Published online Nov 28, 2013. doi: 10.5320/wjr.v3.i3.110
Revised: September 25, 2013
Accepted: October 11, 2013
Published online: November 28, 2013
Processing time: 136 Days and 17.4 Hours
AIM: To evaluate the prognostic factors of long-term survival of more than 3 years in patients with advanced non-small cell lung cancer (NSCLC).
METHODS: We retrospectively analyzed the records of 474 patients with advanced IIIB/IV NSCLC who received chemotherapy as initial treatment between September 2002 and March 2007.
RESULTS: The median survival time (MST) was 12.5 mo and the 3 year and 5 year survival rates were 14.6% and 5.3%, respectively. Long-term survival of more than 3 and 5 years was observed in 65 and 16 patients, respectively. The MST for the 65 patients was 61.5 mo (range, 60.1-81.0 mo). In the 474 patients, a good performance status (PS), female sex, non-smoking status and adenocarcinoma histology were significantly associated with a favorable outcome. Furthermore, female sex, a good PS, non-smoking status and adenocarcinoma histology were significantly correlated with long-term survival of more than 3 years and most of these patients (89.2%, 58/65) received epidermal growth factor receptor-tyrosine kinase inhibitors as any line treatment. Survival analysis of long-term survivors showed that a PS of 0 was an independent prognostic factor for predicting favorable outcomes.
CONCLUSION: Our results suggest that a good PS and adenocarcinoma histology play an important role in long-term survival of more than 3 years. A PS of 0 was an independent prognostic factor for predicting favorable outcomes in patients with advanced NSCLC who survived for more than 3 years.
Core tip: The aim of this study is to evaluate the prognostic factors of long-term survival of more than 3 years in advanced non-small cell lung cancer. Female sex, good performance status (PS), non-smoker and adenocarcinoma were significantly associated with long-term survivors of more than 3 years and most patients received epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKI) at any line treatment. PS of 0 was an independent prognostic factor for predicting favorable prognosis in the long-term survivors of more than 3 years. PS of 0, adenocarcinoma and EGFR-TKI therapy play an important role in the long-term survivors.
- Citation: Kaira R, Kaira K, Shukuya T, Kenmotsu H, Ono A, Murakami H, Tsuya A, Nakamura Y, Naito T, Endo M, Yamamoto N, Takahashi T. Long-term survival of more than 3 years among patients with advanced non-small cell lung cancer treated with chemotherapy. World J Respirol 2013; 3(3): 110-115
- URL: https://www.wjgnet.com/2218-6255/full/v3/i3/110.htm
- DOI: https://dx.doi.org/10.5320/wjr.v3.i3.110
Non-small cell lung cancer (NSCLC) remains a major cause of cancer-related death worldwide. Surgery is the most common curative treatment, but for most patients with NSCLC, the tumor is often inoperable at the time of diagnosis. Platinum-based chemotherapy has resulted in a statistically significant improvement in survival compared to best supportive care[1,2]; however, the prognosis of patients with advanced NSCLC is still poor. Recent phase III trials have reported a median survival time (MST) of 8 to 10 mo and a 1 year survival rate of 30%-35%[3].
In 2004, mutations in the epidermal growth factor receptor (EGFR) gene, conferring increased sensitivity to the chemotherapy drug gefitinib, were reported[4,5]. Recently, two phase III studies of gefitinib as first-line therapy compared with platinum-based chemotherapy showed favorable outcomes in patients with advanced NSCLC harboring EGFR mutations[6,7]. The patients treated with gefitinib had a MST of 30.5 mo and a 2-year survival rate of 61.4%, indicating that some NSCLC patients may survive for more than 2 or 3 years.
