Published online Jul 28, 2013. doi: 10.5320/wjr.v3.i2.38
Revised: May 7, 2013
Accepted: June 1, 2013
Published online: July 28, 2013
Processing time: 121 Days and 23.3 Hours
Lung cancer metastasis is typically determined by histologic similarity between distant and primary lesions. Herein, we present a 70-year-old Japanese woman with an adenocarcinoma in her lung and a squamous cell carcinoma in her femur; both tumors had an identical epidermal growth factor receptor mutation, G719S. This indicated that both tumors had a common origin, despite their histologic dissimilarity. The tumor in the femur was thus identified genetically as a lung cancer metastasis. This case suggests that genetic analysis can determine whether a distant lesion is a lung cancer metastasis, particularly when the histology differs from that of the primary lesion.
Core tip: A tumor in the femur was identified genetically as a lung cancer metastasis. This case suggests that a matching epidermal growth factor receptor (EGFR) mutation in a distant lesion can validate a diagnosis of lung cancer metastasis, even if it differs histologically from the primary lesion. To the best of our knowledge, this is the first report showing the use of EGFR genetic analysis to identify a distant lesion as a lung cancer metastasis. In the era of molecularly-targeted treatments for non-small cell lung cancer, combined pathological diagnosis and genetic analysis could lead to more precise diagnoses and better understanding of histogenesis and more appropriate therapeutic selections.
-
Citation: Kanaji N, Bandoh S, Hayashi T, Haba R, Watanabe N, Ishii T, Kunitomo A, Takahama T, Tadokoro A, Imataki O, Dobashi H, Matsunaga T.
EGFR mutation identifies distant squamous cell carcinoma as metastasis from lung adenocarcinoma. World J Respirol 2013; 3(2): 38-43 - URL: https://www.wjgnet.com/2218-6255/full/v3/i2/38.htm
- DOI: https://dx.doi.org/10.5320/wjr.v3.i2.38
Non-small cell lung cancer (NSCLC) can be classified into several histologic subtypes, including adenocarcinoma and squamous cell carcinoma. Rarely, two histologic subtypes exist within one tumor, such as adenosquamous carcinoma. Metastasis of NSCLC is typically clinically diagnosed based on symptoms, a physical examination, and imaging, such as X-rays and computed tomography (CT). For tissue samples obtained from distant lesions, the diagnosis of metastasis is typically determined by histologic similarity between the distant lesion and the primary lung lesion. Mutations of the epidermal growth factor receptor (EGFR) vary among histologic NSCLC subgroups, and among Asian patients, the incidence is approximately 40%-60% in adenocarcinoma and 3% in squamous cell carcinoma[1-6]. To the best of our knowledge, this is the first report showing the use of an EGFR genetic analysis to identify a distant lesion as a lung cancer metastasis.
