Peer-review started: August 23, 2019
First decision: November 12, 2019
Revised: December 16, 2019
Accepted: December 23, 2019
Article in press: November 12, 2019
Published online: January 8, 2020
Processing time: 126 Days and 7.7 Hours
In the obese patient population, some patients have severe obstructive sleep apnea (OSA) with daytime hypoventilation. Such patients are generally identified on the basis of the presence or absence of daytime hypercapnia, and the condition is called obesity hypoventilation syndrome. However, mechanisms for such daytime hypoventilation remain unclear.
To investigate metabolic syndrome and daytime hypercapnia association based on hypercapnia prevalence in obese OSA patients in a nested case-control study.
Consecutive obese patients (body mass index ≥ 30 kg/m2) who underwent polysomnography due to suspected OSA were included. Among them, patients with severe OSA (apnea hypopnea index ≥ 30/h) were divided into two groups according to the presence or absence of hypercapnia during wakefulness (arterial partial pressure of carbon dioxide ≥ or < 45 Torr, respectively). The characteristics and clinical features of these two groups were compared.
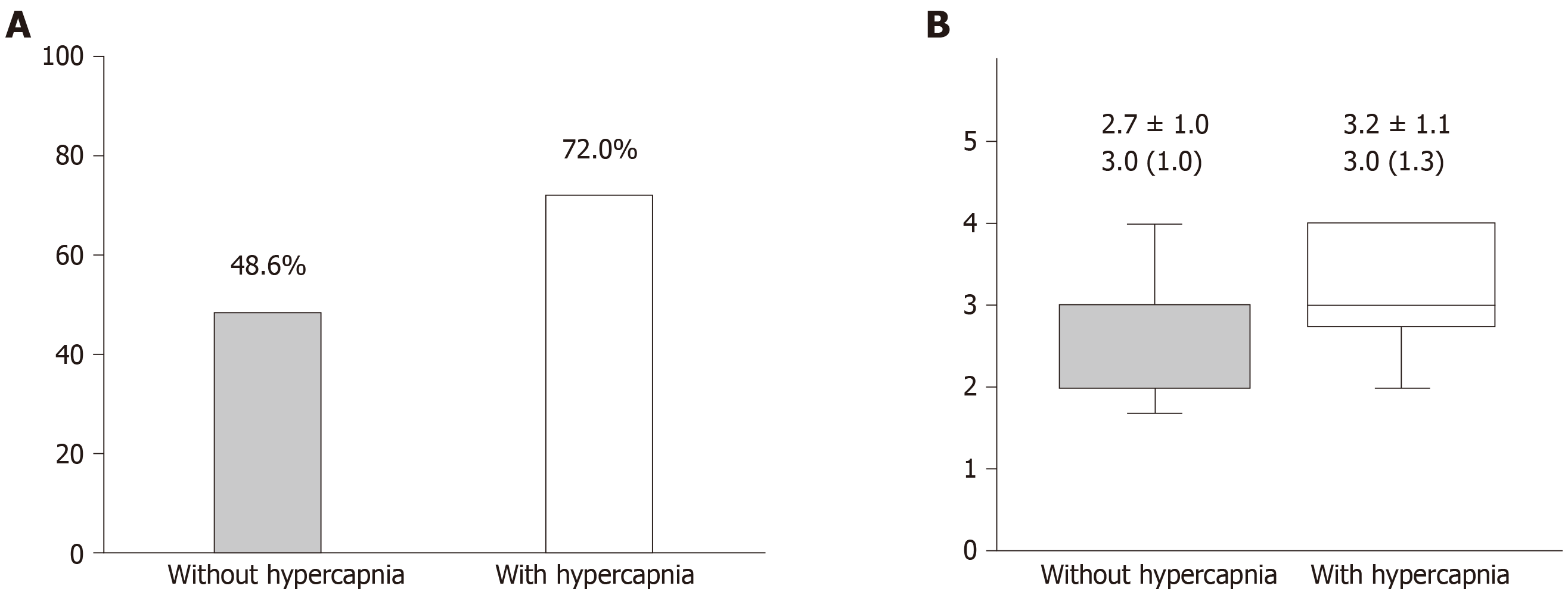
Among 97 eligible patients, 25 patients (25.8%) had daytime hypercapnia. There were no significant differences in age, gender, body mass index, apnea-hypopnea index, and Epworth Sleepiness Scale scores between the two groups. However, patients with hypercapnia had a significantly lower arterial partial pressure of oxygen level (75.8 ± 8.2 torr vs 79.9 ± 8.7 torr, P = 0.042) and higher arterial partial pressure of carbon dioxide level (46.6 ± 2.5 torr vs 41.0 ± 2.9 torr, P < 0.001). Additionally, patients with hypercapnia were more likely to have metabolic syndrome (72.0% vs 48.6%, P = 0.043) and a higher metabolic score (the number of satisfied criteria of metabolic syndrome). In multivariate logistic regression analysis, the presence of metabolic syndrome was associated with the presence of hypercapnia (OR = 2.85, 95%CI: 1.04-7.84, P = 0.042).
