withdrawal of consent. Diphenhydramine, a histamine H2 receptor antagonist and dexamethasone were administered to patients in the CT group as premedication for prophylaxis of hypersensitivity reactions to PTX. No prophylaxis with granulocyte colony-stimulating factors (G-CSF) was designed.
The incidence of AE-IPF as the clinical course of IPF with advanced NSCLC was examined in each group. Regarding first-line CT (CBDCA plus PTX) defined by the protocol, the objective response rate (ORR) were evaluated using the Response Evaluation Criteria in Solid Tumors guidelines[12] and the toxicity was assessed according to the National Cancer Institute Common Toxicity Criteria Version 3.0. Second-line or later CT was not defined by the protocol, however, the incidence of AE-IPF for each CT was recorded. To evaluate AE-IPF, a HRCT scan was performed at least every 2 mo. The relationship between AE-IPF and the parameters, including inflammatory markers and lung function, was compared according to the presence or absence of AE-IPF. An evaluation of the inflammatory markers and the lung function test was conducted every 3 mo.
We assessed the incidence of AE-IPF as the clinical course of IPF with advanced NSCLC according to the presence or absence of CT. The associations between AE-IPF and pre-enrollment parameters, including CRP, LDH, KL-6, SP-D, PaO2, %VC, %DLCO, and desaturation during 6MWT were examined using the Wilcoxon rank-sum test. The progression-free survival (PFS) was defined as the period from the start of CT to an identifiable time for progression. The overall survival (OS) was defined as the period from the entry of this study until death by all causes. Survival curves for the PFS and OS were estimated using the Kaplan-Meier method. The log-rank test was used for the comparison of the survival times. The confidence interval for the response rate was estimated by exact binomial method. All tests were two-tailed and P values less than 0.05 were considered to be statistically significant. All statistical analyses were performed using the Stata 11 software program (Stata Corporation, Texas, United States). The statistical analyses were performed by one of the authors (Shoji Tokunaga), an expert biomedical statistician, assuring the standard of biostatistics required for a clinical research.
From May 2007 through April 2011, 17 CT naive patients with IPF and advanced NSCLC were enrolled in this study. All patients but one chose the CT group. Their characteristics are shown in Table 1. All patients were diagnosed with IPF according to the radiologic pattern on HRCT and other clinical data. All patients were eligible and assessed the incidence of AE-IPF and survival. The median age at the time of diagnosis of lung cancer was 65 years; 16 patients were current or former smokers and all of the patients were male. There were 10 patients with stage IV disease. The most common histologic NSCLC subtype was adenocarcinoma.
| No. of patients | 17 |
| Age (yr) | |
| Median | 65 |
| Range | 43-74 |
| Sex | |
| Male | 16 |
| Female | 1 |
| Performance status | |
| 0 | 6 |
| 1 | 11 |
| Stage at enrollment | |
| IIIA | 2 |
| IIIB | 5 |
| IV | 10 |
| Histology | |
| Adenocarcinoma | 11 |
| Squamous cell carcinoma | 5 |
| Non-small cell carcinoma | 1 |
| Smoking status | |
| Smoker | 16 |
| Non-smoker | 1 |
All of 16 patients in the CT group were assessable for toxicity and tumor response. The toxicities of treatment, with the exception of AE-IPF, are summarized in Table 2. Among the hematological toxicities, the most common toxicity was neutropenia. The grade 3 and 4 neutropenia were observed in 8 patients and 6 patients, respectively, although only one patient developed febrile neutropenia. Seven patients received G-CSF (75 or 100 μg). One patient with grade 3 anemia required a blood transfusion. Among the non-hematologic toxicities, the most common toxicity was grade 2 or less peripheral neuropathy. The tumor responses of CBDCA plus PTX are summarized in Table 3. Six patients had partial responses, 5 had stable diseases and 5 had progressive diseases. The ORR was 38% (95%CI: 15%-65%). The median number of treatment cycles administered was 4 (range, 2 to 6) and the average dose administration of CBDCA plus PTX on the first cycle and the total cycles were AUC 5.5/190 mg/m2 and AUC 5.3/179 mg/m2, respectively. The reasons for protocol discontinuation, with the exception of AE-IPF, were disease progression (n = 6), second dose reduction (n = 1) and suspected drug-induced pneumonitis (n = 1). The median PFS, the median survival time (MST) and the 1-year survival rate were 4.1 mo, 8.7 mo and 35%, respectively.
