Peer-review started: September 29, 2023
First decision: October 9, 2023
Revised: December 12, 2023
Accepted: December 27, 2023
Article in press: December 27, 2023
Published online: January 26, 2024
Processing time: 117 Days and 21.7 Hours
Pulmonary embolism (PE) requires a high degree of clinical suspicion for its diagnosis and can mimic pneumonia due to its clinical, radiological, and laboratory findings. Co-existence of PE and pneumonia can also occur, which is surprisingly more common than appreciated.
Here, we report a case of a young male who initially presented during the peak of the coronavirus disease 2019 pandemic with features of pneumonia. He was kept under observation and was later diagnosed and treated for a right main pulmo
PE and pneumonia share common clinical, radiological, and laboratory findings that may delay the diagnosis of PE. Hypoxia disproportionate to the extent of radiological involvement could be an indicator of an underlying PE.
Core Tip: Pulmonary embolism can present radiologically as pneumonia with similar clinical symptoms and signs. This case highlights the importance of understanding how a pulmonary infarct can present as pneumonia.
- Citation: Mujeeb Rahman KK, Durgeshwar G, Mohapatra PR, Panigrahi MK, Mahanty S. Pulmonary infarct masquerading as community-acquired pneumonia in the COVID-19 scenario: A case report. World J Respirol 2024; 13(1): 1-6
- URL: https://www.wjgnet.com/2218-6255/full/v13/i1/1.htm
- DOI: https://dx.doi.org/10.5320/wjr.v13.i1.1
Pulmonary embolism (PE) mimics pneumonia because of the considerable overlap in their clinical and radiological picture. Moreover, pneumonia may occasionally mask PE, particularly in patients with predominant systemic symptoms such as fever with no evidence of deep vein thrombosis (DVT) or trauma. Here, we discuss a case of a young male who presented during the peak coronavirus disease 2019 (COVID-19) pandemic with initial signs and symptoms of community-acquired pneumonia and, on further evaluation due to clinical worsening, was found to have extensive PE.
A 26-year-old healthy male presented with fever, cough, progressive exertional dyspnea and vomiting for 5 d.
Symptoms started as high-grade fever with a temperature recording of 101 ˚F without rigor or chills and cough noted from the next day. The cough was associated with scanty mucoid sputum, with no history of hemoptysis or copious purulent sputum. Dyspnea progressed slowly over 5 d to modified Medical Research Council grade 2. There were no orthopnea, paroxysmal nocturnal dyspnea, or angina symptoms. He also had 2-3 vomiting episodes per day after the fever. Vomiting was nonbilious, and there was no associated abdominal pain, loose stool or blood in the stool. There was no history of any leg pain, calf swelling, or prolonged immobilization.
He had no other comorbidity, i.e. no history of previous recurrent respiratory infections, asthma, or frequent hospitalizations. There was no history of recent travel outside his native place.
He was a nonsmoker and had been engaged in a painting job for the last 2 years.
On examination, the patient was febrile with a temperature of 100 ˚F, tachypneic with a respiratory rate of 36/min, pulse rate 112/min, blood pressure 110/70 mmHg, and oxygen saturation of 85% at room air. Other respiratory, cardio
His hemogram showed a total white blood cell count of 23090 (neutrophils 88%) lac per cubic millimeter and a platelet count of 3.3 lac per cubic millimeter; blood urea and creatinine were 43 mg/dL (normal range: 5-20 mg/dL), and 1.5 mg/dL (normal range: 0.7-1.3 mg/dL), respectively. The liver function test was normal. The electrocardiogram showed sinus tachycardia.