Among obese patients with severe OSA, 26% of patients had hypercapnia during wakefulness. The presence of metabolic syndrome was independently correlated with the presence of daytime hypercapnia.
Core tip: Daytime hypercapnia presents with severe obstructive sleep apnea in some obese patients, and the condition is called obesity hypoventilation syndrome. However, the prevalence, characteristics, and other clinical features of hypercapnic obese patients with severe obstructive sleep apnea remain unelucidated. Among 97 obese patients with severe obstructive sleep apnea, 25 had daytime hypercapnia, and they were more likely to have the metabolic syndrome. In multivariable analysis, metabolic syndrome was associated with daytime hypercapnia. Although the cause or consequence remains unclear, coexisting metabolic syndrome may play some roles; thus, clinicians should check for metabolic syndrome in obese patients with severe obstructive sleep apnea.
- Citation: Kimura Y, Kasai T, Tomita Y, Kasagi S, Takaya H, Kato M, Kawana F, Narui K. Relationship between metabolic syndrome and hypercapnia among obese patients with sleep apnea. World J Respirol 2020; 10(1): 1-10
- URL: https://www.wjgnet.com/2218-6255/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.5320/wjr.v10.i1.1
Obesity is a growing public health concern, not only in the Western countries but also in the Eastern countries, along with the related comorbid conditions, which lead to an impairment of the patients’ quality of life and an increase in mortality[1]. One such comorbid condition is obstructive sleep apnea (OSA); it is caused by repetitive collapse of the pharynx during sleep and owing to a narrow pharynx due to fat accumulation in the neck, which compromises the pharyngeal lumen[2]. Approximately 10%–20% of obese patients with severe OSA have daytime hypoventilation manifested as daytime hypercapnia, which is generally defined as an arterial atrial pressure of carbon dioxide (PaCO2) ≥ 45 Torr[3], and the combination of such daytime hypercapnia and obesity [i.e., body mass index (BMI) ≥ 30 kg/m2] in patients with OSA is called obesity hypoventilation syndrome (OHS)[4]. It has been reported that patients who are diagnosed with OHS consume more healthcare resources than do eucapnic obese patients with OSA[5], and that patients with OHS are associated with increased morbidity and mortality compared with eucapnic controls because of the cardiometabolic consequences related to OHS[6]. Thus, OHS is distinct from OSA or simple obesity[7]. It is not known why only some obese patients with OSA develop daytime hypoventilation. The presence of excess adipose tissue in the abdomen (i.e., abdominal obesity) and surrounding chest wall may be one cause of hypoventilation; it possibly causes this condition through impeding diaphragm motion and reduced lung compliance[8].
Abdominal obesity plays a central role in metabolic syndrome (MetS), which represents an accumulation of cardiovascular risk factors including high blood pressure, impaired glucose tolerance, and dyslipidemia, and abdominal obesity as an essential condition[9-11]. MetS is also known to be associated with an increased incidence of diabetes and cardiovascular disease[12-17]. Previous studies have suggested that an independent relationship exists between OSA and MetS[18]. If abdominal obesity and/or visceral adiposity play a role in daytime hypoventilation, there may be numerous patients with coexisting MetS and severe OSA with daytime hypercapnia, and the presence of MetS may be associated with daytime hypoventilation. However, data pertaining to the relationship between MetS and daytime hypercapnia in obese patients with severe OSA is absent. The aims of the present study were to investigate the prevalence of daytime hypercapnia in obese patients with severe OSA, to compare obese patients with severe OSA with and without daytime hypercapnia, and to determine whether a relationship exists between MetS and daytime hypercapnia. We hypothesized that obese patients with severe OSA and hypercapnia are more likely to have MetS compared with those without daytime hypercapnia, and that a presence of MetS is associated with daytime hypercapnia.