Previous studies on the long-term survival of patients with advanced NSCLC treated with chemotherapy reported a 2-year survival rate of only 4%-6%[8-10]. However, these studies did not include NSCLC patients who received gefitinib and therefore, these data may no longer be accurate. Recently, we showed that a good performance status (PS), adenocarcinoma histology and EGFR-tyrosine kinase inhibitor (TKI) therapy are important factors associated with long-term survival of more than 5 years[11]. However, our previous study had a small sample size and was preliminary; therefore, further investigations on larger sample sizes were warranted. Moreover, there is no consensus on the definition of long-term survival for patients with advanced NSCLC (whether this should be more than 2, 3, 4 or 5 years). Previous reports have defined long-term survival in patients with advanced NSCLC as more than 2 years[8-10]. However, considering that advanced NSCLC patients responsive to gefitinib have a MST of more than 2 years, the clinical characteristics of patients with long-term survival of more than 3 years should be investigated. Against this background, we conducted a retrospective study to evaluate the prognostic factors associated with long-term survival of more than 3 years among advanced NSCLC patients who received chemotherapy as initial treatment.
We analyzed the records of 474 patients with advanced IIIB/IV NSCLC who received chemotherapy as initial treatment at the Department of Thoracic Oncology of Shizuoka Cancer Center between September 2002 and March 2007. NSCLC patients with recurrence after curative surgery were excluded from this study. The demographic characteristics of the 474 patients are listed in Table 1. The median patient age was 64 years (range, 23-85 years); 323 patients were male and 151 were female; 323 were smokers and 154 had never smoked; 333 had adenocarcinoma histology and 141 had non-adenocarcinoma histology; and 109 patients had clinical stage IIIB disease and 365 had stage IV disease. The Eastern Cooperative Oncology Group (ECOG) PS was 0 in 148 patients, 1 in 240 patients, 2 in 65 patients, 3 in 20 patients, and 4 in 1 patient.
| Variables | No. of patients (n = 474) | |
| Age (yr) | ||
| (median 64) | < 65 | 264 (55.7) |
| (range 23-85) | ≥ 65 | 210 (44.3) |
| Gender | ||
| Male | 323 (68.1) | |
| Female | 151 (31.9) | |
| Performance status | ||
| (ECOG) | 0 | 148 (31.2) |
| 1 | 240 (50.6) | |
| 2 | 65 (13.7) | |
| 3 | 20 (0.04) | |
| 4 | 1 (0.002) | |
| Smoking | ||
| Yes | 320 (67.5) | |
| No | 154 (32.5) | |
| Histology | ||
| Adenocarcinoma | 333 (70.3) | |
| Non-adenocarcinoma | 141 (29.7) | |
| Clinical stage | ||
| IIIB | 109 (23.0) | |
| IV | 365 (77.0) | |
Of these 474 patients, 380 (80.2%) were treated with platinum-doublet regimens, 64 (13.5%) with single-agent regimens, and 30 (6.3%) with EGFR-TKI therapy (gefitinib or erlotinib) as first-line treatment. Two-hundred and thirty-eight patients (50.2%) received EGFR-TKI therapy as any line treatment. Staging was performed for all patients according to the Union for International Cancer Control TNM classification[12]. For TNM staging, all patients underwent computed tomography (CT) of the thorax and upper abdomen, bone scintigraphy or positron emission tomography, and brain CT or magnetic resonance imaging. Histological analysis of the tumors was based on the World Health Organization (WHO) classification of cell types[13].
Survival was recorded from the first day of treatment to the date of death or last follow-up, and the survival curves were calculated using the Kaplan-Meier method[14]. The median follow-up period was 323 d (range, 13-2069 d). Survival time was calculated at more than 3 years after the final registration. Fisher’s exact test was used to examine the association between two categorical variables and probability values of < 0.05 indicated a statistically significant difference. We evaluated the efficacy of chemotherapy using the Response Evaluation Criteria in Solid Tumors, version 1.1. Survival difference was analyzed using the log-rank test. Multivariate analyses were performed using a stepwise Cox proportional hazards model to identify independent prognostic factors. Statistical analysis was performed using JMP 8 (SAS, Institute Inc., Cary, NC, United States) for Windows.