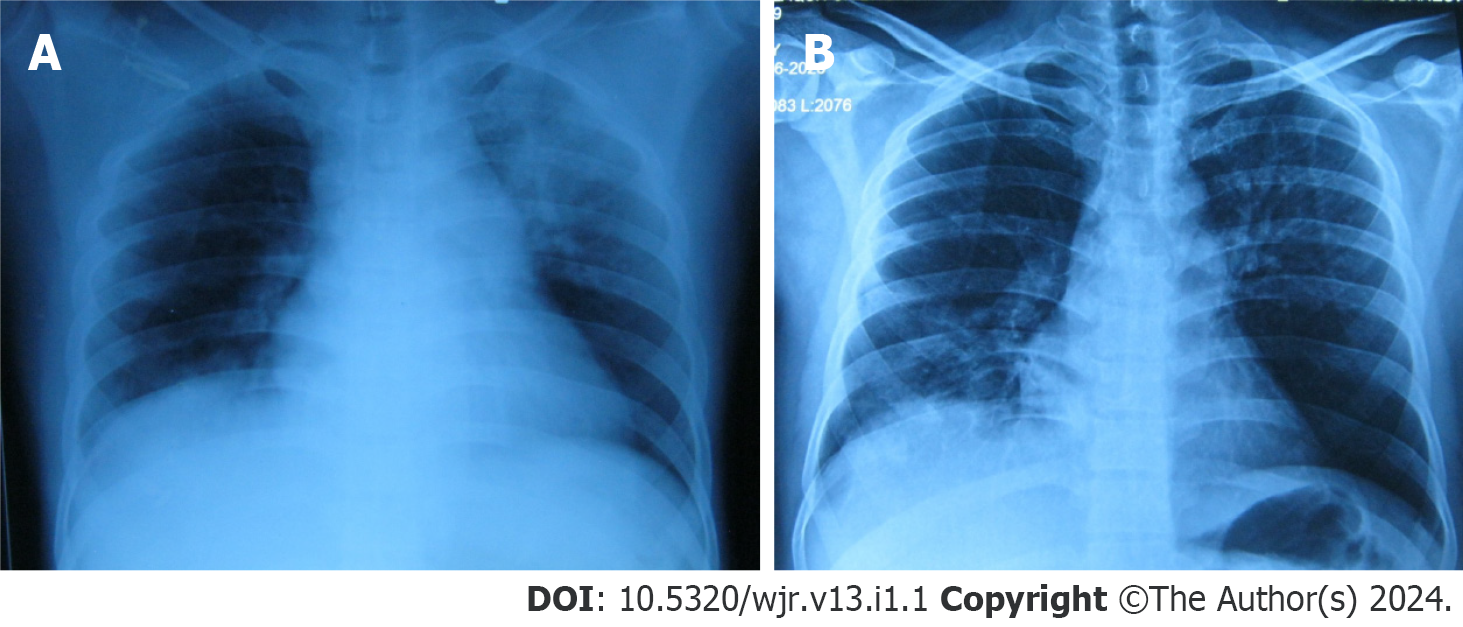
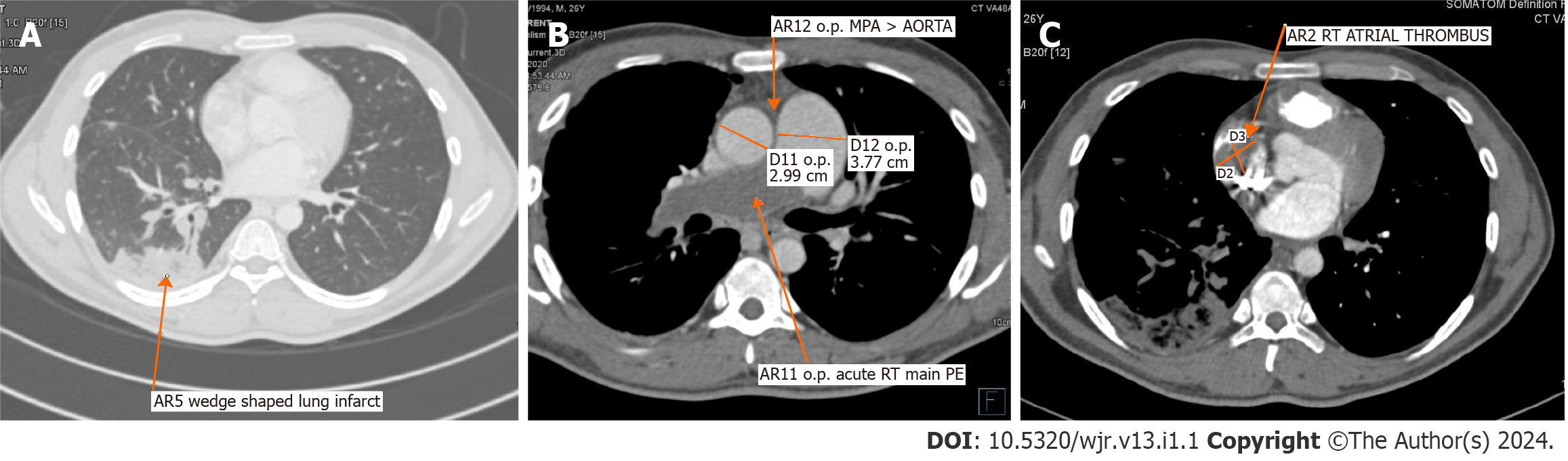
Chest radiograph showed left upper zone inhomogeneous opacity with an air bronchogram and right lower zone opacity obliterating hemi-diaphragm silhouette (Figure 1A). Repeated chest radiographs documented the resolution of the left upper and right lower zone opacities (Figure 1B). Computed tomography (CT)-assisted pulmonary angiography (CTPA) was performed. CTPA revealed a thrombus completely occluding the right main pulmonary artery just after bifurcation and a hypodense filling defect of 2.8 cm × 2 cm in the right atrium, suggestive of thrombus (Figure 2).
