Revised: May 31, 2014
Accepted: June 18, 2014
Published online: August 6, 2014
Processing time: 264 Days and 2.8 Hours
Recurrent acute lymphoblastic leukaemia (ALL) is a common disease for pediatric oncologists and accounts for more deaths from cancer in children than any other malignancy. Although most patients achieve a second remission, about 50% of relapsed ALL patients do not respond to salvage therapy or suffer a second relapse and most children with relapse die. Treatment must be tailored after relapse of ALL, since outcome will be influenced by well-established prognostic features, including the timing and site of disease recurrence, the disease immunophenotype, and early response to retrieval therapy in terms of minimal residual disease (MRD). After reinduction chemotherapy, high risk (HR) patients are clear candidates for allogeneic stem cell transplantation (SCT) while standard risk patients do better with conventional chemotherapy and local therapy. Early MRD response assessment is currently applied to identify those patients within the more heterogeneous intermediate risk group who should undergo SCT as consolidation therapy. Recent evidence suggests distinct biological mechanisms for early vs late relapse and the recognition of the involvement of certain treatment resistance related genes as well cell cycle regulation and B-cell development genes at relapse, provides the opportunity to search for novel target therapies.
Core tip: Selected recent publications regarding the current management of childhood relapsed acute lymophoblastic leukemia have been reviewed. Controversies, current lines of investigation and possible future directions are discussed.
- Citation: Fuster JL. Current approach to relapsed acute lymphoblastic leukemia in children. World J Hematol 2014; 3(3): 49-70
- URL: https://www.wjgnet.com/2218-6204/full/v3/i3/49.htm
- DOI: https://dx.doi.org/10.5315/wjh.v3.i3.49
Despite current cure rates above 75%[1], relapse is the most important obstacle in definite cure of children with acute lymphoblastic leukaemia (ALL)[2]. Depending on certain risk factors, such as age at diagnosis, sex, ethnicity, presenting white blood cell (WBC) count, hematopoietic lineage of the disease, cytogenetic abnormalities, and early response to primary therapy (Table 1)[3], approximately 20% of children diagnosed with ALL will experience relapse after current frontline therapy[4-17]. In a retrospective analysis of 9585 patients registered within 10 consecutive Children’s Cancer Group (COG) studies, the relapsed cohort had a higher percentage of patients who fell in the age range < 1 or ≥ 10 years, as well as a higher proportion of males, patients with initial WBC count > 100000/mL, and African American or Hispanic ethnicity. Slow early response was also associated with a higher risk of relapse. By contrast, there was no significant difference in the distribution of the immunophenotype (B-precursor or T-cell) between the patients who relapsed vs those who did not[16]. Recurrent ALL is a relatively common disease for pediatric oncologists, and given the relatively high prevalence of newly diagnosed ALL, relapsed ALL still has a higher incidence than the new diagnoses of many of the most common pediatric malignancies and represents one of the most common childhood cancer. The number of children with ALL who experience treatment failure each year is similar to the number of children with newly diagnosed acute myeloid leukemia or rhabdomyosarcoma[2,18]. Moreover, relapsed ALL accounts for more deaths from cancer in children than any other malignancy and represents a major cause of death among children[17,19-23]. In the early 1980s, ALL relapse was regarded as an almost incurable disease[24]. Today, most patients achieve a second remission. However, about 50% of relapsed ALL patients do not respond to salvage therapy or suffer a second relapse[20-22,24-26]. For these patients, prognosis is extremely poor with survival rates below 10%[27]. Despite substantial second remission rates and a wide availability of haematopoietic stem cell transplantation (SCT), most children with relapse die[2].