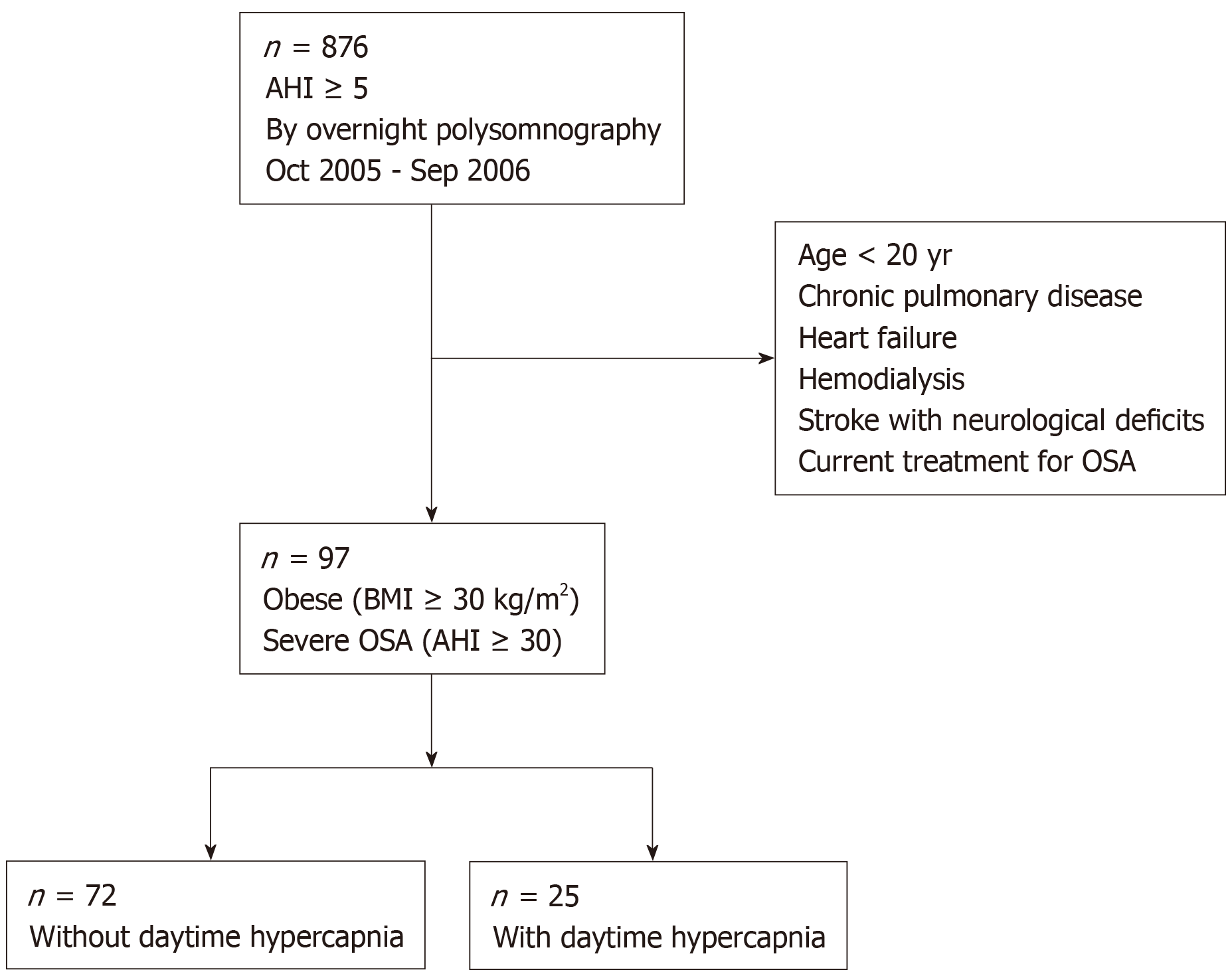
Consecutive obese (body mass index, BMI ≥ 30 kg/m2) Japanese patients who underwent overnight polysomnography between October 2005 and September 2006 at our sleep center (i.e., Toranomon Hospital) due to suspected OSA were targeted. Among them, data of eligible patients with severe OSA (apnea-hypopnea index, AHI ≥ 30 events per hour of sleep) were analyzed. Exclusion criteria were as follows: (1) Age < 20 years; (2) Having chronic pulmonary disease (obstructive and/or restrictive); (3) History of heart failure; (4) History of hemodialysis; (5) History of stroke with neurological deficits; and (6) Undergoing current treatment for OSA.
This nested case-control study was carried out in accordance with the Declaration of Helsinki, and was approved by the Toranomon Hospital Ethics Board. Since in the present study, sleep study, anthropometric data collection, blood sampling including arterial blood gas analysis, which had already been obtained as routine clinical check-up were analyzed, the requirement to obtain informed consent was waived by Toranomon Hospital Ethics Board using opt-out methods.
All subjects underwent full-attended in-hospital overnight polysomnography, using a digital polygraph (SomnoStar® Sleep System; SensorMedics Corp., Yorba Linda, CA) equipped with electroencephalograms, electrooculograms, submental electromyogram, chest and abdominal movement recording, body position monitoring, oronasal airflow monitoring, and oxyhemoglobin saturation (SO2) recording. Generally accepted definitions and scoring methods for respiratory events, sleep stages, and arousals were used[19]. Apneas and hypopneas were classified into obstructive and central[19,20]. The AHI was calculated as the number of apneas and hypopneas per hour of sleep. Subjects with AHI ≥ 5 events per hour of sleep and obstructive AHI ≥ 50% of the total AHI were defined as having OSA. All sleep stages, arousals, and respiratory events were manually scored and interpreted by experienced sleep specialists blinded to information of the subjects.