| Tumor response | CT group (n = 16) |
| Complete response | 0 |
| Partial response | 6 |
| Stable disease | 5 |
| Progressive disease | 5 |
| Response rate | 38% |
| (95%CI) | (15%-65%) |
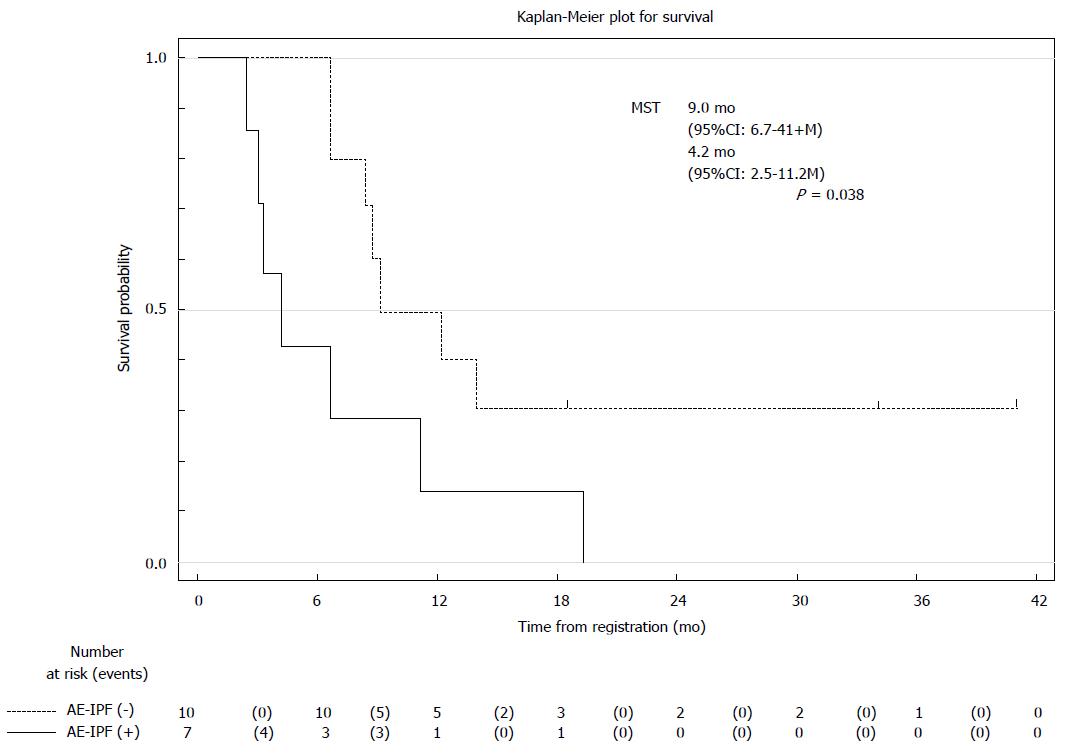
Table 4 summarizes the incidence of AE-IPF, which was observed in 7 (41%) of all patients through the clinical course. All patients but one chose the CT group. AE-IPF was observed in one patient in the BSC group and 6 in the CT group. One patient in the BSC group developed AE-IPF 4 mo after the registry and died 2 mo from AE-IPF. Patients in the CT group received CBDCA plus PTX. In cases 1 and 2, AE-IPF developed within 2 mo of receiving final CT. In cases 3 and 4, AE-IPF developed beyond 3 mo of receiving final CT. In cases 5 and 6, AE-IPF developed within one month of receiving second-line CT. AE-IPF occurred in 2 (12.5%) of 16 patients who received first-line CT (CBDCA plus PTX). AE related to second-line CT was observed in 2 patients (1: pemetrexed; 1: docetaxel). In addition, AE unrelated to CT was observed in 3 patients, 1 in the BSC group and 2 in the CT group. Six of 7 patients who developed AE-IPF died of respiratory failure within 1 mo. The MST according to the absence or presence of AE-IPF was 9.0 and 4.2 mo, respectively (Figure 1).