| Clinical features | High-risk group stratification1 | |
| Age | Infants < 1 yr old | Yes |
| ≥ 10 yr | Yes2 | |
| WBC | ≥ 50 × 109/L | Yes2 |
| Sex | Male | No |
| Ethnicity | Blacks | No |
| Native American | No | |
| Alaskan Native | No | |
| Hispanic | No | |
| CNS status | CNS3 | No |
| Response to therapy | ||
| Morphological response | PPR | Yes |
| Induction failure3 | Yes | |
| MRD ≥ 0.01% | After induction (day 33) | Yes |
| After consolidation (day 78) | ||
| Biology | ||
| Immunophenotype | T-cell | No |
| Early T-cell precursor | Accepted by some study groups | |
| Genetic alterations | BCR-ABL1 | Yes |
| MLL translocation | Yes if age < 1 yr | |
| Hypodiploidy (< 44 chromosomes) | Yes | |
| TCF3-PBX1 (E2A-PBX1) | No | |
| TCF3-HLF | Accepted by some study groups | |
| iAMP21 | Accepted by some study groups | |
| BCR-ABL1-like ALL4 | No | |
| IKZF1 mutation or deletion | No |
After remission reinduction, recommendations for continuation therapy include ongoing intensive chemotherapy with or without radiation therapy or SCT. As in newly diagnosed patients, treatment must be tailored after relapse of ALL, since outcome will be influenced by several risk factors. Decisions regarding optimal postremission therapy in relapsed ALL are frequently based on well-established prognostic features, including the timing and site of disease recurrence, the disease immunophenotype, and, more recently, on evaluation of early response in terms of minimal residual disease (MRD) at the end of the reinduction phase[20,26,28-31]. With conventional approaches (intensive chemotherapy and/or SCT), disease free survival (DFS) rates range approximately from 20% to 50% depending on the study, time to end point, and the patient population. Though slightly different variables were measured, results from different study groups showed similar poor outcomes for patients in second complete remission (CR2)[16,20-22,24,25,32-34]. Thus, there is a relative lack of success in the induction of durable second remissions using conventional chemotherapy combinations and the benefits of SCT vs aggressive chemotherapy for different patient groups remain unclear. Although the best treatment approach for relapsed ALL remains uncertain, there is agreement that when relapse occurs early, leukemia-free survival remains dismal; most children still die despite aggressive chemo-radiotherapy approaches, including transplantation, and novel salvage regimens are needed[18,21,24,35,36]. Relapsed ALL represents the focus of considerable pediatric research and alternative treatment options exploring distinct mechanisms of action are being pursued[17,37]. New studies clearly need to address how to effectively treat relapsed patients and maintain durable remissions[16].
Site of relapse and length of first remission are the major criteria for the classification of patients after first relapse. According to the site of relapse, patients are commonly classified as isolated marrow, concurrent marrow, isolated central nervous system (CNS), isolated testicular and other extramedullary relapses with or without CNS involvement (Table 2)[16].
| Site of relapse | Ref. | |||
| BM | > 25% blasts in the BM (M3 marrow) and/or blasts cell in the PB | Isolated BM | No evidence of ALL in the CNS or any other site | [21,24,26, 28,30,36,38] |
| Concurrent or combined | ≥ 5% blasts in the BM in combination with EM ALL | [22,24,26, 28,30,38,39] | ||
| Isolated CNS | ≥ 5 cells/mm3 with leukemic blasts in a cytocentrifuge preparation of the cerebro-spinal fluid demonstrating leukemic blasts (cytomorphological) without major blood contamination ( ≤ 20 erythrocytes/mm3)1 OR clinical signs of CNS disease OR a leukaemic mass found on cranial computed tomography or magnetic resonance imaging | < 5% blasts in the BM, no blasts in the PB and absence of leukemic infiltrations elsewhere | [24,25,28,30,36,38,39] | |
| Isolated testicular2 | Leukemic infiltrations in the testis demonstrated by biopsy (both microscopically and immunologically) | < 5% blasts in the BM, no blasts in the PB and absence of leukemic infiltrations elsewhere | [24] | |
| Other extramedullary | Leukemic infiltrations demonstrated by biopsy (both microscopically and immunologically) | < 5% blasts in the BM, no blasts in the peripheral blood and absence of leukemic infiltrations elsewhere | [24,38] | |
| Length of first CR | ||||
| COG classification | [16,26,28, 29,36] | |||
| Early | Within 36 mo from initial diagnoses | Very early | < 18 mo from initial diagnoses | |
| Intermediate | 18-36 mo from initial diagnosis | |||
| Late | ≥ 36 mo from initial diagnosis | |||
| BFM classification | ||||
| Early | Occurring within 6 mo of the completion of frontline therapy | Very early | Within 18 mo from diagnosis | [42] |
| Late | More than 6 mo after the completion of frontline therapy | |||
| Response evaluation after relapse | ||||