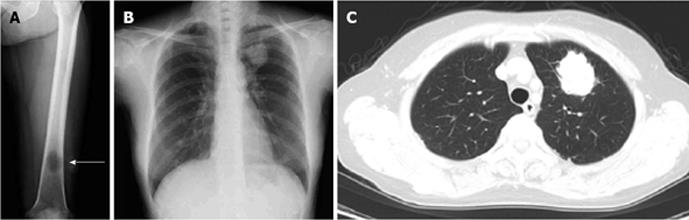
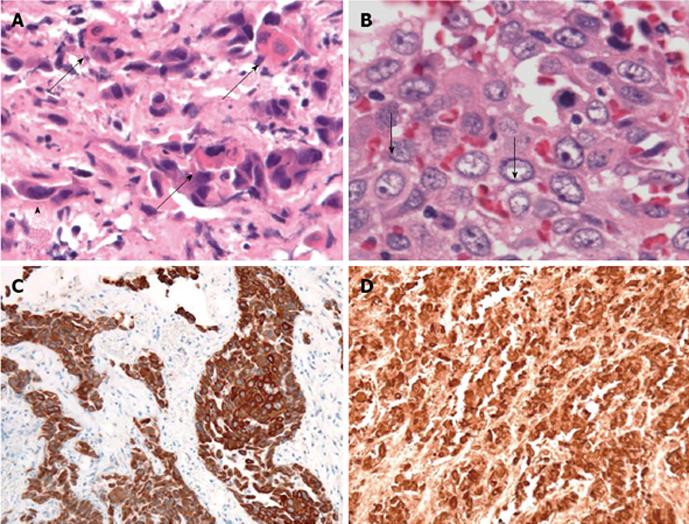
A 70-year-old Japanese woman was admitted to our hospital in July 2012 for further evaluation of pain in her left femur, which had gradually progressed over 6 mo. She had never smoked, and had no past history of illness. A physical examination disclosed tenderness on her left femur. Lymphadenopathy and clubbing were not present. SpO2 was 97%. Her vesicular sound was normal, and no crackles were auscultated. A left femur X-ray showed an osteolytic lesion, suggestive of metastasis (Figure 1A). A chest X-ray showed a mass shadow in the left lung field; CT showed a 4-cm tumor in the left upper lobe, suggesting that the primary lesion was lung cancer (Figure 1B and C). Among tumor markers, carcinoembryonic antigen (CEA; 23.4 ng/mL; normal range: < 5.0 ng/mL), cytokeratin 19 fragment (CYFRA; 11.6 ng/mL; normal range: < 3.5 ng/mL), and squamous cell carcinoma antigen (SCC; 1.9 ng/mL; normal range: < 1.5 ng/mL) showed values consistent with NSCLC. Shortly after a diagnostic bronchoscopic examination was planned, the patient’s left femur fractured. Under general anesthesia, a biopsy was taken from under the osteosynthesis. The pathological findings included carcinoma, with single cell keratosis, tadpole cells, and intercellular bridges (Figure 2A and B). The cancer cells were positive for cytokeratin (CK) 5/6 and p63 (Figure 2C and D) and negative for thyroid transcription factor-1 (data not shown). Based on these findings, the tumor in the femur was diagnosed as a poorly differentiated squamous cell carcinoma; we observed no pathological findings that suggested adenocarcinoma.
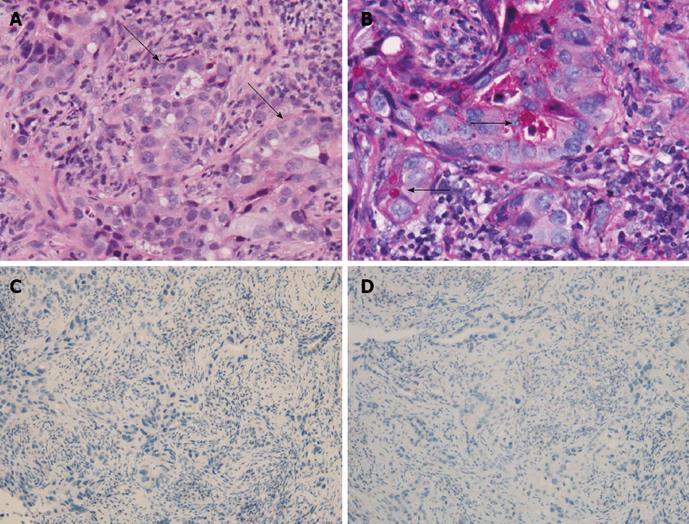
Because squamous cell lung cancer in never-smokers is relatively rare, a transbronchial tumor biopsy was also performed. The pathologic findings showed poorly differentiated adenocarcinoma including glandular formation (Figure 3A). These cancer cells were positive for D-periodic acid-Schiff, suggesting mucin production (Figure 3B), and negative for CK5/6 and p63 (Figure 3C and D). No findings suggestive of squamous cell carcinoma were observed in the lung biopsy sample. Therefore, the pathologic diagnosis of the lung tumor was completely different from that of the femur. No primary lesions other than the lesion found in the lung were detected in any other part of her body.