PE, hypoxic respiratory failure, shock due to massive PE.
Considering the current pandemic, the patient was initially treated as a COVID-19 suspect, and a nasopharyngeal swab test was performed. Empirically, antibiotics (intravenous Co-amoxiclav and oral azithromycin) were initiated with high-flow oxygen at 10 L/min and enoxaparin 40 mg subcutaneously once daily. His nasopharyngeal swab used for reverse transcription polymerase chain reaction (RT-PCR) for COVID-19 was negative, and he was transferred to the pulmonary ward for further management. Sputum and blood culture were sterile. Sputum for Xpert MTB/Rif was negative. He gradually improved clinically, and by day 4, his fever subsided, leukocytosis decreased, and oxygen requirement reduced to 4 L/min. On the 5th d of admission, he had new onset hemoptysis with minimal quantity. Repeated chest radiographs documented the resolution of the opacities in the left upper and right lower zones (Figure 1B). Repeated electrocardiograms showed sinus tachycardia and T wave inversion from V1 to V3. However, Troponin I was negative.
In view of persisting hypoxia, radiological opacity, and higher Wells score, CTPA was performed. CTPA revealed a thrombus completely occluding the right main pulmonary artery just after bifurcation, with a hypodense filling defect of 2.8 cm × 2 cm in the right atrium, suggestive of a thrombus (Figure 2). Deep vein screening and compression ultrasound were normal. Echocardiography revealed the dilatation of the right atrium and right ventricle with increased right ventricular systolic pressure. A PE was confirmed. As the patient was hemodynamically stable, an injection of Enoxaparin 40 mg was initiated subcutaneously twice daily. The antibiotic was escalated to piperacillin and tazobactam 4.5 gm every six hours and linezolid 600 mg twice daily in view of pulmonary opacity and elevated total white cell count of 21180 cells per cubic millimeter. On the 4th d of anticoagulation, the patient had increasing tachypnea, and oxygen requirement increased from 4 L/min to 10 L/min. Over the next six hours, the patient's dyspnea further increased. Therefore, he was intubated and mechanically ventilated due to severe hypoxia despite a high oxygen flow. Finally, he was transferred to the intensive care unit. The patient was initially hemodynamically stable except for sinus tachycardia. However, after 12 h, the patient developed hypotension, requiring fluid resuscitation and noradrenaline support. Therefore, the patient was immediately considered for thrombolysis after counseling on the benefits and risks of thrombolysis. Due to financial constraints, the family could only afford to give him streptokinase treatment. Injection streptokinase 2.5 Lac IU (international unit) loading dose followed by one lac IU per hour was started. After 3 h of thrombolysis, there was a progressive worsening of hypotension, and the patient developed pulmonary edema and refractory shock and passed away on the 14th d of admission.
Died on 14th d of admission.
In the present case, a young male admitted with a presumptive diagnosis of COVID-19 pneumonia was later diagnosed with PE. We missed the diagnosis of PE initially in the context of rising COVID-19 cases and time elapsed while waiting for RT-PCR results. The initial clinical diagnosis was non-COVID pneumonia due to the presence of fever, elevated total leukocyte count, and initial clinical improvement with antibiotics. There was the possibility of concomitant PE and pneumonia. PE is often misdiagnosed as pneumonia because of similar clinical presentations. Several retrospective studies have already discussed pneumonia patients who have about 2–3-fold increased risk of venous thrombosis[1].