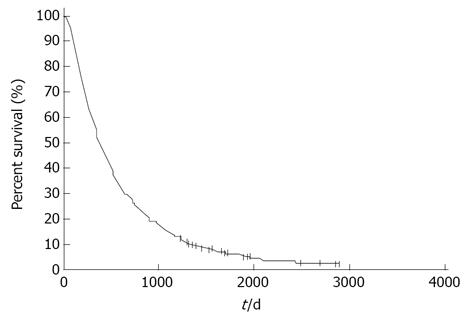
Figure 1 shows the survival curves for all 474 patients. The MST was 12.5 mo and the 1 year, 2 years, 3 years, 4 years and 5 years survival rates were 50.8%, 26.3%, 14.6%, 8.2% and 5.3%, respectively. Long-term survival of more than 3 years was observed in 65 patients and long-term survival of more than 5 years was observed in 16 patients. Univariate analysis showed that a good PS, female sex, non-smoking status and adenocarcinoma histology were significantly associated with a favorable outcome. Multivariate analysis demonstrated that a good PS and adenocarcinoma histology were independent prognostic factors for predicting a favorable prognosis (Table 2).
| Variables | Univariate analysis(log-rank test)P value | Multivariate analysis(Cox’s proportional hazard models)P value | |
| Age (yr) | < 65/≥ 65 | 0.4158 | 0.5172 |
| Gender | Male/Female | < 0.0001 | 0.0982 |
| PS | 0-1/2-4 | < 0.0001 | < 0.0001 |
| Smoking | Yes/No | < 0.0001 | 0.1363 |
| Histology | AC/Non-AC | < 0.0001 | < 0.0001 |
| Clinical stage | IIIB/IV | 0.2151 | 0.5216 |
Of the 474 patients, 65 (33 men and 32 women) with a median age of 65 years (range, 35-78 years) survived for more than 3 years, with a MST of 61.5 mo (range, 60.1-81.0 mo). The PS, clinical stage and histology of the patients were as follows: 35 patients had a PS of 0, 25 had a PS of 1, and 5 had a PS of 2-3; 18 patients had stage IIIB disease and 47 had stage IV disease; 65 patients had adenocarcinoma histology. With regard to metastatic sites, 23 patients had bone metastases, 18 had brain metastases, and 9 had synchronous brain and bone metastases. With regard to the efficacy of first-line treatment, a partial response was noted in 29% of cases (19/65). The median treatment-free interval between first and second-line therapy was 518 d (range, 26-1901 d) and the median total number of therapeutic lines was 4 (range, 1-13 lines).
We then compared the demographic characteristics of patients who did (n = 65) and did not (n = 409) achieve survival of more than 3 years (Table 3). Female sex, a good PS, non-smoking status and adenocarcinoma histology were significantly correlated with long-term survival of more than 3 years. The treatment regimens of patients who did and did not achieve long-term survival were also compared. Among patients who did not achieve long-term survival, 325 (79.4%) received platinum-doublet regimens, 59 (14.4%) received single-agent therapy, and 25 (6.1%) received EGFR-TKI therapy as first-line treatment. In contrast, among long-term survivors, 55 (84.6%) received platinum-doublet regimens, 5 (7.7%) received single-agent therapy, and 5 (7.7%) received gefitinib as first-line treatment. Demographic characteristics did not differ significantly between patients who did and did not achieve long-term survival, according to the first-line regimen. As any line treatment, 180 patients (44.0%) who did not achieve long-term survival and 58 patients (89.2%) who did achieve long-term survival received EFGR-TKI; this difference was statistically significant (P < 0.0001).
| Variables | < 3 yr (n=409) | ≥3 yr (n=65) | P value | |
| Age | < 65/≥65 | 231/178 | 21/34 | 0.2267 |
| Gender | Male/Female | 290/119 | 33/32 | 0.0024 |
| PS | 0-1/2-4 | 329/80 | 60/5 | 0.0225 |
| Smoking | Yes/No | 291/118 | 35/30 | 0.0090 |
| Histology | AC/Non-AC | 268/141 | 65/0 | < 0.0001 |
| Clinical stage | IIIB/IV | 91/318 | 18/47 | 0.3428 |
With regard to response to first-line chemotherapy among the 65 patients who achieved long-term survival, 17 patients were responders and 48 patients were non-responders, resulting in a response rate of 26%.