MetS was diagnosed according to the Japanese definition[10]. Briefly, central obesity was defined as waist circumference ≥ 85 cm for males and ≥ 90 cm for females, high triglycerides was defined as serum triglycerides ≥ 150 mg/dL), low high-density lipoprotein (HDL) cholesterol was defined as serum HDL cholesterol < 40 mg/dL, high blood pressure was defined as systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg, and high fasting blood sugar was defined as serum blood sugar ≥ 110 mg/dL. In subjects who were on specific medications for the abovementioned components, each component was counted as positive for the diagnosis. Subjects with at least two components in addition to central obesity were defined as having MetS. Metabolic score was defined as the number of each component of the MetS criteria.
Fasting venous blood samples were obtained after an overnight fast, in the early morning and at the time of the sleep study. The glycated hemoglobin (HbA1c) value was estimated according to the National Glycohemoglobin Standardization Program equivalent value[21]. Arterial blood for blood gas analysis was obtained under room air conditions just before starting the sleep study with the subjects in the supine position, maintained for 20 min before sampling. Arterial partial pressure of carbon dioxide (PaCO2) ≥ 45 Torr was regarded as daytime hypercapnia, and all eligible patients were divided into two groups according to the presence or absence of hypercapnia during wakefulness (i.e., PaCO2 ≥ 45 Torr as cases or PaCO2 < 45 Torr as controls). Blood pressure (BP) was measured by trained nurses with the patient in a sitting position, in the early morning following the sleep study. The Epworth Sleepiness Scale (ESS) score was computed to assess subjective sleepiness[22]. All medications used by the subjects at the time of sleep study were recorded.
The null hypothesis for this study was that the proportions of MetS in patients with and without daytime hypercapnia were equal among obese patients with severe OSA. Because there are no previous data regarding proportions of MetS in patients with and without daytime hypercapnia, a medium effects size (i.e., 0.3) was applied. Assuming a 2-tailed Type I error of 0.05, we required a total sample size of 88 with a power of 0.8.
Continuous variables are expressed as mean ± SD or median (interquartile range), and were compared between patients with and without daytime hypercapnia using a Student’s t-test or Mann-Whitney U-test. Categorical variables are shown as numbers and percentages and were compared using χ2 or Fisher’s exact test. Associations between the presence of hypercapnia and subjects’ characteristics, features, and sleep study results were analyzed using univariable logistic regression analyses including the presence of hypercapnia as a dependent variable and age, gender, BMI, total cholesterol, HbA1c, uric acid, C-reactive protein, ESS score, awake PaO2, sleep study results (total sleep time, total, obstructive, and central AHI, the percent of time spent SO2 < 90%, lowest SO2, arousal index, sleep stages), metabolic score, and MetS as independent variables. Then, variables with P < 0.10 in univariable analysis were entered into two separate multivariate logistic regression models (P < 0.10 for inclusion and P > 0.05 for exclusion). One included MetS as one of the independent variables and the other included metabolic score as one of the independent variables with the presence of hypercapnia as a dependent variable. A P value of < 0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS version 23.0 (IBM Corp., Tokyo, Japan).
Figure 1 shows a flow chart of the patient recruitment process. During the study period, 876 patients were diagnosed with OSA (i.e., AHI ≥ 5 events per hour of sleep) with overnight polysomnography. Among them, 97 patients were eligible for the present study and their data were analyzed.
Out of 97 patients, 25 patients (25.8%) had daytime hypercapnia. Table 1 lists the characteristics of the patients. Overall, middle aged men with an abdominal obesity were enrolled in this study. We found that apart from PaO2 and PaCO2, the characteristics were similar between the two groups. In patients with daytime hypercapnia, PaO2 was significantly lower compared with those without daytime hypercapnia, in addition to the significantly higher PaCO2 by definition.
| OSA without hypercapnia (n = 72) | OSA with hypercapnia (n = 25) | P value | |
| Age, yr | 44.6 ± 11.2 | 45.6 ± 12.3 | 0.731 |
| Men, n (%) | 67 (93) | 24 (96) | 0.960 |
| BMI, kg/m2 | 33.8 ± 4.1 | 33.6 ± 3.4 | 0.790 |
| Waist circumference, cm | 109.3 ± 10.4 | 110.7 ± 11.6 | 0.567 |
| Systolic blood pressure, mmHg | 118.6 ± 35.5 | 129.3 ± 30.6 | 0.183 |
| Diastolic blood pressure, mmHg | 71.5 ± 22.1 | 76.6 ± 18.9 | 0.313 |
| Total cholesterol, mg/dL | 195.6 ± 39.9 | 202.2 ± 25.3 | 0.439 |
| Triglycerides, mg/dL | 150.0 (94.0) | 164.0 (93.0) | 0.351 |
| HDL cholesterol, mg/dL | 43.7 ± 7.8 | 41.9 ± 9.1 | 0.360 |
| Fasting blood glucose, mg/dL | 110.3 ± 29.8 | 114.6 ± 38.5 | 0.567 |
| HbA1c % | 6.4 ± 1.4 | 6.3 ± 1.2 | 0.923 |
| Uric acid, mg/dL | 5.7 ± 3.0 | 6.7 ± 3.4 | 0.173 |
| CRP, mg/dL | 0.1 (0.2) | 0.2 (0.3) | 0.275 |
| Epworth Sleepiness Scale | 11.0 (8.0) | 10.5 (9) | 0.862 |
| Awake PaO2, Torr | 79.9 ± 8.7 | 75.8 ± 8.2 | 0.042 |
| Awake PaCO2, Torr | 41.0 ± 2.9 | 46.6 ± 2.5 | <0.001 |
| Medications, n (%) | |||
| Antihypertensive | 18 (25) | 8 (32) | 0.675 |
| Antidyslipidemic | 1 (1) | 2 (8) | 0.162 |
| Antidiabetic | 4 (6) | 1 (4) | 0.999 |
| Antihyperuricemic | 5 (7) | 1 (4) | 0.999 |
The sleep study results are summarized in Table 2. Interestingly, there were no significant differences in the frequency and type of apnea and hypopnea, hypoxia parameters during sleep, and the sleep stages between the two groups. However, the prevalence of MetS and the metabolic scores were greater in patients with daytime hypercapnia than in patients without it (Figure 2).