Due to the lower sensitivity of RT-PCR for COVID-19, our clinical suspicion continued due to the prevailing trend of an ever-increasing COVID pandemic[2]. Fever, leukocytosis, elevated C-reactive protein, and procalcitonin, which are commonly used to differentiate PE from pneumonia, often exist in both conditions[3]. In one study, 80% of angiographically proven PE reported leukocytosis, and two-thirds reported fever[4]. Initial radiological workup like chest radiograph may often help in diagnosing pneumonia. Still, data from ICOPER study, including 2000 PE patients, reported normal chest radiographs in one-quarter of the patients, and patchy infiltrations were present in 17% of patients[5]. Radiographic findings of PE have been extensively documented but are frequently nonspecific, i.e. normal findings, focal oligemia, pleural effusion, variable zones of atelectasis, pulmonary consolidations, large hilar vessels, and elevated hemi diaphragm[6]. Airspace consolidation may result from infarction and actual necrosis of the lung tissue but more commonly occurs from "incomplete infarction" and hemorrhage into the air spaces, and the size of infarction can vary from barely perceptible to 10 cm in diameter[7]. Elevated D-dimer with PE probability scoring from the 1st d of treatment may help to make a decision regarding CTPA. But D-dimer can also be elevated in pneumonia and sepsis along with another inflammatory marker. This elevated D-dimer is associated with intensive care unit admission and increased 30-d mortality[8]. ECG changes like right ventricular strain, S1Q3T3 pattern, right bundle branch block, and atrial fibrillation can also be seen in community-acquired pneumonia[9].
In the initial clinical evaluation of this case, there was no sign of DVT, and on the 1st d, Well's score was only 1.5 (low probability for PE). Bedside echocardiography is usually performed and helps to detect early signs of PE like enlarged right ventricular size with reduced functionality, abnormal septal wall movement, and tricuspid regurgitation. Right ventricular thrombi can be visualized in echocardiography, similar to our case, and usually signify a poor prognosis[10]. A timely CT pulmonary angiography helps confirm the diagnosis. Secondly, there are large-scale autopsy studies, including 1455 cases, which demonstrated concomitant presentation of PE and pneumonia, and out of 54 patients identified with anatomically major PE at autopsy, only 30% had correct ante-mortem diagnosis. However, the accuracy was more pronounced in postoperative patients. In 80% of cases in which lung scanning and pulmonary angiography were examined, there was an increased tendency for a correct diagnosis of PE. Among 21 patients with autopsy-proved major PE who also had pneumonia, PE was not diagnosed before death, indicating the importance of this discussion[11]. Both hereditary and acquired conditions can increase the risk of venous thromboembolism (VTE). Published guidelines of various bodies recommend screening for thrombophilia in some selected patients with unprovoked VTE. Various guidelines and committees recommended not testing thrombophilia in provoked VTE. Compared to provoked, unprovoked VTE has an estimated 30% 5-year recurrence risk when the patient is off anticoagulation[12]. Five disorders of anticoagulation system factor: V G1691A "Leiden" mutation (FVL), prothrombin G2021A mutation (PGM), deficiency of natural anticoagulant protein C and protein S (PS), and antithrombin (AT) deficiency have been called the "classic thrombophilias"[13]. In addition to the above-mentioned classic thrombophilia, there are many inherited mutations like homocysteine metabolism, von Willebrand factor, overactivity of factor VIII, abnormal factor IX, XI, and fibrinogen[14]. Performing thrombophilia testing in acute thrombosis and patients already on anticoagulant medication is technically challenging. Loss of function of PS, PC, and AT is influenced by anticoagulation and acute thrombosis[15]. Testing the genotypic abnormality is only available in specific centers and only for a few conditions. In acute VTE situations, due to the possibility of unreliable results, it is best to avoid testing for natural anticoagulation deficiency like AT, PC, and PS. Hereditary thrombophilia testing in large case-controlled registry data has found limited utility in predicting the risk of recurrence of unprovoked VTE[16]. Considering limited data, the 10th American College of Chest Physicians guideline and others recommend extended anticoagulation in all cases unless the risk of bleeding is unacceptably high[17]. In situations where thrombophilia testing is required, a two-step approach is better. First-line testing of FVL, PGM, cardiolipin, and beta–2 glycoprotein-1 antibodies are tested before stopping anticoagulation. If these tests are normal, anticoagulation can be discontinued, and remaining thrombophilia tests like Lupus anticoagulant, PC, PS, and AT are performed. Due to limited evidence recommendation, financial limitations, lack of in-house availability, and COVID-19 pandemic logistic issues, we did not test thrombophilia in this case. As the patient had some clinical and radiological improvement with antibiotics, the PE diagnosis was delayed. Hypoxia that is out of proportion to clinical-radiological assessment is the key to early detection of PE, as in the present case.
