Published online Aug 8, 2021. doi: 10.5320/wjr.v11.i1.12
Peer-review started: March 7, 2021
First decision: May 6, 2021
Revised: May 17, 2021
Accepted: July 14, 2021
Article in press: July 14, 2021
Published online: August 8, 2021
Processing time: 149 Days and 14.6 Hours
Venous thromboembolism, which includes deep venous thrombosis and pulmonary embolism, is a well-known causal disorder with high morbidity and mortality rates. Inherited or acquired conditions affecting components of coagulation and fibrinolysis systems have been linked to venous thromboembolism pathogenesis as they may lead to a pro-inflammatory state in human bodies. Toxoplasmosis is a zoonosis that potentially leads to acute systemic cachectic-inflammatory effects in experimental animal models but is not yet proven in humans. It is known that venous thrombosis can occur during acute inflammatory/infectious diseases, although it is not well established with regard to toxoplasmosis alone.
A 70-year-old Caucasian man and his 32-year-old son developed general malaise, chills, fever, and myalgia, having established a diagnosis of toxoplasmosis. Twenty days later, they presented dry cough leading to further investigations that revealed an incidental deep venous thrombosis plus pulmonary embolism in them both. Thrombophilia screening showed both patients had a factor V Leiden mutation heterozygosis. Father and son completely recovered without any sequalae after anticoagulant treatment. They have not presented symptom recurrence of either medical disorder during 1 year of follow-up.
Toxoplasmosis may enhance the risk of venous thromboembolism in patients showing factor V Leiden mutation heterozygosis.
Core Tip: This paper describes two closely related patients simultaneously infected by Toxoplasma gondii who concurrently presented their first venous thromboembolism episode. Further investigation revealed a factor V Leiden mutation in them both. The association between these two morbid conditions suggests that systemic infection/ inflammation may enhance the risk of venous thromboembolism in people carrying this kind of pro-thrombophilia mutation. As far as we know, this is the first report of that association. We believe that this paper provides an insight into this important issue bringing potential help for clinical decision-making processes by doctors around the world.
- Citation: Hannun P, Hannun W, Yoo HH, Resende L. Like father, like son: Pulmonary thromboembolism due to inflammatory or hereditary condition? Two case reports. World J Respirol 2021; 11(1): 12-17
- URL: https://www.wjgnet.com/2218-6255/full/v11/i1/12.htm
- DOI: https://dx.doi.org/10.5320/wjr.v11.i1.12
Inflammation and coagulation are intrinsically related processes that communicate at various levels; either can be the cause or consequence of the other[1,2]. The mechanism that links inflammation (originally infectious or not) and thrombosis is complex and not fully understood. The most commonly accepted hypothesis states that aseptic inflammations or infection-related inflammations promote the secretion of several cellular mediators, mostly related to the innate immune response. Inflammation activates endothelial cells, leukocytes, and platelets, resulting in cell dysfunction and/or injury and platelet aggregation[2]. Concomitantly, an infection can result in antiphospholipid antibodies that trigger the overproduction of tissue factor and thromboxane A2[3]. All together this could initiate the blood clotting cascade[1,2].
Toxoplasmosis, a common zoonosis caused by the Toxoplasma gondii protozoan, is a systemic infection that may cause cachectic-inflammatory effects in experimental animal models[4] and even the development of antiphospholipid syndrome in humans[3,5]. Hence, toxoplasmosis by itself might eventually provoke a venous thromboembolism (VTE) event in a given patient.
In addition to having toxoplasmosis, our studied patients carried factor V Leiden (FVL) mutation heterozygosis, a well-known cause of thrombophilia. The combination of these two morbid conditions might have increased the risk of VTE in both patients.
A 70-year-old Caucasian man and his 32-year-old son had developed general malaise, chills, fever, and myalgia. Dry cough appeared approximately 20 d after initial symptoms in both patients.
At the beginning of 2019, a 70-year-old Caucasian man and his 32-year-old son had lunch together at a steakhouse in São Paulo City, Brazil. After 5 d, the older man developed general malaise, chills, fever, and myalgia; he also noticed enlarged cervical and occipital lymph nodes. One week later, he took a 10 h flight to the United States remaining abroad for 10 d without any clinical improvement. During this time, his son who had stayed in Brazil, started having similar symptoms. About 20 d later they both developed a persistent dry cough. One month after initial symptoms, they were hospitalized after being radiologically diagnosed with pulmonary thromboembolism.