| OSA without hypercapnia (n = 72) | OSA with hypercapnia (n = 25) | P value | |
| TST, min | 333.5 ± 82.7 | 330.6 ± 69.7 | 0.876 |
| Total AHI, events/h | 60.8 ± 20.5 | 65.6 ± 26.1 | 0.353 |
| Obstructive | 58.0 ± 21.9 | 64.8 ± 26.6 | 0.206 |
| Central | 0.3 (0.9) | 0.3 (0.6) | 0.815 |
| Time spent SO2< 90%/TST, % | 46.1 (54.7) | 56.9 (60.0) | 0.128 |
| Lowest SO2, % | 69.5 ± 9.2 | 68.8 ± 7.8 | 0.764 |
| Arousal index, events/h | 55.2 ± 24.1 | 59.1 ± 25.1 | 0.491 |
| Sleep stages, %TST | |||
| N1 | 40.5 ± 16.7 | 45.2 ± 18.7 | 0.245 |
| N2 | 44.8 ± 13.2 | 39.4 ± 15.1 | 0.094 |
| N3 | 2.3 (9.0) | 6.1 (9.7) | 0.817 |
| REM | 9.1 (6.2) | 10.8 (5.7) | 0.494 |
In univariable analysis, only the lower awake PaO2 levels, presence of MetS, and the metabolic scores were significantly associated with presence of daytime hypercapnia (Table 3). Thus, two separate multivariable logistic regression models, one which included awake PaO2 and MetS and another which included awake PaO2 and metabolic scores, were created (Table 3). In the multivariable models, the presence of MetS and the increased metabolic scores were associated with the presence of daytime hypercapnia (OR = 2.85; P = 0.042, and OR = 1.66; P = 0.048, respectively) in obese patients with severe OSA.
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Awake PaO2 (1 Torr increase) | 0.94 (0.89-0.99) | 0.047 | 0.94 (0.89-0.99) | 0.043 |
| Metabolic syndrome | 2.72 (1.01-7.30) | 0.047 | 2.85 (1.04-7.84) | 0.042 |
| Awake PaO2 (1 Torr increase) | 0.94 (0.89-0.99) | 0.047 | 0.94 (0.89-1.00) | 0.052 |
| Metabolic score (1-point increase) | 1.65 (1.01-2.68) | 0.044 | 1.66 (1.01-2.74) | 0.048 |
The present study provides several insights into the relationship between MetS and daytime hypercapnia in obese patients with severe OSA. Firstly, among obese patients with severe OSA, 25.8% had daytime hypercapnia. Secondly, in patients with daytime hypercapnia, prevalence of MetS and the metabolic scores were greater than in patients without daytime hypercapnia. Furthermore, awake PaO2 was significantly lower in patients with daytime hypercapnia than in those without. In addition, despite no differences in the study results including AHI, PaCO2 was significantly higher. Thirdly, in the multivariable analysis, a significant relationship between MetS and daytime hypercapnia was observed in obese patients with severe OSA. Finally, a significant dose-response relationship was observed between metabolic scores and daytime hypercapnia in obese patients with severe OSA. Therefore, this study is the first to show that the presence of MetS and increased metabolic scores were associated with a greater risk of daytime hypercapnia in these patients. The importance of the present study is that physicians will be able to identify patients at risk for OHS, on noting MetS or high metabolic scores in obese patients with severe OSA, without determining arterial blood gas. Because patients with OHS consume greater levels of healthcare resources[5], and are associated with increased morbidity and mortality compared with eucapnic controls[6], distinguishing patients with and without OHS is highly valuable.
The prevalence of daytime hypercapnia in obese patients with OSA was reported to be 10%–20%[3,23,24]. In Japan, there are two reports outlining the prevalence of OHS; one of these reports showed that daytime hypercapnia was present in 9% of patients with OSA (AHI ≥ 20 events per hour of sleep), and in 32% of patients with obesity and OSA (AHI ≥ 20 events per hour of sleep and BMI ≥ 30 kg/m2)[25]. In another report, Harada and colleagues showed that daytime hypercapnia (PaCO2 ≥ 45 Torr) was present in 2.3% of patients with OSA (AHI ≥ 5 events per hour of sleep) and 12.3% of obese patients with OSA (AHI ≥ 5 events per hour of sleep and BMI ≥ 30 kg/m2)[26]. In the present study, approximately one fourth (25.8%) of the participants had daytime hypercapnia. This prevalence is greater than those found in the previous studies in the Japanese population and may be due to the inclusion of only obese (BMI ≥ 30 kg/m2) and severe OSA (AHI ≥ 30 events per hour of sleep) patients in this study (Table 4). Because our center is one of the biggest sleep centers in Japan, the possibility of a referral bias may exist. Therefore, this should be taken into account while interpreting the prevalence of daytime hypercapnia among obese patients with OSA in the present study.
In the present study, however, compared to patients without daytime hypercapnia, PaO2 was significantly lower, probably in association with daytime hypoventilation, and prevalence of MetS and metabolic scores were significantly greater in those with daytime hypercapnia. MetS is strongly associated with abdominal obesity and/or visceral adiposity[9,10,27]. As previously reported, abdominal obesity has an independent effect on hypoventilation[28]. Abdominal obesity with excess adiposity surrounding the chest wall can restrict respiration by impairing diaphragm motion and reducing lung compliance[8], and is associated with a reduction in PaO2[29], thereby inducing daytime hypoxia[30] in addition to daytime hypercapnia. Such mechanisms may play a central role in the daytime hypercapnia prevalent in our patient population. However, considering the fact that there was no significant difference in waist circumference between patients with and without hypercapnia, MetS by itself or along with other factors, rather than abdominal obesity alone may contribute to the development of daytime hypercapnia. For instance, one of the MetS components, accumulation of each MetS component, quality of abdominal/visceral fat, or mediators/products from the adipose tissue are potential contributors. Nevertheless, whether daytime hypercapnia can be reversed by the reduction of waist circumference may be of interest.
Leptin, a circulating protein known to inhibit appetite centrally and stimulate ventilation, is produced mainly by the adipose tissue[7]. However, plasma leptin levels are high in obese individuals; it is hypothesized that these individuals may have leptin resistance, and consequently have central leptin deficiency[7,31]. Patients with OSA and OHS had higher plasma levels of leptin than did weight-matched controls without either OSA or OHS[32,33]. Furthermore, leptin levels may be a better predictor of hypercapnia than degree of adiposity[33], as higher leptin levels are associated with a reduced response to hypercapnia in severely obese individuals[34]. In contrast, hyperleptinemia and leptin resistance were observed in patients with MetS independent of obesity[35-37]. In addition, increased levels of leptin and leptin resistance are associated with the development and worsening of each component of MetS independent of obesity[38]. Thus, hyperleptinemia and leptin resistance may play a key role in the relationship between MetS and daytime hypercapnia in obese patients with severe OSA independent of abdominal obesity. Furthermore, coexistence of MetS and high metabolic scores with daytime hypercapnia might explain the increased morbidity and mortality in patients with OHS[6].
The present study has several limitations. Firstly, the number of enrolled participants was relatively small, from a single center, and included only a Japanese patient population. Secondly, this study was a cross-sectional study; thus, a causal relationship between MetS and daytime hypercapnia could not be determined. Thirdly, although we assumed that leptin resistance may play a key role in the relationship between MetS and daytime hypercapnia in our patients, circulating levels of leptin were not measured. This was a pilot study; however, it may contribute to generating further hypotheses that hyperleptinemia contributes to the daytime hypoventilation in obese patients with severe OSA. Finally, although patients with chronic pulmonary disease were excluded, there may be some patients with subclinical impairment of pulmonary function. Thus, a lack of pulmonary function test data is one of the major limitations and the present results should be interpreted with caution.
In conclusion, we have shown that approximately one fourth of obese patients with severe OSA had daytime hypercapnia and that the presence of MetS and increased number of MetS components were independently correlated to daytime hypercapnia. However, whether this relationship is causal or consequential remains unclear. Coexistence of MetS may play a role, and its determination may be important in obese patients with severe OSA. Physicians should consider the possibility of daytime hypercapnia in cases of obese patients with severe OSA presenting with coexisting MetS.
In the obese patient population, some patients have severe obstructive sleep apnea (OSA) with daytime hypoventilation. Such patients are generally identified on the basis of the presence or absence of daytime hypercapnia, and the condition is called obesity hypoventilation syndrome (OHS). However, mechanisms for such daytime hypoventilation remain unclear.
Because patients with OHS consume greater levels of healthcare resources, and are associated with increased morbidity and mortality compared with eucapnic controls, identifying patients with OHS is highly valuable.
To investigate metabolic syndrome and daytime hypercapnia association based on hypercapnia prevalence in obese OSA patients in a nested case-control study.
Consecutive obese patients (body mass index ≥ 30 kg/m2) who underwent polysomnography due to suspected OSA were included. Among them, patients with severe OSA (apnea hypopnea index ≥ 30/h) were divided into two groups according to the presence or absence of hypercapnia during wakefulness (arterial partial pressure of carbon dioxide ≥ or < 45 Torr, respectively).
Among 97 eligible patients, 25 patients (25.8%) had daytime hypercapnia. There were no significant differences in age, gender, body mass index, apnea-hypopnea index, and Epworth Sleepiness Scale scores between the two groups. However, patients with hypercapnia had a significantly lower arterial partial pressure of oxygen level (75.8 ± 8.2 torr vs 79.9 ± 8.7 torr, P = 0.042) and higher arterial partial pressure of carbon dioxide level (46.6 ± 2.5 torr vs 41.0 ± 2.9 torr, P < 0.001). Additionally, patients with hypercapnia were more likely to have metabolic syndrome (MetS) (72.0% vs 48.6%, P = 0.043) and a higher metabolic score (the number of satisfied criteria of metabolic syndrome). In multivariate logistic regression analysis, the presence of MetS was associated with the presence of hypercapnia (OR = 2.85, 95%CI: 1.04-7.84, P = 0.042).
This study is the first to show that the presence of MetS and increased metabolic scores were associated with a greater risk of daytime hypercapnia in these patients. The importance of the present study is that physicians will be able to identify patients at risk for OHS, on noting MetS or high metabolic scores in obese patients with severe OSA, without determining arterial blood gas. Physicians should consider the possibility of daytime hypercapnia in cases of obese patients with severe OSA presenting with coexisting MetS. In addition, we have suggested that hyperleptinemia and leptin resistance may play a key role in the relationship between MetS and daytime hypercapnia in obese patients with severe OSA independent of abdominal obesity.
Although a causal relationship between MetS and daytime hypercapnia remains to be elucidated, and although circulating levels of leptin were not measured in the present study, this study may contribute to generating hypotheses that hyperleptinemia in association with MetS contributes to the daytime hypoventilation in obese patients with severe OSA. Thus, further large-scale prospective studies including measurement of circulating levels of leptin are needed.
| 1. | Kinlen D, Cody D, O'Shea D. Complications of obesity. QJM. 2018;111:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 2. | Taranto Montemurro L, Kasai T. The upper airway in sleep-disordered breathing: UA in SDB. Minerva Med. 2014;105:25-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care. 2010;55:1347-1362; discussion 1363-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Masa JF, Pépin JL, Borel JC, Mokhlesi B, Murphy PB, Sánchez-Quiroga MÁ. Obesity hypoventilation syndrome. Eur Respir Rev. 2019;28:180097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 5. | Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest. 2001;120:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 179] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Nowbar S, Burkart KM, Gonzales R, Fedorowicz A, Gozansky WS, Gaudio JC, Taylor MR, Zwillich CW. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 298] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med. 2011;183:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Hodgson LE, Murphy PB, Hart N. Respiratory management of the obese patient undergoing surgery. J Thorac Dis. 2015;7:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 9. | Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5418] [Article Influence: 258.0] [Reference Citation Analysis (1)] |
| 10. | [Definition and the diagnostic standard for metabolic syndrome--Committee to Evaluate Diagnostic Standards for Metabolic Syndrome]. Nihon Naika Gakkai Zasshi. 2005;94:794-809. [PubMed] |
| 11. | Kasai T, Miyauchi K, Kubota N, Tamura H, Kojima T, Yokoyama K, Kurata T, Daida H. The relationship between the metabolic syndrome defined by various criteria and the extent of coronary artery disease. Atherosclerosis. 2008;197:944-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Kasai T, Miyauchi K, Kurata T, Ohta H, Okazaki S, Miyazaki T, Kajimoto K, Kubota N, Daida H. Prognostic value of the metabolic syndrome for long-term outcomes in patients undergoing percutaneous coronary intervention. Circ J. 2006;70:1531-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Kasai T, Miyauchi K, Kajimoto K, Kubota N, Dohi T, Kurata T, Amano A, Daida H. The adverse prognostic significance of the metabolic syndrome with and without hypertension in patients who underwent complete coronary revascularization. J Hypertens. 2009;27:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kasai T, Miyauchi K, Kajimoto K, Kubota N, Yanagisawa N, Amano A, Daida H. Relationship between the metabolic syndrome and the incidence of stroke after complete coronary revascularization over a 10-year follow-up period. Atherosclerosis. 2009;207:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 623] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 16. | Kajimoto K, Kasai T, Miyauchi K, Hirose H, Yanagisawa N, Yamamoto T, Takazawa K, Niinami H, Hosoda Y, Daida H, Amano A. Metabolic syndrome predicts 10-year mortality in non-diabetic patients following coronary artery bypass surgery. Circ J. 2008;72:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Kajimoto K, Miyauchi K, Kasai T, Yanagisawa N, Yamamoto T, Kikuchi K, Nakatomi T, Iwamura H, Daida H, Amano A. Metabolic syndrome is an independent risk factor for stroke and acute renal failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2009;137:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Wang F, Xiong X, Xu H, Huang H, Shi Y, Li X, Qian Y, Zou J, Yi H, Guan J, Yin S. The association between obstructive sleep apnea syndrome and metabolic syndrome: a confirmatory factor analysis. Sleep Breath. 2019;23:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and technical Specification. 1st ed. Weschester, Illinois: American Academy of Sleep Medicine, 2007. |
| 20. | Kasai T, Narui K, Dohi T, Ishiwata S, Yoshimura K, Nishiyama S, Yamaguchi T, Momomura S. Efficacy of nasal bi-level positive airway pressure in congestive heart failure patients with cheyne-stokes respiration and central sleep apnea. Circ J. 2005;69:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H; Committee on the Standardization of Diabetes Mellitus‐Related Laboratory Testing of Japan Diabetes Society. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3:39-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 719] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 22. | Takegami M, Suzukamo Y, Wakita T, Noguchi H, Chin K, Kadotani H, Inoue Y, Oka Y, Nakamura T, Green J, Johns MW, Fukuhara S. Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on item response theory. Sleep Med. 2009;10:556-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 23. | Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath. 2007;11:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Laaban JP, Chailleux E. Daytime hypercapnia in adult patients with obstructive sleep apnea syndrome in France, before initiating nocturnal nasal continuous positive airway pressure therapy. Chest. 2005;127:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Akashiba T, Akahoshi T, Kawahara S, Uematsu A, Katsura K, Sakurai S, Murata A, Sakakibara H, Chin K, Hida W, Nakamura H. Clinical characteristics of obesity-hypoventilation syndrome in Japan: a multi-center study. Intern Med. 2006;45:1121-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Harada Y, Chihara Y, Azuma M, Murase K, Toyama Y, Yoshimura C, Oga T, Nakamura H, Mishima M, Chin K; Japan Respiratory Failure Group. Obesity hypoventilation syndrome in Japan and independent determinants of arterial carbon dioxide levels. Respirology. 2014;19:1233-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Fujita T. The metabolic syndrome in Japan. Nat Clin Pract Cardiovasc Med. 2008;5 Suppl 1:S15-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Zavorsky GS, Hoffman SL. Pulmonary gas exchange in the morbidly obese. Obes Rev. 2008;9:326-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Busetto L, Sergi G. Visceral fat and respiratory complications. Diabetes Obes Metab. 2005;7:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol (1985). 2010;108:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 496] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 31. | Berger S, Polotsky VY. Leptin and Leptin Resistance in the Pathogenesis of Obstructive Sleep Apnea: A Possible Link to Oxidative Stress and Cardiovascular Complications. Oxid Med Cell Longev. 2018;2018:5137947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Phipps PR, Starritt E, Caterson I, Grunstein RR. Association of serum leptin with hypoventilation in human obesity. Thorax. 2002;57:75-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 143] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Shimura R, Tatsumi K, Nakamura A, Kasahara Y, Tanabe N, Takiguchi Y, Kuriyama T. Fat accumulation, leptin, and hypercapnia in obstructive sleep apnea-hypopnea syndrome. Chest. 2005;127:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Campo A, Frühbeck G, Zulueta JJ, Iriarte J, Seijo LM, Alcaide AB, Galdiz JB, Salvador J. Hyperleptinaemia, respiratory drive and hypercapnic response in obese patients. Eur Respir J. 2007;30:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Hodge AM, Boyko EJ, de Courten M, Zimmet PZ, Chitson P, Tuomilehto J, Alberti KG. Leptin and other components of the Metabolic Syndrome in Mauritius--a factor analysis. Int J Obes Relat Metab Disord. 2001;25:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Moreno LA, Pineda I, Rodríguez G, Fleta J, Giner A, Juste MG, Sarría A, Bueno M. Leptin and metabolic syndrome in obese and non-obese children. Horm Metab Res. 2002;34:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Huang KC, Lin RC, Kormas N, Lee LT, Chen CY, Gill TP, Caterson ID. Plasma leptin is associated with insulin resistance independent of age, body mass index, fat mass, lipids, and pubertal development in nondiabetic adolescents. Int J Obes Relat Metab Disord. 2004;28:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Franks PW, Brage S, Luan J, Ekelund U, Rahman M, Farooqi IS, Halsall I, O'Rahilly S, Wareham NJ. Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes Res. 2005;13:1476-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited Manuscript
Specialty type: Respiratory system
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barzilay J, Gheita T, Tung TH S-Editor: Ma YJ L-Editor: A E-Editor: Qi LL