| Period (d) | ||
| From registry (last chemotherapy) to AE-IPF | From AE-IPF to death | |
| A group (n =1) | 136 | 66 |
| B group (n =16) | ||
| Case 1 CBDCA/PTX 2 cycles | 56 (27) | 20 |
| Case 2 CBDCA/PTX 2 cycles | 77 (47) | 16 |
| Case 3 CBDCA/PTX 3 cycles | 124 (101) | 6 |
| Case 4 CBDCA/PTX 3 cycles | 317 (249) | 24 |
| Case 5 CBDCA/PTX 3 cycles | ||
| → 2nd line PEM 1 cycle | 83 (12) | 18 |
| Case 6 CBDCA/PTX 3 cycles | ||
| → 2nd line PEM 4 cycles | 583 (14) | 8 |
Table 5 shows the relationship between AE-IPF and each pre-enrollment parameter, including CRP, LDH, KL-6, SP-D, PaO2, %VC, %DLCO, and desaturation during 6MWT. However, none of these factors were associated with the incidence of AE-IPF.
| AE-IPF | P1 | ||
| + (n = 7) | - (n = 10) | ||
| CRP (mg/dL) | 0.51 (0.14-15.0) | 3.43 (0.15-11.1) | 0.77 |
| LDH (IU/L) | 191 (132-399) | 205 (163-969) | 0.73 |
| KL-6 (U/mL) | 603 (285-1373) | 683 (381-2340) | 0.56 |
| SP-D (ng/dL) | 88.3 (69.1-457) | 101 (58.9-139) | 1.00 |
| PaO2 (mmHg) | 77.1 (75.0-85.3) | 76.8 (69.0-91.7) | 0.78 |
| %VC (%) | 100.1 (83.7-131.1) | 83.6 (68.0-115.7) | 0.07 |
| %DLCO (%) | 58.9 (49.5-78.3) | 65.3 (58.3-92.2) | 0.25 |
| 6MWT: Minimum SpO2 (%) | 93 (90-98) | 93 (90-95) | 0.77 |
To the best of our knowledge, this is the first report to prospectively observe the clinical course of IPF with advanced NSCLC. In our study, AE-IPF was observed in 7 (41%) of all patients during the median 18 mo of follow-up. AE-IPF has been recognized as a well-known phenomenon that develops during the natural course of IPF. Recent placebo-controlled studies reported that the incidence of AE during the natural course of IPF was approximately 5%-15% of patients with IPF annually[4,13-15]. There have been few reports concerning AE-IPF following CT. Therefore, we conducted a prospective observational study of IPF with advanced NSCLC for each group of patients receiving CT or the BSC according to the patient’s preference for the purpose of excluding a potential selection bias by the treating physicians; we found it difficult to ethically conduct a randomized controlled trial to research the clinical course of IPF with advanced NSCLC and the association between AE-IPF and CT. In fact, all patients but one chose the CT group, despite the explanation of the potential fatal toxicity due to AE-IPF. AE related to CT was defined as AE which occurred within three months after final CT and CT-unrelated AE beyond three months. AE-IPF occurred in 2 (12.5%) of 16 patients who received first-line CT (CBDCA plus PTX). AE related to second-line CT was observed in 2 patients (1: pemetrexed; 1: docetaxel). In addition, AE unrelated to CT was observed in 3 patients, 1 in the BSC group and 2 in the CT group.
Kenmotsu et al[16] reported that the incidence of AE related to CT was higher among the patients with a UIP pattern than among those with a non-UIP pattern (30% vs 8%), taken from evidence gleaned from the HRCT scans for the diagnosis of IPF; nevertheless, AE related to CT was defined as AE which occurred within four weeks after final CT[16]. A recent prospective study for idiopathic interstitial pneumonias with advanced NSCLC (6 IPF and 12 NSIP patients) showed that the incidences of AE related to first-line (CBDCA plus PTX) and second-line CT were 5.6% and 18%, respectively, and 2 of 6 IPF patients developed AE[17]. The incidence of AE-IPF was higher among IPF patients with advanced NSCLC than among those without NSCLC.