The older man had a prostatic carcinoma in situ operated 5 years earlier; he was also a former smoker.
The younger had only a right shoulder capsuloplasty 9 years earlier; he was a non-smoker.
The older man had current controlled hypertension and type II diabetes. There was no history of family disorders.
The relevant findings were enlarged cervical and occipital lymph nodes in both patients and muscular stiffness in the right calf of the younger man.
There were no respiratory signs or low oxygen saturation levels on room air.
The following laboratory tests were normal in both patients: Blood cell count, prothrombin time, activated partial thromboplastin time, four hereditary alterations for thrombophilia (protein C, protein S, and antithrombin serum levels; G20210A prothrombin gene mutation search), and three acquired alterations for thrombophilia (anticardiolipin antibodies, anti-beta- 2-glycoprotein I antibodies, and lupus anticoagulant).
Tests with abnormal results in both patients were (Table 1): Presence of high serum C-reactive protein, presence of positive IgM antibodies for Toxoplasma gondii, and presence of FVL mutation heterozygous status (gene F5, c.691G>A, p.R506Q).
| Laboratory tests and imaging studies | Father (older patient) | Son (younger patient) | Normal values and conditions |
| C-reactive protein (mg/L) | 4.6 | 44.8 | < 1.2 |
| Toxoplasma gondii IgM antibodies (UI/mL) | 19.8 | 30.8 | < 0.5 |
| FVL mutation status (gene F5, c.691G>A, p.R506Q) | Heterozygosis | Heterozygosis | Unmutated |
| D-dimer (ng/mL) | 5.724 | Not performed | < 500 |
| Lower limbs Doppler ultrasound | Absent blood flow in anterior tibial, posterior tibial, and fibular veins in the left leg | Absent blood flow in anterior tibial, posterior tibial, fibular, and popliteal veins in the right leg | Presence of blood flow in the veins |
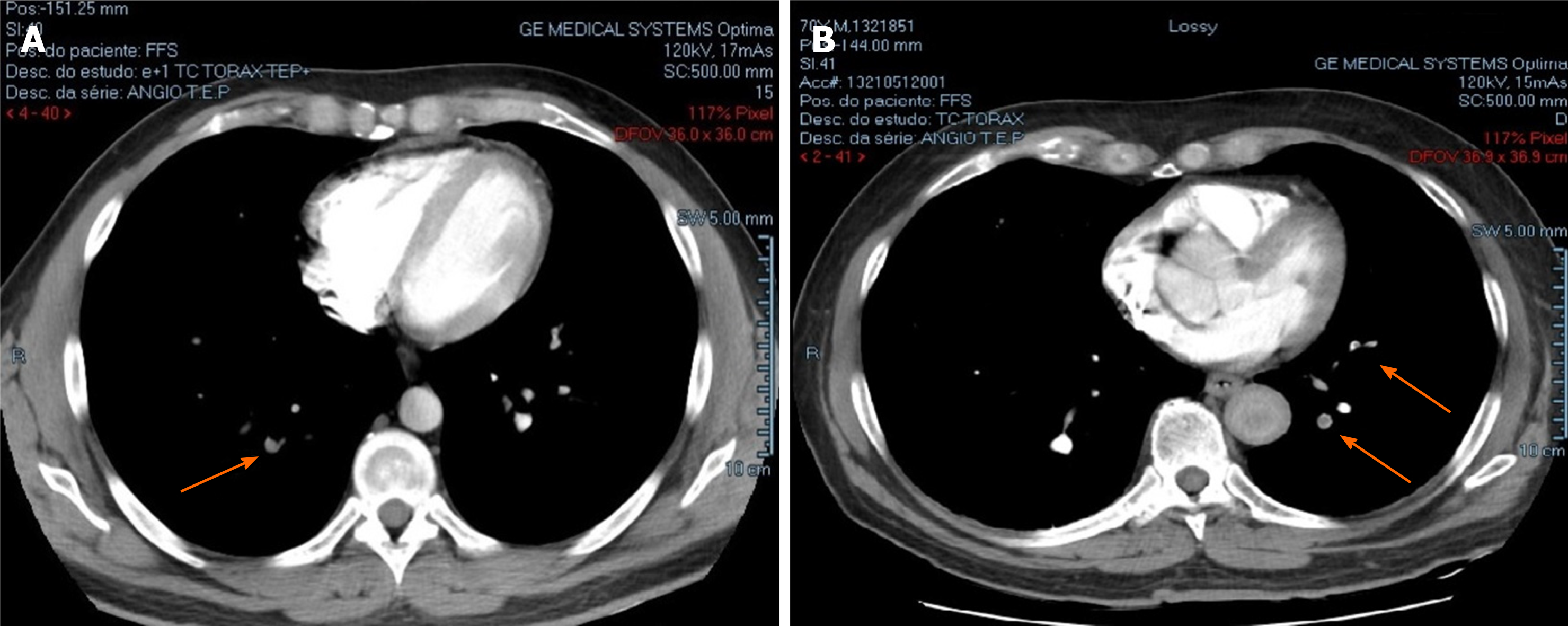
| Chest CT scan | Filling defects in segmental and subsegmental arterial branches in upper and lower lobes of both lungs (Figure 1A) | Filling defects in segmental and subsegmental arterial branches in lower lobes of both lungs and in apical subsegmental artery of right lung(Figure 1B) | Absence of filling defects in the arteries |
D-dimers were only analyzed in the older patient with a high result.