PE and pneumonia share common clinical, radiological, and laboratory findings that may delay PE diagnosis. Our case suggests that persisting hypoxia, even after clinical and radiological improvement, even during the peak of the COVID pandemic, and hypoxia disproportionate to the extent of radiological involvement could be an indicator of an underlying PE.
| 1. | Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 330] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | Jamal AJ, Mozafarihashjin M, Coomes E, Powis J, Li AX, Paterson A, Anceva-Sami S, Barati S, Crowl G, Faheem A, Farooqi L, Khan S, Prost K, Poutanen S, Taylor M, Yip L, Zhong XZ, McGeer AJ, Mubareka S; Toronto Invasive Bacterial Diseases Network COVID-19 Investigators. Sensitivity of Nasopharyngeal Swabs and Saliva for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2. Clin Infect Dis. 2021;72:1064-1066. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 3. | Müller B, Harbarth S, Stolz D, Bingisser R, Mueller C, Leuppi J, Nusbaumer C, Tamm M, Christ-Crain M. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis. 2007;7:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 265] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Murray HW, Ellis GC, Blumenthal DS, Sos TA. Fever and pulmonary thromboembolism. Am J Med. 1979;67:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Elliott CG, Goldhaber SZ, Visani L, DeRosa M. Chest radiographs in acute pulmonary embolism. Results from the International Cooperative Pulmonary Embolism Registry. Chest. 2000;118:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Jacoby CG, Mindell HJ. Lobar consolidation in pulmonary embolism. Radiology. 1976;118:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Kearon C, Hirsh J. The diagnosis of pulmonary embolism. Haemostasis. 1995;25:72-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Ge YL, Liu CH, Wang N, Xu J, Zhu XY, Su CS, Li HL, Zhang HF, Li ZZ, Zhang X, Chen H, Yu HL, Fu AS, Wang HY. Elevated Plasma D-Dimer in Adult Community-Acquired Pneumonia Patients is Associated with an Increased Inflammatory Reaction and Lower Survival. Clin Lab. 2019;65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Stein PD, Matta F, Ekkah M, Saleh T, Janjua M, Patel YR, Khadra H. Electrocardiogram in pneumonia. Am J Cardiol. 2012;110:1836-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Payus AO, Rajah R, Febriany DC, Mustafa N. Pulmonary Embolism Masquerading as Severe Pneumonia: A Case Report. Open Access Maced J Med Sci. 2019;7:396-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 11. | Goldhaber SZ, Hennekens CH, Evans DA, Newton EC, Godleski JJ. Factors associated with correct antemortem diagnosis of major pulmonary embolism. Am J Med. 1982;73:822-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 188] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Boutitie F, Pinede L, Schulman S, Agnelli G, Raskob G, Julian J, Hirsh J, Kearon C. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants' data from seven trials. BMJ. 2011;342:d3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 13. | Mannucci PM, Franchini M. Classic thrombophilic gene variants. Thromb Haemost. 2015;114:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Simone B, De Stefano V, Leoncini E, Zacho J, Martinelli I, Emmerich J, Rossi E, Folsom AR, Almawi WY, Scarabin PY, den Heijer M, Cushman M, Penco S, Vaya A, Angchaisuksiri P, Okumus G, Gemmati D, Cima S, Akar N, Oguzulgen KI, Ducros V, Lichy C, Fernandez-Miranda C, Szczeklik A, Nieto JA, Torres JD, Le Cam-Duchez V, Ivanov P, Cantu-Brito C, Shmeleva VM, Stegnar M, Ogunyemi D, Eid SS, Nicolotti N, De Feo E, Ricciardi W, Boccia S. Risk of venous thromboembolism associated with single and combined effects of Factor V Leiden, Prothrombin 20210A and Methylenetethraydrofolate reductase C677T: a meta-analysis involving over 11000 cases and 21000 controls. Eur J Epidemiol. 2013;28:621-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Sundquist K, Wang X, Svensson PJ, Sundquist J, Hedelius A, Larsson Lönn S, Zöller B, Memon AA. Plasminogen activator inhibitor-1 4G/5G polymorphism, factor V Leiden, prothrombin mutations and the risk of VTE recurrence. Thromb Haemost. 2015;114:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3522] [Article Influence: 352.2] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: ERS-European Respiratory Society, No. 360284; Indian Chest Society.
Specialty type: Respiratory system
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Pan L, China; Yamaguchi K, Japan S-Editor: Liu JH L-Editor: Filipodia P-Editor: Zhao S