| CR3 | M1 marrow | (< 5% blasts by bone marrow aspirate) in absence of clinical signs of disease with no evidence of circulating blasts or extramedullary disease and a recovered bone marrow4 | [19,22,25, 28,30,38] | |
| M2 marrow | presence of 5% to 25% blasts in the BM aspirate by conventional morphology | [28] | ||
| M3 marrow | presence of > 25% blasts in the BM aspirate by conventional morphology | [28] | ||
| CNS remission | < 5 WBC cells/mL regardless of cytologic evaluation | [36] | ||
| Remission of testicular relapse | Defined clinically by return to normal testicular size | [36] | ||
| Reinduction failure | Reinduction treatment not resulting in CR | [19] | ||
| Refractory patients | Surviving patients after reinduction failure | [19] | ||
| Relapse after a new remission | A pathologically confirmed M3 marrow (≥ 25% leukemic blasts) or the presence of leukemia in any other site (e.g., CNS, PB) | [19] | ||
| Treatment failure5 | All cases of relapse and reinduction failure | [19] | ||
| MRD response | positive | Identification of ≥ 0.01% blasts (1/10000) in the BM using flow cytometry–based assays | [28] | |
| negative | < 0.01% blasts in the BM using flow cytometry–based assays | [28] | ||
Marrow relapse is generally defined as a bone marrow (BM) showing greater than 25% blasts (M3) by conventional morphology and/or blasts cells in the peripheral blood[21,24,26,28,30,36,38]. Isolated BM (medullary) relapse is like marrow relapse but without evidence of ALL in the CNS, testicles or any other site. Combined BM relapse is defined as ≥ 5% blasts in the BM in combination with extramedullary ALL[22,24,26,28,30,38,39]. Accordingly, isolated extramedullary relapses are those with a clinically-overt extramedullary manifestation of leukemia and less than 5% marrow infiltration[24]. Isolated CNS relapse is defined as ≥ 5 cells/mm3 in a cytocentrifuge preparation of the cerebro-spinal fluid demonstrating leukemic blasts (cytomorphological) without major blood contamination (≤ 20 erythrocytes/mm3) or clinical signs of CNS disease or a leukemic mass found on cranial computed tomography or magnetic resonance imaging, and < 5% blasts in the bone marrow, no blasts in the peripheral blood and the absence of leukemic infiltrations elsewhere (Table 2)[22,25,28,30,36,38,39]. Some studies require the demonstration of the presence of leukemic cells in the cerebrospinal fluid (CSF) in two consecutive CSF samples taken with an interval of at least 24 h[38,40]. Evidence suggests that submicroscopic involvement of the BM with leukemia is a frequent finding in patients with “apparently” isolated CNS relapse[41].
Isolated relapse elsewhere (testicle, skin, bone, orbita, mediastinum, lymph nodes, and tonsils) can be defined as leukemic infiltrations demonstrated by biopsy (both microscopically and immunologically), with < 5% blasts in the BM, no blasts in the peripheral blood and an absence of leukemic infiltrations elsewhere (Table 2)[38]. In some studies, testicular relapse was diagnosed in case of uni- or bilateral painless enlargement of the testicles[22,33]. In the case of unilateral testicular relapse, it is recommended to rule out a subclinical involvement of the contralateral testis[24].
Although a cut-off point between early and late relapses is often made at 3-6 mo after treatment cessation, the definitions for “early”vs“late” relapse differ slightly among different study groups. The COG categorizes relapses as “early” (recurrence occurring within 36 mo after initial diagnoses) or “late” (occurring ≥ 36 mo after initial diagnosis). Early relapses are further classified as “very early”, if they occur < 18 mo, or “intermediate”, if they occur 18-36 mo after initial diagnosis[16,26,28,29,36]. For the Berlin-Frankfurt-Münster (BFM) group, the time-point of relapse is defined in relation to the date of primary diagnosis and the date of completion of primary therapy (i.e., the end of the antileukemic therapy of the frontline protocol). Although completion of primary therapy often corresponds to the end of the maintenance therapy, in a few patients, it may correspond to the end of a short and intensive first line treatment, or to the end of an inadequately short primary treatment. For the BFM group, the end of frontline therapy is as much or even more important than the duration of the first remission. Therefore, those relapses occurring within 6 mo of the completion of frontline therapy are classified as “early”, while “late” relapses are those occurring more than 6 mo after the completion of frontline therapy. The concept of “very early” relapse coincides with that of the COG classification (i.e., relapses occurring within 18 mo after diagnosis)[42]. Thus, assuming that, for most patients, the duration of contemporary frontline treatment is 24 mo, there is a six months gap between the COG and the BFM criteria for the definition of late relapse so that relapses occurring between 30 and 36 mo after initial diagnosis should be considered as “late” within BFM trials, while COG trials should consider them as early relapses.