In a Japanese case-controlled study, preexisting ILD was reported to be an independent risk factor for developing AE[18]. The incidence of AE related to treatment is considered to be more than AE unrelated to treatment. Minegishi retrospectively demonstrated that the incidence of AE for patients receiving CT or the BSC was 20.0% and 31.3%, respectively, and the higher incidence of AE in the BSC group appeared to be dependent on selection bias based on a poor PS[19].
The etiology of AE-IPF is unknown. In this study, the associations between AE-IPF and pre-enrollment parameters, including CRP, LDH, KL-6, SP-D, PaO2, %VC, %DLCO, and desaturation during 6MWT, which were considered to be markers of IPF progression, were investigated, however no significant differences between patients who did and those who did not develop AE-IPF were observed among these factors. Inflammatory cytokines induced by CT agents, which are considered to be one of the causes of AE, worsen inflammation in the lung tissue[20]. Without CT, lung cancer has been reported to produce inflammatory cytokines[21], thus lung cancer itself may be a risk factor of AE, which might explain the higher incidence of IPF patients with advanced NSCLC.
CBDCA plus PTX is most widely used as a standard regimen for advanced NSCLC. A randomized phase III study in Japanese patients without IPF reported that the ORR, median PFS, OS and 1-year survival rate in CBDCA plus PTX, were 32.4%, 4.5 mo, 12.3 mo and 51.0%, respectively[22]. The ORR (38%) and median PFS (4.1 mo) in this study were comparable to Japanese phase III study. However, the MST (8.7 mo) and 1-year survival (35%) would be regarded as unsatisfactory for patients without IPF. The results of this study were comparable to the prospective study by Minegishi[17], which demonstrated that the ORR, median PFS, MST and 1-year survival rate were 61%, 5.3 mo, 10.6 mo, and 22%, respectively. The incidence of neutropenia (grade > 3) in our study was higher than the data reported by Minegishi and is likely due to the PTX administration schedule of the PTX plus CBDCA regimen, in which PTX was administered every 3 wk, not weekly. Febrile neutropenia was observed in one patient. Seven patients received G-CSF, which could lead to pulmonary toxicities[23], however, no patients developed AE related to G-CSF. Regarding patients treated with second-line CT, AE occurred in 2 patients (1: pemetrexed; 1: docetaxel) comparable to the report by Kenmotsu et al[16]. In this study, 6 of 7 patients who developed AE-IPF died of respiratory failure within one month. AE-IPF has a fatal toxicity with a poor prognosis, as observed in previous reports[5,6].
One major limitation associated with this study was that all patients were diagnosed with IPF and AE-IPF according to evidence from the HRCT scans of the chest and other clinical features. The diagnosis of IPF and AE-IPF in this study was confirmed centrally by three independent respirologists. HRCT findings were consistent with the UIP pattern defined by the international evidence-based guideline on the diagnosis and management of IPF[24]. Another major limitation of this study was the small sample size and that only one patient chose to receive BSC. This study was terminated early due to poor accrual. The association of IPF and lung cancer is well recognized and IPF patients have a higher incidence of lung cancer than the general population. However, a good PS in IPF patients with advanced NSCLC is limited. In the entry criteria of this study, %VC, %DLCO, or desaturation during the 6MWT as poor prognostic factors of patients with IPF were added to PaO2 as normal pulmonary function to prevent AE-IPF, which might be less easily enrolled. This study was not a randomized controlled trial, thus all patients but one chose CT, despite the explanation of potential fatal toxicity due to AE-IPF. IPF patients with advanced NSCLC and almost good PS did not wish to receive BSC, which we considered to reflect the clinical practice, and thus it was difficult to ethically conduct a randomized controlled trial to compare CT with BSC.
In conclusion, we showed that the incidence of AE-IPF was higher among IPF patients with advanced NSCLC than among those without NSCLC. CBDCA plus PTX regimen was tolerable and effective even for IPF patients. However, AE-IPF has a fatal toxicity with or without regimen in IPF patients with advanced NSCLC. Our understanding of AE-IPF with advanced NSCLC is poor. Further studies are required to establish an optimal treatment plan that is safe and effective for IPF patients with advanced NSCLC.
Acute exacerbation (AE) of idiopathic pulmonary fibrosis (IPF) has been generally recognized. Little is known, however, about the natural history of IPF and the frequency of AE-IPF with advanced non-small cell lung cancer (NSCLC).