Univariate analysis did not identify any statistically significantly prognostic factors (Table 4). We excluded 5 patients with a PS of 2-3 from these 65 patients, thus evaluating 60 patients with a PS of 0-1 by univariate and multivariate analyses (Table 5). A PS of 0 was found to be an independent prognostic factor for predicting a favorable outcome.
| Variables | No. of patients | Univariate analysis (log-rank test) P value | |
| Age | < 65/≥ 65 | 31/34 | 0.9448 |
| Gender | Male/Female | 33/32 | 0.3467 |
| PS | 0-1/2-3 | 60/5 | 0.7468 |
| Smoking | Yes/No | 35/30 | 0.9835 |
| Clinical stage | IIIB/IV | 18/47 | 0.7627 |
| Variables | No. of patients | Univariate analysis (log-rank test) P value | Multivariate analysis (Cox’s proportionalhazard models) P value | |
| Age | < 65/≥ 65 | 29/31 | 0.8099 | 0.9421 |
| Gender | Male/Female | 31/29 | 0.4133 | 0.3676 |
| PS | 0/1 | 35/25 | 0.0158 | 0.0244 |
| Smoking | Yes/No | 33/27 | 0.9062 | 0.5170 |
| Clinical stage | IIIB/IV | 18/42 | 0.8139 | 0.6781 |
The present study showed that advanced NSCLC patients who survived for more than 3 years had a good PS and adenocarcinoma histology. Most patients who survived for more than 3 years received platinum-containing chemotherapy as initial treatment and EGFR-TKI as any line chemotherapy. Multivariate analysis of long-term survivors showed that a PS of 0 was an independent prognostic factor for predicting a favorable outcome.
A previous study reported that the best prognostic factors for long-term survivors were non-metastatic disease status and response to chemotherapy[14]. In this previous study, 1052 patients treated with platinum-based chemotherapy were analyzed and the 2 years and 5 years survival rates were 7.4% and 1.8%, respectively. All patients who survived for more than 5 years had limited disease and were treated by complementary thoracic radiation and/or surgery. Other recent studies also reported that a good PS, adenocarcinoma histology and EGFR-TKI therapy contributed to long-term survival of more than 2 years[9,11]. As EGFR-TKI therapy contributes to prolonged survival, previous reports on long-term survivors who did not receive this treatment may not be useful. Therefore, retrospective studies that include a treatment history of EGFR-TKI therapy are needed to identify the prognostic factors for a favorable outcome.
Our study suggested that a PS of 0, but not 1, was an important factor for predicting long-term survival of more than 3 years. In the ECOG experience, the rate of survival for more than 2 years in patients with metastatic NSCLC was 4.0% and pretreatment characteristics associated with long-term survival were an initial PS of 0, no bone metastases, female sex, no subcutaneous metastases, no larger cell histology, a prior weight loss of less than 5%, and no liver metastases[15]. The experience of the South West Oncology Group also documented that a good PS, female sex and an age of more than 70 years were significant independent survival predictors[16]. A good PS is known to be closely associated with a favorable outcome after chemotherapy in patients with advanced NSCLC. Although adenocarcinoma histology, use of EGFR-TKI, and an initial good PS are essential in order to achieve survival for more than 3 years in cases of advanced NSCLC, the outcome of patients with a PS of 0 may be different from that of patients with a PS of 1.
In July 2002, gefitinib was approved for pretreated NSCLC patients in Japan in clinical practice. Recently, Satouchi et al[17] reported on the predictive factors associated with the prognostic benefits of gefitinib, showing that survival was significantly better for female sex, adenocarcinoma histology, never-smoked status, a favorable PS and EGFR mutation positivity. Recent clinical studies demonstrated that the use of gefitinib or erlotinib resulted in significantly longer survival than platinum-based chemotherapy in patients with advanced NSCLC harboring EGFR mutation[18,19] and the MST of patients treated with gefitinib was approximately 3 years (27.7 mo)[18]. In multivariate analysis, EGFR mutation positivity and a PS of 0-1 have been described as independent predictors of a favorable prognosis.