Patients are considered to have achieved a complete response (CR) if reinduction treatment results in an M1 marrow (< 5% blasts by BM aspirate) in the absence of clinical signs of disease with no evidence of circulating blasts or extramedullary disease and a recovered BM (Table 2)[19,22,25,28,30,38]. M2 and M3 marrow response are defined as the presence of 5% to 25% and > 25% blasts in the BM aspirate by conventional morphology, respectively[28]. Recovery of the BM is assumed in cases where the white blood cell (WBC) count is > 2.0 × 109/L and platelet count > 50 × 109/L[38]. Other studies considered the recovery of peripheral counts if absolute neutrophil count is ≥ 750-1000/μL with platelet count ≥ 75000-100000/μL)[19,28,29]. Some studies consider patients without platelet recovery but fulfilling the remaining criteria for CR as “CR without platelets”[19,28,43]. CNS remission is commonly defined as < 5 WBC cells/mL regardless of the cytologic evaluation and remission of testicular relapse is defined clinically by a return to normal testicular size[36]. Reinduction treatment not resulting in CR is generally termed reinduction failure and surviving patients are termed refractory[19]. Regarding MRD response, the identification of ≥ 0.01% blasts (1/10000) in the BM using flow cytometry-based assays is generally assumed as MRD positive (negative < 0.01%)[28]. After a new remission is achieved, relapse is defined as a pathologically confirmed M3 marrow (≥ 25% leukemic blasts) or the presence of leukemia in any other site (e.g., CNS, peripheral blood)[19]. Relapses and reinduction failures are collectively termed treatment failures within most studies. Treatment failures, the development of a second malignant neoplasm, or death from any cause are generally considered events for DFS analysis[19].
Age and WBC at primary diagnosis of ALL are the most important clinical prognostic factors. Infants < 1 year old and children ≥ 10 years have the worse prognosis (Table 1)[3]. Risk factors predicting CNS relapse after the first CR include T-cell immunophenotype, hyperleukocytosis, high-risk genetic abnormalities, and the presence of leukemic cells in the CSF at the time of diagnosis[44].
Understanding the biological factors contributing to relapse will probably contribute to identify new agents able to increase the chances of a sustained second remission and cure. Studying the biology of these diseases at diagnosis, in minimal residual disease states after selection by chemotherapy, and at relapse, provides a unique opportunity to dissect pathways and identify potential therapeutic strategies for relapsed childhood ALL and may improve our understanding of how to use current therapy as well as identifying new targets[16,37,45].