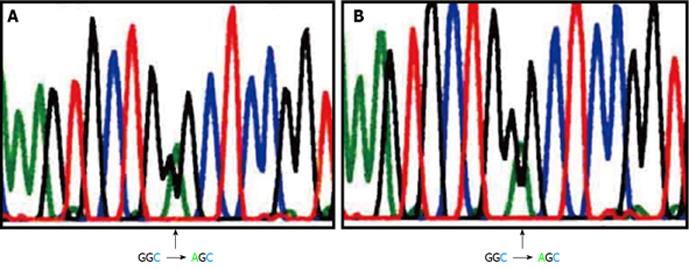
We analyzed the EGFR mutation status in tumor samples from the lung and the femur as described previously[4]. Briefly, RNA was extracted from cell samples, and cDNA was synthesized. Reverse transcriptase-polymerase chain reaction (PCR) for EGFR was performed, and the PCR products were sequenced. The G719S mutation in exon 18 was detected in both samples (Figure 4). This mutation was not detected in circulating white blood cells (data not shown). Based on this genetic analysis, we determined that the lung and femur tumors had the same origin. She was thus diagnosed as having lung cancer with metastasis to the femur (cT2aN2M1b, stage IV). Chemotherapy with carboplatin (area under the curve 5, day 1) and docetaxel (60 mg/m2, day 1) was administered, and the fractured left femur was irradiated (total 30 Gy). After four cycles of chemotherapy, the primary lesion markedly regressed, and all tumor markers, including CEA, CYFRA, and SCC, decreased to within the normal ranges. She exhibited a partial response to chemotherapy.
In general, EGFR mutations have been reported to be almost exclusively found in carcinomas of the lung, although they are observed with a low frequency in other solid tumors[7]. In the present case, (1) primary lung cancer did exist; and (2) the EGFR mutation G719S was identified in both the primary and distant lesions, despite their different histology, adenocarcinoma and squamous cell carcinoma, respectively. This finding proved that these histologically different tumors had the same origin, facilitating a diagnosis of lung cancer and its metastasis.
Adenosquamous carcinoma has components of both adenocarcinoma and squamous cell carcinoma in one tumor and is a rare NSCLC subtype detected in 0.4%-4% of lung cancers[8,9]. A study of Korean patients found EGFR mutations in 11 (44%) of 25 adenosquamous carcinomas[9], which is in agreement with their frequency in Asian patients with adenocarcinoma[1-4,6]. Interestingly, in all 11 adenosquamous carcinomas, when the two tissue types were manually microdissected from each tumor, the adenocarcinoma and squamous cell carcinoma components had the same EGFR mutation[9]. This finding indicates that adenosquamous carcinoma components are more likely to originate from common monoclonal progenitor cells than from a polyclonal pathway[9].
In the present case, the lung biopsy sample showed adenocarcinoma with no squamous cell carcinoma. However, the lung tumor might have contained a squamous cell component because the tissue sample obtained by bronchoscopy was a small part of the total tumor. Possible lung tumor histology includes adenocarcinoma, adenosquamous carcinoma, and adenocarcinoma with squamous differentiation. However, the femur tumor was a squamous cell carcinoma with no adenocarcinoma component. The most likely course is that a squamous cell component of the lung tumor spread to the femur; alternatively, this specific bone environment might favor squamous cell differentiation from undifferentiated progenitor cells. Regarding the mechanisms of histological differentiation, some interesting studies have been reported. Lung adenocarcinoma phenotypes have been reproduced by introducing an active form of PIK3CA, cyclin-D1, or a dominant-negative form of liver kinase B1 in combination with genetic alterations including human telomerase overexpression, inactivation of the pRB and p53 pathways, and KRAS activation[10]. p63 regulates cell proliferation and differentiation[11] and is a well-known marker of squamous differentiation[12]. Although the actual factors involved in squamous and adenomatous differentiation in the current case are unknown, these molecular mechanisms may have been involved.