It is currently unclear whether EGFR mutation is a prognostic factor in NSCLC patients not treated with EGFR-TKI. Therefore, Kosaka et al[20] examined the prognostic significance of EGFR mutation in a large cohort of patients with surgically treated lung adenocarcinoma. In their study, univariate analysis demonstrated that patients with EGFR mutations have favorable survival compared to those without EGFR mutations (P = 0.0046). However, EGFR mutation positivity was not independently associated with poor outcome in cases of resectable lung adenocarcinoma not treated with EGFR-TKI and was a predictive factor for cases treated with gefitinib, but not for pulmonary adenocarcinoma not treated with gefitinib. In the present study, one of the limitations was that the EGFR mutation status had not been analyzed in all patients. Therefore, whether a PS of 0 is a useful factor for predicting favorable prognosis compared with EGFR mutation positivity remains unknown. Accordingly, further study is warranted for the confirmation of our results.
While PS is an important factor in determining outcomes in cases of NSCLC, there is limited available data on the distribution of PS among NSCLC patients. Recently, Kawaguchi et al[21] showed that PS is an independent favorable prognostic factor in a large-scale retrospective study of 26957 patients with NSCLC. In their study, most patients with a PS of 0 presented with stage I disease and were never-smokers and overall survival differed significantly between patients with a PS of 0 and those with a PS of 1. Moreover, outcomes differed significantly in patients with advanced NSCLC between those with a PS of 0 and those with a PS of 1. Qi et al[22] also reported that pretreatment quality of life was an independent prognostic factor for overall survival in patients with advanced NSCLC. These reports suggest that PS before treatment may be closely associated with long-term survival in patients with advanced NSCLC. Furthermore, outcomes are thought to differ between treated NSCLC patients with a PS of 0 and those with a PS of 1, but no data are available in the literature on the long-term survival of chemotherapy-treated patients with advanced NSCLC. At present, three major scales are used to measure PS in oncology, Karnofsky PS, ECOG PS and WHO PS, although the ECOG PS scale has been shown to be more effective than the Karnofsky PS scale in discriminating between patients with different prognoses[23]. Our results indicate a strong relationship between a PS of 0 and long-term survival in advanced NSCLC patients treated with chemotherapy, indicating that the ECOG PS scale may be an appropriate measure for predicting long-term survival.
In conclusion, our results suggest that a good PS and adenocarcinoma histology play an important role in long-term survival of more than 3 years. A PS of 0 was an independent prognostic factor for predicting favorable outcomes in patients with advanced NSCLC who survived for more than 3 years. In the future, a large-scale study including EGFR mutation analysis might be considered for determining the prognostic factors of patients with advanced NSCLC who are treated with chemotherapy and achieve long-term survival of more than 3 years.
Surgery is the most common curative treatment but for most patients with non-small cell lung cancer (NSCLC), the tumor is often inoperable at the time of diagnosis. Recent phase III trials have reported a median survival time of 8 to 10 mo and a 1 year survival rate of 30%-35%.
The authors’ results suggest that a good performance status (PS) and adenocarcinoma histology play an important role in long-term survival of more than 3 years. A PS of 0 was an independent prognostic factor for predicting favorable outcomes in patients with advanced NSCLC who survived for more than 3 years.
PS of 0, adenocarcinoma and epidermal growth factor receptor-tyrosine kinase inhibitors therapy play an important role in the long-term survivors.
This is a flawless manuscript, very well written, which can be useful for the readers and investigators in lung cancer.