It has generally been assumed that relapse is the consequence of the emergence of a drug-resistant leukemia subclone which was already present at diagnosis and that was selected during frontline therapy. During initial therapy, this minor population would exhibit only moderate reduction relative to the bulk of the diagnostic leukemic cells but would rapidly expand before clinical relapse[45]. Although most relapsed patients achieve a second CR2 with drug combinations involving the same agents used at primary diagnosis, those patients who fail to enter in remission are not likely to be salvaged using different drug combinations, suggesting intrinsic drug resistance[45]. The equivalent post-relapse survival for patients undergoing different intensity regimens as first line therapy, suggests that the malignant cells responsible for relapse are present at diagnosis and mutate to a resistant phenotype through the acquisition of spontaneous mutations that are dependent on intrinsic genomic instability rather than treatment exposures[17]. Lesion specific backtracking studies revealed that in most cases the relapse clone existed as a minor sub-clone within the diagnostic sample prior to the initiation of therapy suggesting that the relapse clone was selected for during treatment. In only a minority (6%) of ALL cases did the relapse clone represent the emergence of a genetically distinct and thus unrelated second leukemia[46]. These findings indicate that the diagnosis and relapse clones originated from a common ancestral clone and acquired distinct copy number abnormalities (CNAs) before emerging as the predominant clones at diagnosis or relapse. In this model, relapse emerges from a drug-resistant subclone present at initial diagnosis that is selected during treatment regardless of the nature of the frontline therapy delivered[17]. This data support the hypothesis that many relapses may be the result of the selection of a relatively resistant clone already present at initial diagnosis rather than the generation of a novel clone by mutation[18,47]. Resistant leukemia subclones are probably present at primary diagnosis in those patients destined for early relapse. Early-relapse mechanisms appear to be more homogeneous and are suggestive of the selection of a resistant, more proliferative clone (Table 3)[48]. Alternatively, the acquisition of resistance-conferring mutations induced by initial treatment might be responsible for the relative drug resistance noted at relapse[45]. For subsequent relapses and treatment attempts a significant decrease in CR rates is expected[19], which suggests the emergence of new mechanisms of resistance. According to this model, genomic studies carried out in samples from children at diagnosis and relapse demonstrated the acquisition of new genetic alterations at relapse, often involving cell proliferation and B-cell development pathways[45,46,48,49].
| Clinical data | Biological explanation | Ref. | Biological evidence | Ref. | |
| Early relapse | Patients failing to achieve CR2 with the same agents used at primary diagnosis usually do not respond to different drug combinations | Intrinsic drug resistance: the malignant cells responsible for relapse are present at diagnosis and are selected for during treatment | Yang et al[45], 2008 | Genome-wide analysis of DNA CNAs and LOH on matched diagnostic and relapse BM samples revealed that the majority (94%) of relapse cases was related to the presenting diagnostic leukemic clone | Mullighan et al[46], 2008 |
| Equivalent post-relapse survival for patients undergoing different intensity regimens at primary diagnosis | The malignant cells responsible for relapse are present at diagnosis and mutate to a resistant phenotype through the acquisition of spontaneous mutations | Freyer et al[17], 2011 | Primary diagnosis and relapse clones originates from a common ancestral clone and acquire distinct CNAs before emerging as the predominant clone at diagnosis or relapse | ||
| Decrease in CR rates after subsequent relapses and treatment attempts | Acquisition of resistance-conferring mutations induced by initial treatment | Ko et al[19], 2010 | Adquisition of new genetic alterations at relapse, often involving cell proliferation and B-cell development pathway | Bhojwani et al[48], 2006 Yang et al[45], 2008 Mullighan et al[46], 2008 Hogan et al[49], 2011 | |
| Late relapse | The distribution of patients experiencing early and late relapses were highly skewed towards NCI HR in the former group and NCI standard risk in the latter | Late relapse represents de novo development of a second leukaemia from a common premalignant clone | Nguyen et al[16], 2008 | Distinct patterns of gene expression in pairs of relapsed samples from patients who relapse early from those relapsing later | Bhojwani et al[48], 2006 |
| Pattern of NOTCH1 mutations and genome-wide copy number showed a common clonal origin between diagnosis and early relapse but not for late relapses of T-cell ALL | Szczepanski et al[53], 2011 | ||||
| Distinct pattern of deletions at the non-translocated TEL allele at primary diagnosis and relapse of TEL-AML1-positive ALL | Zuna et al[51], 2004 |
By contrast, late relapses may represent de novo development of a second leukemia from a common premalignant clone. Data regarding patients relapsing after being primarily treated within 10 Children Cancer Group (CCG) trials showed how the distribution of patients experiencing early, intermediate and late relapses were highly skewed toward National Cancer Institute (NCI) HR patients in the former group and NCI SR in the latter group. Although SR patients receive less intense therapy, these data suggest that intrinsic differences in the biology of the leukemic blasts are correlated with different mechanisms and the timing of relapse[16]. Distinct gene expression profiles were revealed for pediatric relapsed ALL patients at both early and late time points[49]. The analysis of microsatellite markers showed that some very late relapses of TEL/AML1+ positive leukemia most likely represent a new event that occurs in a quiescent precursor leukemia cell harboring an otherwise silent fusion gene that has escaped eradication during initial therapy[50]. Moreover, analysis of deletions at the non-translocated TEL allele study of relapsed TEL-AML1-positive ALL samples showed that the relapsed clone was related but distinct from the clone at initial diagnosis. This might explain the clinical responsiveness of many cases of late or off-treatment TEL/AML1+ ALL relapses[51]. Paired samples from patients experiencing early relapse are more similar in expression patterns than paired samples from those relapsing later[48]. Staal et al[52] using genome-wide expression array on purified leukemic cells, found that genes involved in a late or an early relapse identified clearly distinct pathways. Analyses of the TCR gene rearrangement status, pattern of NOTCH1 mutations, and genome-wide copy number showed a common clonal origin between diagnosis and early relapses of T-cell ALL but not for the few cases of T-cell ALL late relapses, suggesting that these recurrences should be considered as a second T-ALL rather than a resurgence of the original clone[53]. These findings are suggestive of a model whereby late relapse is due to the acquisition of diverse secondary events that might occur in a distinct subpopulation such as a leukemic stem cell[48].