Currently, the clinical diagnosis of lung cancer metastasis is typically based on symptoms, a physical examination, and imaging such as X-ray and CT; pathologists’ assessments of tissue samples are fundamental to this process, as they can confirm histological similarities between distant and primary lesions, using, for example, immunostains specific to tissue types. However, a distant tumor with a histology that differs from the primary lesion can be difficult to diagnose as a metastasis. In the present case, the presence of an identical EGFR mutation facilitated the identification of a distant lesion as a metastasis, despite its completely different histology.
We previously reported the significance of detecting an EGFR mutation in the cerebrospinal fluid of a patient with carcinomatous meningitis, whose primary lung lesion was not approached because of his poor performance status; detection of an EGFR mutation contributed to the diagnosis of lung cancer and facilitated the determination of an appropriate therapeutic protocol[13]. We found several previous reports showing the usefulness of p53 analysis in primary and metastatic lesions. In one study, a p53 mutation analysis determined that the ovarian tumor was a metastasis from the sigmoid colon[14]. Similarly, an identical p53 mutation confirmed that a neck nodal metastasis originated from oral squamous cell carcinoma that had been treated over five years prior[15]. The present case supports the use of genetic analysis in diagnosing distant lesions and suggests that it could be expanded to include mutations other than those in EGFR and p53, such as anaplastic lymphoma kinase[16], ret proto-oncogene[17,18], and c-ros oncogene 1, receptor tyrosine kinase[18]. Moreover, genetic analyses could help determine the origin of tumors of so-called unknown origin.
A short deletion in exon 19 and L858R in exon 21 are two major EGFR mutations that constitute approximately 80% of total EGFR mutations[3]. A mutation on codon G719 is an uncommon EGFR mutation that was detected only in 15 (2.4%) of 627 cases with EGFR mutations[3]. This amino acid substitution mutation includes G719A, G719C, G719D, and G719S, the last of which was detected in the present case. The efficacy of EGFR-tyrosine kinase inhibitor (TKI) treatment for tumors with G719 mutations was previously evaluated, and the response rate was 50% (4 of 8 cases without complex mutations)[3]. Although few cases were evaluated, progressive disease was observed in 3 cases (38%), suggesting a relatively lower effectiveness of EGFR-TKIs[3].
The frequency of EGFR mutations in squamous cell carcinoma is reportedly approximately 3%, which is much lower compared to adenocarcinoma or adenosquamous carcinoma[5]. Therefore, pulmonologists might wonder whether testing for an EGFR mutation found in only 3% of samples is cost-effective. In this regard, smoking history could be considered. The frequency of EGFR mutations is lower in smokers than in never-smokers (approximately 14% vs 60%)[19]. However, in one study of 87 patients with squamous cell carcinoma, all 3 patients with EGFR mutations were smokers[5]. We have been analyzed EGFR mutation status in all squamous cell carcinomas as well as adenocarcinomas.
In conclusion, a matching EGFR mutation in a distant lesion can validate a diagnosis of lung cancer metastasis, even if it differs histologically from the primary lesion. In the era of molecularly-targeted treatments for NSCLC, a combined pathological diagnosis and genetic analysis could lead to more appropriate therapeutic selections, more precise diagnoses, and a better understanding of histogenesis.
