Published online Nov 28, 2013. doi: 10.5320/wjr.v3.i3.44
Revised: July 4, 2013
Accepted: July 17, 2013
Published online: November 28, 2013
Processing time: 241 Days and 11.2 Hours
Incomplete development of the lung secondary to extreme prematurity or pulmonary hypoplasia causes significant morbidity and mortality during the neonatal period. Currently, the management is primarily supportive with no specific treatment to stimulate the growth and development of the lung. Mechanical forces generated inside the fetal lung by constant distention pressure and “breathing-like movements” are a major determinant of fetal lung development. However, the mechanisms by which lung cells sense these mechanical signals to promote lung development are not well-defined. Tracheal ligation has been used not only experimentally but also in human fetuses affected by severe congenital diaphragmatic hernia to stimulate lung growth and decrease the degree of pulmonary hypoplasia. Past investigations suggested that the increase of intratracheal pressure after tracheal ligation releases soluble factors that are critical for lung development. Studies from our laboratory have shown that mechanical strain of fetal type II epithelial cells, simulating mechanical forces in utero, promotes differentiation via release of epidermal growth factor receptor ligands heparin binding epidermal growth factor-like growth factor and transforming growth factor alpha. The identification of growth factors released by mechanical forces that are important for normal lung development could lead to novel treatments to accelerate lung development.
Core tip: Identification of soluble factors released to the lumen of the lung after tracheal occlusion could lead to new therapeutic opportunities to accelerate lung development in newborns affected by extreme prematurity or pulmonary hypoplasia.
- Citation: Sanchez-Esteban J. Growth factors and fetal lung development mediated by mechanical forces. World J Respirol 2013; 3(3): 44-47
- URL: https://www.wjgnet.com/2218-6255/full/v3/i3/44.htm
- DOI: https://dx.doi.org/10.5320/wjr.v3.i3.44
Pulmonary hypoplasia secondary to congenital diaphragmatic hernia, oligohydramnios, etc., is an important cause of neonatal morbidity and mortality. Indeed, pulmonary hypoplasia is the most common finding (up to 26%) in neonatal autopsies[1]. Furthermore, more than 20000 babies are born every year in the United States before 27 wk of gestation (canalicular stage of lung development). These disorders have in common an incomplete development of the lungs. In addition to the risk of death, these conditions can also cause severe respiratory distress at birth and serious long-term morbidities[2]. Currently, the management is primarily supportive and there is not specific treatment to stimulate the growth/development of the lungs.
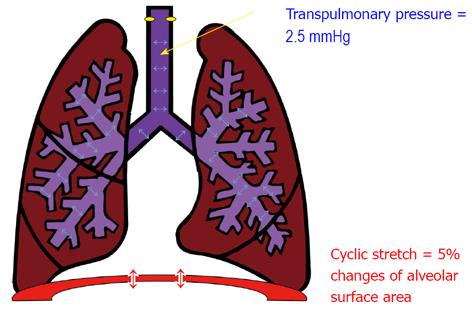
The lungs are unique in that their growth and development depends primarily on extrinsic factors and specifically on mechanical forces[3-7]. During gestation, the epithelium of the lung secretes fluid creating a constant distension pressure in the lumen of the lung of approximately 2.5 mmHg[8]. Moreover, the fetus makes episodic breathing movements (FBM) starting in the first trimester and increasing in frequency up to 30% of the time by birth[9] (Figure 1). It is clear from experimental animals that drainage of lung fluid volume[10] or abolition of FBM[11,12] lead to lung hypoplasia. Therefore, both tonic hydrostatic distension and cyclic mechanical deformation provide physical signals necessary for normal fetal lung development. However, the mechanisms by which lung cells sense these mechanical signals to promote lung development are not well-defined.
Tracheal ligation has been used experimentally[13] and in humans fetuses affected by congenital diaphragmatic hernia[14] as a mechanism to increase the intraluminal pressure of the lung, accelerate development and minimize the degree of pulmonary hypoplasia. However, this approach has a high rate of complications such as preterm labor, premature rupture of membranes and even death[15] and limitations and inability to be used in other forms of pulmonary hypoplasia. Therefore, a different approach to this problem is to investigate how mechanical forces promote lung development and use that information to stimulate lung development.