By comparing matched diagnosis and relapse samples, Bhojwani et al[48] found that certain genes involved in cell proliferation, protein biosynthesis, carbohydrate metabolism, and DNA replication/repair were among those highly expressed in relapsed vs newly diagnosed blasts. By contrast, some of the genes down-regulated at relapse compared with initial diagnosis included proapoptotic genes, antiproliferative genes and a putative tumor suppressor gene[48].
Treatment resistance related genes, such as CDKN2A/B and MSH6, ETV6, and cell cycle regulation and B-cell development (PAX5, EBF1, IKZF1) were shown to emerge at relapse, providing the opportunity to search for novel target therapies[37,45,48]. The discovery of these new genetic alterations associated with high rates of relapse (and shorter first remission), such as the rearrangement of CRLF2, IKZF1 deletions/mutations and JAK family mutations, offers the potential for the identification of patients at diagnosis who should be treated more aggressively and with agents targeting those molecular lesions[45,54-56].
During the last 2 decades several study groups such the Acute Lymphoblastic Leukemia-Relapse Study of the BFM Group (ALL-REZ BFM) have performed prospective controlled phase III trials to establish standardized treatment protocols with the primary goal of improving the prognosis of children with relapsed ALL and to evaluate risk factors, thereby allowing for risk-adapted treatment intensity[24].
Time to relapse (length of first remission), site of relapse and ALL-immunophenotype are well-established risk factors that can predict survival and constitute the most important prognostic determinants that can be used to stratify patients with a first relapse into different treatment groups[2,16,17,20,25-27,32,34,35,57].
Before relapse, the median duration of the first complete remission (CR1) has been reported to be around 2.5 years[20,25,35]. Most ALL relapses occur during treatment or within the first 2 years after treatment completion, although relapses have been reported to occur even 10 years after diagnosis[2,18].
In a large series of 854 ALL relapses reported by the Nordic Society for Pediatric Hematology and Oncology (NOPHO), the median time from diagnosis to first relapse was 28 mo (range, 2-227 mo)[33]. According to Chessells et al[32] 74% of relapses occurred in the first 3 years after diagnosis, 4% after 6 years, and only 1% occurred more than 10 years after diagnosis. Reissmüller et al[22] reported a relative incidence of very early (within 18 mo from diagnosis), early (after 18 mo from diagnosis up to 6 mo after cessation of primary treatment) and late relapses (more than 6 mo after cessation of front-line therapy) of 41%, 22% and 37%, respectively. In a retrospective analysis of 1961 relapsed patients registered within 10 consecutive CCG studies, the duration of the CR1 for patients who relapsed varied according to NCI risk group at primary diagnosis, with shorter duration of remission coinciding largely with higher risk features at diagnosis[16]. The duration of the CR1 has been reported to vary with the site of relapse[34,35,41]. In the study reported by Malempati et al[34], the mean interval between day 28 of primary induction and relapse for all patients was 32.8 mo, CNS relapses tended to occur earlier (mean 23.1 mo), and testicular recurrences tended to occur later (40.5 mo) than BM relapses (mean 36.2 mo).