The authors aimed to investigate the natural history of IPF with advanced NSCLC and the relationship between AE-IPF and chemotherapy (CT).
This is the first report to prospectively observe the clinical course of IPF with advanced NSCLC.
IPF patients with advanced NSCLC had a higher AE incidence than those without NSCLC.
IPF is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause by progressive worsening of dyspnea and lung function and is associated with a poor prognosis. AE-IPF often results in respiratory failure and has a fatal toxicity. The etiology of AE-IPF is unknown, however, CT agents are considered to be one of various factors associated with it.
This is a well performed study on a relevant subject. The presentation is good, the quality of written English as well.
| 1. | American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161:646-664. [PubMed] |
| 2. | Turner-Warwick M, Lebowitz M, Burrows B, Johnson A. Cryptogenic fibrosing alveolitis and lung cancer. Thorax. 1980;35:496-499. [PubMed] |
| 3. | Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med. 2000;161:5-8. [PubMed] |
| 4. | Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040-1047. [PubMed] |
| 5. | Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J. 2006;27:143-150. [PubMed] |
| 6. | Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE, Lasky JA, Loyd JE, Noth I, Olman MA. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636-643. [PubMed] |
| 7. | Jezek V, Fucik J, Michaljanic A, Jezkova L. The prognostic significance of functional tests in cryptogenic fibrosing alveolitis. Bull Eur Physiopathol Respir. 1980;16:711-720. [PubMed] |
| 8. | Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, Murray S, Kazerooni EA, Gross BH, Lynch JP. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:1084-1090. [PubMed] |
| 9. | Egan JJ, Martinez FJ, Wells AU, Williams T. Lung function estimates in idiopathic pulmonary fibrosis: the potential for a simple classification. Thorax. 2005;60:270-273. [PubMed] |
| 10. | Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest. 1993;103:1808-1812. [PubMed] |
| 11. | Akira M, Hamada H, Sakatani M, Kobayashi C, Nishioka M, Yamamoto S. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR Am J Roentgenol. 1997;168:79-83. [PubMed] |
| 12. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [PubMed] |
| 13. | King TE, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, Stähler G, Leconte I, Roux S, Raghu G. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:75-81. [PubMed] |
| 14. | Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 737] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 15. | Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 838] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 16. | Kenmotsu H, Naito T, Kimura M, Ono A, Shukuya T, Nakamura Y, Tsuya A, Kaira K, Murakami H, Takahashi T. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol. 2011;6:1242-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Minegishi Y, Sudoh J, Kuribayasi H, Mizutani H, Seike M, Azuma A, Yoshimura A, Kudoh S, Gemma A. The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non-small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer. 2011;71:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Kudoh S, Kato H, Nishiwaki Y, Fukuoka M, Nakata K, Ichinose Y, Tsuboi M, Yokota S, Nakagawa K, Suga M. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177:1348-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 383] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 19. | Minegishi Y, Takenaka K, Mizutani H, Sudoh J, Noro R, Okano T, Azuma A, Yoshimura A, Ando M, Tsuboi E. Exacerbation of idiopathic interstitial pneumonias associated with lung cancer therapy. Intern Med. 2009;48:665-672. [PubMed] |
| 20. | Sheppard MN, Harrison NK. New perspectives on basic mechanisms in lung disease. 1. Lung injury, inflammatory mediators, and fibroblast activation in fibrosing alveolitis. Thorax. 1992;47:1064-1074. [PubMed] |
| 21. | Collard HR, Calfee CS, Wolters PJ, Song JW, Hong SB, Brady S, Ishizaka A, Jones KD, King TE, Matthay MA. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L3-L7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y, Fukuoka M. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18:317-323. [PubMed] |
| 23. | Adach K, Suzuki M, Sugimoto T, Suzuki S, Niki R, Oyama A, Uetsuka K, Nakamaya H, Doi K. Granulocyte colony-stimulating factor exacerbates the acute lung injury and pulmonary fibrosis induced by intratracheal administration of bleomycin in rats. Exp Toxicol Pathol. 2002;53:501-510. [PubMed] |
| 24. | Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5557] [Cited by in RCA: 5432] [Article Influence: 362.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Goldmann T, Tanabe S S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK