©The Author(s) 2025.
World J Obstet Gynecol. Dec 18, 2025; 14(3): 112710
Published online Dec 18, 2025. doi: 10.5317/wjog.v14.i3.112710
Published online Dec 18, 2025. doi: 10.5317/wjog.v14.i3.112710
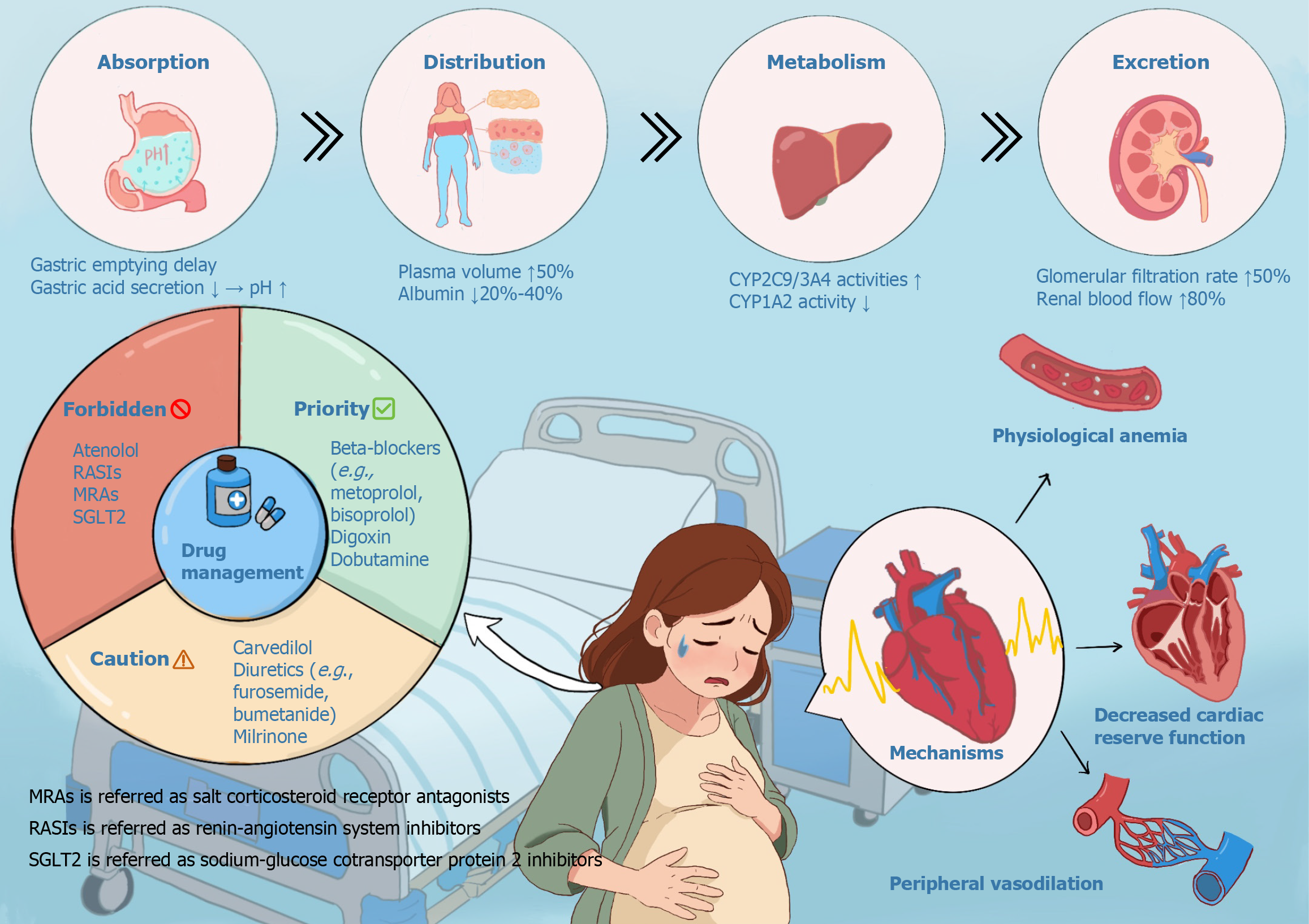
Figure 1 Graphical Abstract.
It summarizes the complex interplay between profound physiological changes during pregnancy, their impact on pharmacokinetics (PK), subsequent medication safety classifications, and the pathophysiology of peripartum heart failure (HF). Key maternal adaptations include altered gastric absorption, significantly increased renal elimination, and modulated hepatic enzyme activity. The underlying pathophysiology for HF during pregnancy involves physiological anemia, decreased cardiac reserve function, and peripheral vasodilation, which collectively increase cardiovascular strain. These shifts necessitate a stringent drug classification system: "Forbidden" denotes agents with high fetal risk, while "Caution" requires vigilant monitoring. Understanding this triad – physiology, PK, and risk categorization – is essential for safe pharmacotherapy in pregnant patients, particularly those with or at risk of cardiac dysfunction. MRAs: Mineralocorticoid receptor antagonists; RASIs: Renin-angiotensin system inhibitors; SGLT2: Sodium-glucose cotransporter 2.
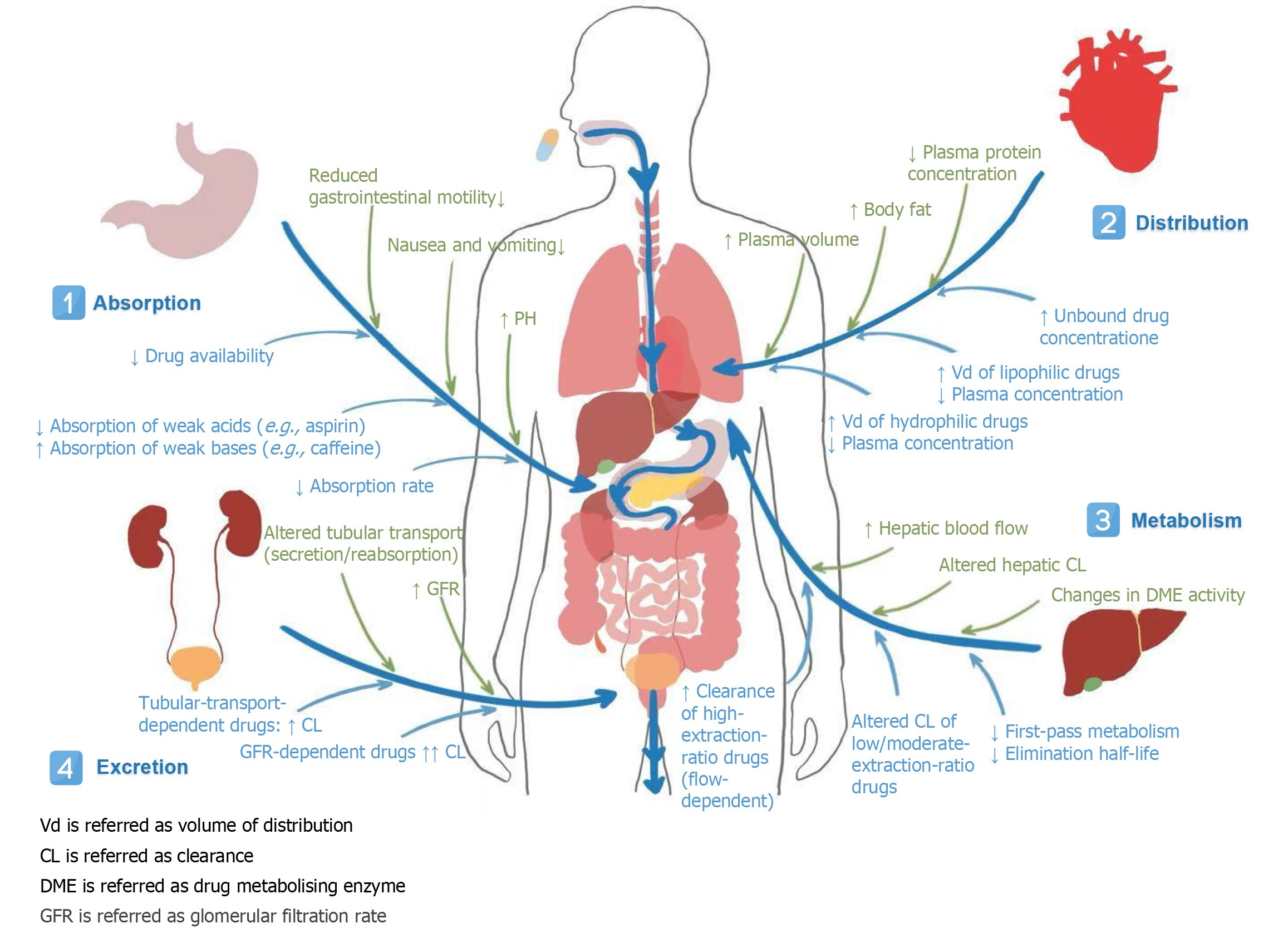
Figure 2 Effect of physiological changes during pregnancy on the pharmacokinetics of heart failure drugs.
It summarizes key physiological changes during pregnancy and their impact on drug pharmacokinetics across absorption, distribution, metabolism, and excretion. Major alterations include reduced gastrointestinal motility, increased plasma volume and body fat, modified hepatic enzyme activity (e.g., induction of CYP3A4, inhibition of CYP1A2), and elevated glomerular filtration rate. These changes significantly affect drug levels: Absorption rates decrease, volume of distribution increases for hydrophilic drugs, unbound drug concentration rises, and clearance is enhanced for many agents (e.g., digoxin, atenolol). Understanding these adaptations is crucial for dosing adjustments and therapeutic drug monitoring in pregnant patients. CL: Clearance; DME: Drug metabolising enzyme; GFR: Glomerular filtration rate; Vd: Volume of distribution.
- Citation: Cheng X, Yin XL, Shan YQ, Wang SY, Xia YB, Xu B, Xu TC. Navigating heart failure medications in obstetric practice. World J Obstet Gynecol 2025; 14(3): 112710
- URL: https://www.wjgnet.com/2218-6220/full/v14/i3/112710.htm
- DOI: https://dx.doi.org/10.5317/wjog.v14.i3.112710