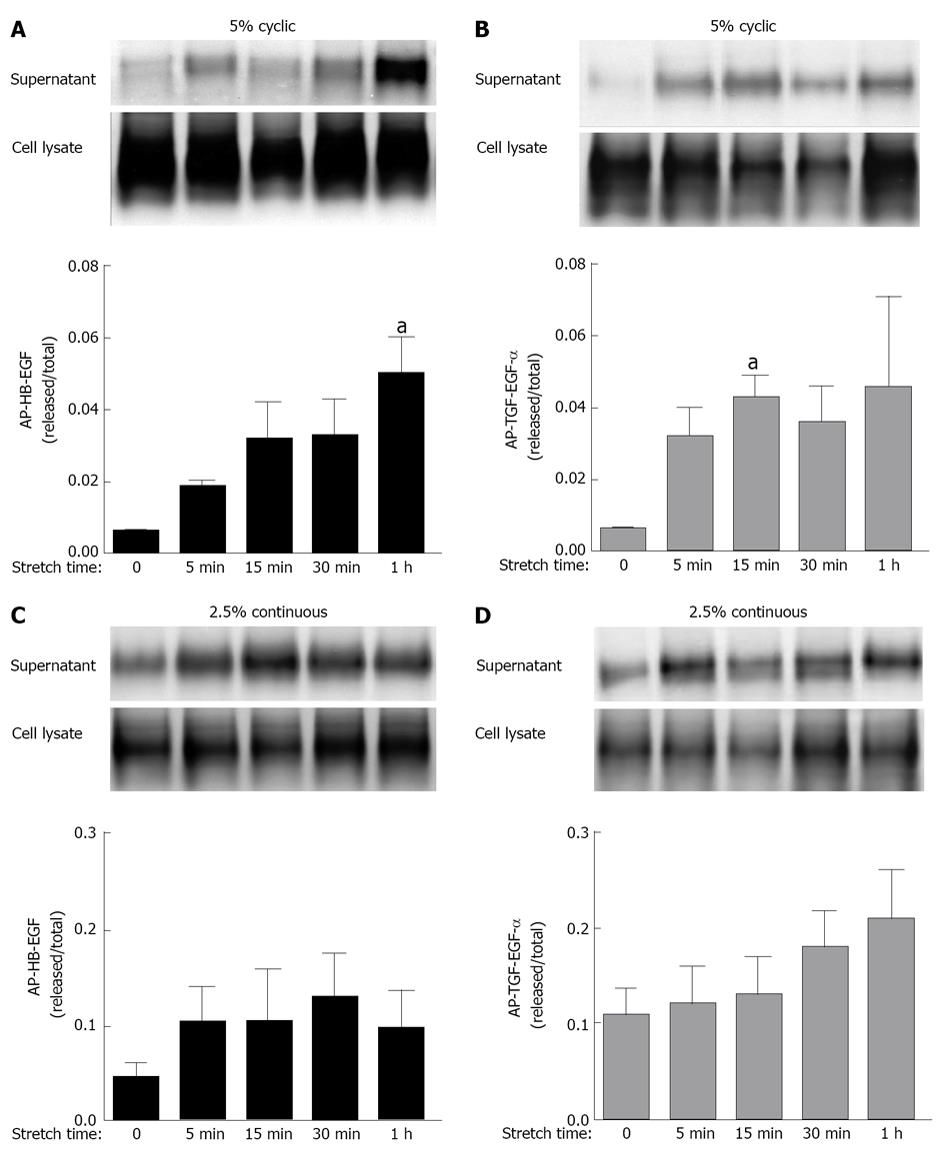
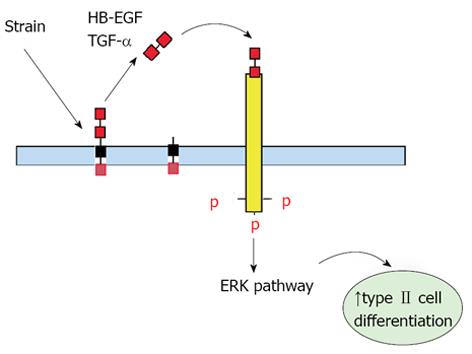
Past investigations in fetal lambs have shown that lung fluid composition after tracheal ligation was critical to promote lung development, since acceleration of growth and differentiation was not observed when lung fluids were replaced with normal saline[16,17]. These studies suggest that increased intratracheal pressure after tracheal ligation releases soluble factors that are important for lung development. This hypothesis is supported by previous in vitro studies from our laboratory in which fetal type II epithelial cells isolated during the canalicular stage of lung development were exposed to mechanical strain mimicking mechanical forces in lung development. Our data showed (Figure 2) that mechanical strain cleavages and releases the soluble, mature forms of epidermal growth factor receptor (EGFR) ligands heparin binding epidermal growth factor-like growth factor and transforming growth factor alpha (TGF-α)[18,19]. Release of these soluble factors bind and activate the EGFR via autocrine or paracrine signaling and promote differentiation of type II cells via the extracellular signal-regulated kinase signaling pathway (Figure 3).
The identification of soluble factors released by mechanical forces that are important for normal lung development could lead to novel avenues to accelerate lung development. Potential translational research applications would be prenatal administration to fetuses affected by pulmonary hypoplasia secondary to congenital diaphragmatic hernia or oligohydramnios or fetuses with borderline viability (22-24 wk) and at risk for delivery. Another theoretical application would be postnatal administration via the endotracheal tube. This is just an example on how the information obtained from these in vitro mechanistic studies could have the potential for clinical applicability. However, the therapeutic applicability of TGF-α in human neonatal and adult lung diseases is questionable since animal studies have demonstrated that transgenic overexpression of TGF-α disrupts neonatal lung development[20] and induces adult lung fibrosis[21]. In addition, increased epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma[22]. Therefore, before considering their use in humans, rigorous experiments in animal models are required first to demonstrate the effectiveness of this therapy and the lack of untoward side effects.