P- Reviewers Araujo A, Boonsarngsuk V, Melloni G, Pompeo E S- Editor Wen LL L- Editor Roemmele A E- Editor Liu XM
| 1. | NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617-4625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 457] [Cited by in RCA: 450] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 2. | Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S, Olak J, Stover D, Strawn JR, Turrisi AT. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22:330-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1108] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 3. | Socinski MA, Morris DE, Masters GA, Lilenbaum R. Chemotherapeutic management of stage IV non-small cell lung cancer. Chest. 2003;123:226S-243S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7278] [Cited by in RCA: 7559] [Article Influence: 343.6] [Reference Citation Analysis (0)] |
| 5. | Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8739] [Cited by in RCA: 8874] [Article Influence: 403.4] [Reference Citation Analysis (0)] |
| 6. | Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M; West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung harbouring mutations of the epidermal growth factor receptor (WJTOG 3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2837] [Cited by in RCA: 3314] [Article Influence: 207.1] [Reference Citation Analysis (0)] |
| 7. | Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3961] [Cited by in RCA: 4454] [Article Influence: 278.4] [Reference Citation Analysis (0)] |
| 8. | Satoh H, Ishikawa H, Yamashita YT, Naito T, Takahashi H, Kamma H, Ohtsuka M, Hasegawa S. Analysis of long-term survivors after platinum containing chemotherapy in advanced non-small cell lung cancer. Anticancer Res. 1998;18:1295-1298. [PubMed] |
| 9. | Satoh H, Ishikawa H, Ohara G, Kagohashi K, Kurishima K, Ohtsuka M, Hizawa N. Long-term survivors after chemotherapy in advanced non-small cell lung cancer. Anticancer Res. 2007;27:4457-4460. [PubMed] |
| 10. | Okamoto T, Maruyama R, Shoji F, Asoh H, Ikeda J, Miyamoto T, Nakamura T, Miyake T, Ichinose Y. Long-term survivors in stage IV non-small cell lung cancer. Lung Cancer. 2005;47:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Kaira K, Takahashi T, Murakami H, Tsuya A, Nakamura Y, Naito T, Endo M, Yamamoto N. Long-term survivors of more than 5 years in advanced non-small cell lung cancer. Lung Cancer. 2010;67:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Travis WD, Brambilla E, Muller-Hermelink HK. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press 2004; 53-58. |
| 13. | Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E, Sobin LH. Histological typing of lung and pleural tumors, 3rd ed. World Health Organization international histological classification of tumors. Berlin: Springer Verlag 1999; . |
| 14. | Sculier JP, Paesmans M, Libert P, Bureau G, Dabouis G, Thiriaux J, Michel J, Van Cutsem O, Schmerber J, Giner V. Long-term survival after chemotherapy containing platinum derivatives in patients with advanced unresectable non-small cell lung cancer. European Lung Cancer Working Party. Eur J Cancer. 1994;30A:1342-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Finkelstein DM, Ettinger DS, Ruckdeschel JC. Long-term survivors in metastatic non-small-cell lung cancer: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1986;4:702-709. [PubMed] |
| 16. | Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9:1618-1626. [PubMed] |
| 17. | Satouchi M, Negoro S, Funada Y, Urata Y, Shimada T, Yoshimura S, Kotani Y, Sakuma T, Watanabe H, Adachi S. Predictive factors associated with prolonged survival in patients with advanced non-small-cell lung cancer (NSCLC) treated with gefitinib. Br J Cancer. 2007;96:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 2013;24:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 19. | Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4228] [Cited by in RCA: 4421] [Article Influence: 315.8] [Reference Citation Analysis (6)] |
| 20. | Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T. Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol. 2009;4:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, Saito R, Maruyama Y, Kawahara M, Ignatius Ou SH. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5:620-630. [PubMed] |
| 22. | Qi Y, Schild SE, Mandrekar SJ, Tan AD, Krook JE, Rowland KM, Garces YI, Soori GS, Adjei AA, Sloan JA. Pretreatment quality of life is an independent prognostic factor for overall survival in patients with advanced stage non-small cell lung cancer. J Thorac Oncol. 2009;4:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 457] [Article Influence: 15.2] [Reference Citation Analysis (0)] |