P- Reviewers Wang Y, Yeudall WA S- Editor Gou SX L- Editor A E- Editor Lu YJ
| 1. | Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919-8923. [PubMed] |
| 2. | Mu XL, Li LY, Zhang XT, Wang MZ, Feng RE, Cui QC, Zhou HS, Guo BQ. Gefitinib-sensitive mutations of the epidermal growth factor receptor tyrosine kinase domain in chinese patients with non-small cell lung cancer. Clin Cancer Res. 2005;11:4289-4294. [PubMed] |
| 3. | Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17:3812-3821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 4. | Kanaji N, Bandoh S, Ishii T, Kushida Y, Haba R, Kohno K, Dobashi H, Ohnishi H, Matsunaga T. Detection of epidermal growth factor receptor mutations in a few cancer cells from transbronchial cytologic specimens by reverse transcriptase-polymerase chain reaction. Mol Diagn Ther. 2011;15:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Miyamae Y, Shimizu K, Hirato J, Araki T, Tanaka K, Ogawa H, Kakegawa S, Sugano M, Nakano T, Mitani Y. Significance of epidermal growth factor receptor gene mutations in squamous cell lung carcinoma. Oncol Rep. 2011;25:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Sun PL, Seol H, Lee HJ, Yoo SB, Kim H, Xu X, Jheon S, Lee CT, Lee JS, Chung JH. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features. J Thorac Oncol. 2012;7:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Masago K, Asato R, Fujita S, Hirano S, Tamura Y, Kanda T, Mio T, Katakami N, Mishima M, Ito J. Epidermal growth factor receptor gene mutations in papillary thyroid carcinoma. Int J Cancer. 2009;124:2744-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Toyooka S, Yatabe Y, Tokumo M, Ichimura K, Asano H, Tomii K, Aoe M, Yanai H, Date H, Mitsudomi T. Mutations of epidermal growth factor receptor and K-ras genes in adenosquamous carcinoma of the lung. Int J Cancer. 2006;118:1588-1590. [PubMed] |
| 9. | Kang SM, Kang HJ, Shin JH, Kim H, Shin DH, Kim SK, Kim JH, Chung KY, Kim SK, Chang J. Identical epidermal growth factor receptor mutations in adenocarcinomatous and squamous cell carcinomatous components of adenosquamous carcinoma of the lung. Cancer. 2007;109:581-587. [PubMed] |
| 10. | Sasai K, Sukezane T, Yanagita E, Nakagawa H, Hotta A, Itoh T, Akagi T. Oncogene-mediated human lung epithelial cell transformation produces adenocarcinoma phenotypes in vivo. Cancer Res. 2011;71:2541-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev. 2006;20:3185-3197. [PubMed] |
| 12. | Conde E, Angulo B, Redondo P, Toldos O, García-García E, Suárez-Gauthier A, Rubio-Viqueira B, Marrón C, García-Luján R, Sánchez-Céspedes M. The use of P63 immunohistochemistry for the identification of squamous cell carcinoma of the lung. PLoS One. 2010;5:e12209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Kanaji N, Bandoh S, Nagamura N, Kushida Y, Haba R, Ishida T. Significance of an epidermal growth factor receptor mutation in cerebrospinal fluid for carcinomatous meningitis. Intern Med. 2007;46:1651-1655. [PubMed] |
| 14. | Yamano T, Morii E, Arai I, Takada T, Kubota K, Sato M, Inoue T, Okada Y, Hara T, Aozasa K. Diagnosis of primary versus metastatic ovarian adenocarcinoma using p53 gene mutation analysis. Int J Clin Oncol. 2010;15:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Hoekstra JW, Kummer JA, van Es RJ. Late (& gt; 5 years) regional lymph node metastasis of oral squamous cell carcinoma (SCC), proven by p53 mutation analysis. J Craniomaxillofac Surg. 2008;36:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561-566. [PubMed] |
| 17. | Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, Sakamoto H, Tsuta K, Furuta K, Shimada Y. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 678] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 18. | Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, Asaka R, Hamanaka W, Ninomiya H, Uehara H. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 1047] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 19. | Sasaki T, Rodig SJ, Chirieac LR, Jänne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010;46:1773-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 429] [Article Influence: 26.8] [Reference Citation Analysis (0)] |