P- Reviewers: Ledford JG, Wu S S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Husain AN, Hessel RG. Neonatal pulmonary hypoplasia: an autopsy study of 25 cases. Pediatr Pathol. 1993;13:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997-1003. [PubMed] |
| 3. | Joe P, Wallen LD, Chapin CJ, Lee CH, Allen L, Han VK, Dobbs LG, Hawgood S, Kitterman JA. Effects of mechanical factors on growth and maturation of the lung in fetal sheep. Am J Physiol. 1997;272:L95-105. [PubMed] |
| 4. | Liu M, Post M. Invited review: mechanochemical signal transduction in the fetal lung. J Appl Physiol. 2000;89:2078-2084. [PubMed] |
| 5. | Sanchez-Esteban J, Cicchiello LA, Wang Y, Tsai SW, Williams LK, Torday JS, Rubin LP. Mechanical stretch promotes alveolar epithelial type II cell differentiation. J Appl Physiol. 2001;91:589-595. [PubMed] |
| 6. | Sanchez-Esteban J, Tsai SW, Sang J, Qin J, Torday JS, Rubin LP. Effects of mechanical forces on lung-specific gene expression. Am J Med Sci. 1998;316:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol. 2000;119:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 142] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Scarpelli EM, Condorelli S, Cosmi EV. Lamb fetal pulmonary fluid. I. Validation and significance of method for determination of volume and volume change. Pediatr Res. 1975;9:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Harding R. Fetal breathing movements. The lung: Scientific fountations. 2nd ed., Philadelphia, Lippincott-Raven 1997; 2093-2104. |
| 10. | Moessinger AC, Harding R, Adamson TM, Singh M, Kiu GT. Role of lung fluid volume in growth and maturation of the fetal sheep lung. J Clin Invest. 1990;86:1270-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 171] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Goldstein JD, Reid LM. Pulmonary hypoplasia resulting from phrenic nerve agenesis and diaphragmatic amyoplasia. J Pediatr. 1980;97:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 46] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Wigglesworth JS, Desai R. Effect on lung growth of cervical cord section in the rabbit fetus. Early Hum Dev. 1979;3:51-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 112] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | De Paepe ME, Johnson BD, Papadakis K, Luks FI. Lung growth response after tracheal occlusion in fetal rabbits is gestational age-dependent. Am J Respir Cell Mol Biol. 1999;21:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Deprest J, Gratacos E, Nicolaides KH. Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound Obstet Gynecol. 2004;24:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Jani JC, Nicolaides KH, Gratacós E, Valencia CM, Doné E, Martinez JM, Gucciardo L, Cruz R, Deprest JA. Severe diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol. 2009;34:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 291] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 16. | Papadakis K, Luks FI, De Paepe ME, Piasecki GJ, Wesselhoeft CW. Fetal lung growth after tracheal ligation is not solely a pressure phenomenon. J Pediatr Surg. 1997;32:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Luks FI, Roggin KK, Wild YK, Piasecki GJ, Rubin LP, Lesieur-Brooks AM, De Paepe ME. Effect of lung fluid composition on type II cellular activity after tracheal occlusion in the fetal lamb. J Pediatr Surg. 2001;36:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Huang Z, Wang Y, Nayak PS, Dammann CE, Sanchez-Esteban J. Stretch-induced fetal type II cell differentiation is mediated via ErbB1-ErbB4 interactions. J Biol Chem. 2012;287:18091-18102. [PubMed] |
| 19. | Wang Y, Maciejewski BS, Soto-Reyes D, Lee HS, Warburton D, Sanchez-Esteban J. Mechanical stretch promotes fetal type II epithelial cell differentiation via shedding of HB-EGF and TGF-alpha. J Physiol. 2009;587:1739-1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Hardie WD, Bruno MD, Huelsman KM, Iwamoto HS, Carrigan PE, Leikauf GD, Whitsett JA, Korfhagen TR. Postnatal lung function and morphology in transgenic mice expressing transforming growth factor-alpha. Am J Pathol. 1997;151:1075-1083. [PubMed] |
| 21. | Korfhagen TR, Swantz RJ, Wert SE, McCarty JM, Kerlakian CB, Glasser SW, Whitsett JA. Respiratory epithelial cell expression of human transforming growth factor-alpha induces lung fibrosis in transgenic mice. J Clin Invest. 1994;93:1691-1699. [PubMed] |
| 22. | Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, Korfhagen TR, Sivaprasad U, Ericksen M, Gibson AM. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am J Physiol Lung Cell Mol Physiol. 2011;300:L414-L421. [PubMed] |