Imaging studies are shown in Table 1.
In the father, pulmonary computed tomography angiography showed bilateral pulmonary embolism (Figure 1A), and a further Doppler ultrasound (US) found deep venous thrombosis (DVT) in the left lower limb (anterior tibial, posterior tibial, and fibular veins). In the son, pulmonary computed tomography angiography and Doppler US respectively showed a bilateral pulmonary embolism (Figure 1B) and DVT in the right lower limb (anterior tibial, posterior tibial, fibular, and popliteal veins).
The final diagnosis of both patients was identical, corresponding to pulmonary VTE associated with acute toxoplasmosis plus FVL heterozygous mutation status.
Although clinically stable, both patients were admitted to the hospital to start anticoagulant therapy with enoxaparin (60 mg, subcutaneously, twice a day for 4 d) followed by rivaroxaban (15 mg, orally, twice a day) according to local standards. They were discharged completely free of symptoms, under oral anticoagulation for 1 year.
Regarding toxoplasmosis, treatment of immunocompetent adults with lymphadenopathic toxoplasmosis is rarely indicated due to its usually self-limited evolution[6]. The only prescription being bed rest.
Father and son have totally recovered without sequelae. After 1 year of follow-up, patients have not presented a recurrence of either morbid condition.
Toxoplasmosis is a usually asymptomatic zoonosis with wide geographical distribution. According to the São Paulo Sanitary Surveillance Agency, at the beginning of 2019 there was an increased number of acute toxoplasmosis cases in São Paulo City, including asymptomatic cases only detected by positive serological tests due to a foodborne disease outbreak. Of the 165 notified cases, only 9% had severe complications, none being DVT or pulmonary embolism[7].
The relationship between toxoplasmosis and venous thrombosis is uncertain. There is evidence of direct[8,9] and indirect[3,5] toxoplasmosis effects that may lead to thrombotic events. Toxoplasmosis was described as resulting in cerebral vein thrombosis in severe cases of its congenital form in humans[8]. Placental thrombosis was also seen in abortions during experimental Toxoplasma gondii infection in sheep[9]. However, indirect toxoplasmosis effects have been more consistently reported in literature, especially human Toxoplasma-induced antiphospholipid syndrome[3,5]. However, our studied cases did not show positivity for any antibodies related to antiphospholipid syndrome.
Being a systemic infectious disease, the potential for toxoplasmosis to trigger a venous thrombosis via an inflammatory pathway cannot be neglected[1,2], especially in patients with FVL mutation heterozygous status, the most common type of inherent thrombophilia[10]. In association with a pre-existing prothrombotic state, the inflammatory and pro-hemostatic environment caused by the infection may have caused endothelial dysfunction and changes in coagulation homeostasis resulting in VTE[2]. Considering a scenario without direct endothelial damage, it is also possible that the inflammation itself had increased the genetic expression of tissue factor (factor III), thus initiating the extrinsic coagulation pathway[2]. These two hypothetical situations would favor activation of mutated factor V, therefore amplifying thrombus formation given the mutated factor V resistance to activated protein C[2].
Some experimental and clinical studies corroborate the interaction between congenital thrombophilia and systemic infections/sepsis leading to exacerbation of thrombotic activity[11-13]. Although they differ regarding the impact of the thrombophilia gene mutation heterozygosis on survival of infected individuals, most agree that there is a significant increase in thrombin generation markers and in the conversion of fibrinogen to fibrin, both of which represent more frequent and severe episodes of thrombosis[11-13].
Regarding other predisposing factors for DVT, only the older of the two patients had a long period of immobility during a flight concurrently with toxoplasmosis. It is also important to highlight that both patients had undergone previous surgeries with no venous thrombosis.
Considering father and son had been living with the FVL heterozygous mutation throughout their lives without any thrombotic events, it does not seem a simple coincidence that they both simultaneously had VTE with concurrent toxoplasmosis.
This paper reports the unusual clinical cases of two closely related patients carrying the hereditary thrombophilia FVL heterozygous mutation who have concomitantly experienced the so far only VTE episode during concurrent Toxoplasma gondii acute infection. Their sharing of these three events does not seem to be mere coincidence and suggests a potential synergistic effect between toxoplasmosis and heterozygous mutation state for FVL as triggers of the thromboembolic events.
| 1. | Perez-Pujol S, Aras O, Escolar G. Factor v leiden and inflammation. Thrombosis. 2012;2012:594986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Aksu K, Donmez A, Keser G. Inflammation-induced thrombosis: mechanisms, disease associations and management. Curr Pharm Des. 2012;18:1478-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Sène D, Piette JC, Cacoub P. Antiphospholipid antibodies, antiphospholipid syndrome and infections. Autoimmun Rev. 2008;7:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Hatter JA, Kouche YM, Melchor SJ, Ng K, Bouley DM, Boothroyd JC, Ewald SE. Toxoplasma gondii infection triggers chronic cachexia and sustained commensal dysbiosis in mice. PLoS One. 2018;13:e0204895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 5. | Shapira Y, Agmon-Levin N, Selmi C, Petríková J, Barzilai O, Ram M, Bizzaro N, Valentini G, Matucci-Cerinic M, Anaya JM, Katz BS, Shoenfeld Y. Prevalence of anti-Toxoplasma antibodies in patients with autoimmune diseases. J Autoimmun. 2012;39:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Montoya J, Boothroyd J, Kovacs J. Toxoplasma gondii. In: Mandell G, Bennett J, Dolin R, Eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: Churchill Livingstone Elsevier, 2017: 3122-3153. |
| 7. | Coordenadoria de Vigilância em Saúde de São Paulo. Toxoplasmose: Boletim epidemiológico, São Paulo, n 1, 2019. [cited 2021 Mar 7]. Available from: https://www.prefeitura.sp.gov.br/cidade/secretarias/upload/saude/boletim_toxoplasmose_2019.pdf. |
| 8. | Wiebe B, Fremerey C, Ehlen M. 539 Severe form of Congenital Toxoplasmosis with Extensive Cerebral findings. Arch Dis Childhood. 2012;97:A156-A7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Castaño P, Fuertes M, Ferre I, Fernández M, Ferreras Mdel C, Moreno-Gonzalo J, González-Lanza C, Katzer F, Regidor-Cerrillo J, Ortega-Mora LM, Pérez V, Benavides J. Placental thrombosis in acute phase abortions during experimental Toxoplasma gondii infection in sheep. Vet Res. 2014;45:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Campello E, Spiezia L, Simioni P. Diagnosis and management of factor V Leiden. Expert Rev Hematol. 2016;9:1139-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Kondaveeti S, Hibberd ML, Booy R, Nadel S, Levin M. Effect of the Factor V Leiden mutation on the severity of meningococcal disease. Pediatr Infect Dis J. 1999;18:893-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Kerlin BA, Yan SB, Isermann BH, Brandt JT, Sood R, Basson BR, Joyce DE, Weiler H, Dhainaut JF. Survival advantage associated with heterozygous factor V Leiden mutation in patients with severe sepsis and in mouse endotoxemia. Blood. 2003;102:3085-3092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Elmas E, Suvajac N, Jilma B, Weiler H, Borggrefe M, Dempfle CE. Factor V Leiden mutation enhances fibrin formation and dissolution in vivo in a human endotoxemia model. Blood. 2010;116:801-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Respiratory system
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Protopapas AA S-Editor: Zhang H L-Editor: Filipodia P-Editor: Li JH