Timing of relapse has emerged as the most significant predictor of outcome and the most important factor for a second relapse is the duration of the first remission. Early relapse has worse prognoses than late relapse[16,17,20,22,25,32-35,38,57]. Some late relapses are thought to arise from a common precursor that retains the chemosensitivity of the original clone, which could explain the high cure rates achieved with chemotherapy alone in late relapses[30]. Ko et al[19] found CR rates of 83% for early first relapse and 93% for late first relapse. Breaking down early relapse into very early relapse (< 18 mo from diagnosis) and intermediate (18 to 36 mo from diagnosis), they found CR rates of 78% and 86%, respectively. EFS rates reported for early relapses ranged from 5% to 18% and 19% to 57% for late relapses[16,19,20,22,24,26,28]. Even when intensive salvage strategies including SCT are employed, longer-term EFS rates for early relapses are only 10% to 20%, compared with 40% to 50% for late relapses. These outcomes have been remarkably consistent over recent decades, irrespective of differences in the components of salvage regimens[21,24,28].
The majority of relapses (60% to 80%) involve the bone marrow (BM) alone or together with extramedullary involvement, and more than 70% of relapses involving the BM are isolated BM relapses. Isolated CNS or testicular relapse or, much less frequently, relapse involving other extramedullary sites may also occur (Table 4)[20,22,32,34].
Bone marrow relapses are associated with a worse outcome than extramedullary relapses, with overall long-term survival rates of approximately 25%[16,17,19,22,33-34]. Survival at 3 to 6 years after relapse has been found to range from approximately 20% for isolated marrow relapse to 50%-80% for isolated extramedullary relapse, with combined-site (i.e., marrow plus extramedullary) relapses having an intermediate outcome[16,20,22,25,32,34,40]. In extramedullary relapses, a clear distinction also has to be made for early relapses vs late relapses. Regarding early relapse, survival rates are higher for patients with isolated CNS relapse than for patients with either isolated or combined BM relapse, and this is also true for intermediate and late relapsing patients. Survival rates were also significantly higher for patients with concurrent marrow relapses compared to those with isolated marrow relapses[16,24].
Thus, involvement of an extramedullary site in patients with BM relapse has been identified as a favourable prognostic feature compared to patients without extramedullary involvement. To explain this fact, it has been hypothesized that combined BM relapses originate from the involved extramedullary compartment, in which the leukemic cells could survive the front-line chemotherapy because they were protected by the blood-brain/testis barrier. Thus, relapses in extramedullary sites are often considered as relapses from malignant cells treated with suboptimal drug levels; due to their homing on these sanctuaries. Therefore, they may be more sensitive to chemotherapy than clones originating directly from the BM[24]. Five-year survival rates for isolated CNS range between 43.5% for early, and 78.2% for late relapses[16,40].
In the case of a testicular relapse, isolated relapse patients fare better, with an EFS of 58% vs 28% for combined relapses[20]. In the COG analysis reported by Nguyen et al[16], overall 5-year post-relapse survival rate after early isolated testicular relapse was lower (13%) than after intermediate (52%) or late (59.9%) relapses although this difference was not statistically significant.
The immunological lineage of the disease (B-cell precursor vs T-cell ALL) is another well recognized risk factor in childhood relapsed ALL. Late relapses of T-ALL are rare and make up approximately 10% of all recurrences[53]. The BFM group demonstrated that children with T-cell ALL BM relapses have a much worse prognosis than B-cell precursor ALL (BCP), irrespective of the time between diagnosis and recurrence[42]. In a report by investigators at St. Jude Children’s Research Hospital, CR2 rates for this population were 60%, with a 5-year EFS of only 5% compared to 28.7% for B-cell lineage[25]. Other studies confirmed that the prognosis of patients with a first relapse of T-ALL is dismal, with only 15% to 25% of patients achieving durable remissions after second-line treatment[16,24]. Thus, apart from the fact that T-cell recurrences tend to occur early, T-cell immunophenotype itself is associated with a very poor outcome after relapse regardless of site and time to relapse[16,20-22,24-26,28,32].
Minimal residual disease (MRD), measured either by flow cytometry or real-time polymerase chain reaction (PCR), may supplement morphologic response[29,58,59]. Rates of MRD positivity after reinduction for relapsed ALL are much higher than those observed in first-line ALL clinical trials[28]. The prognostic significance of MRD response at relapse has been assessed in several studies[28,31,60]. Persistence of MRD after re-induction/consolidation therapy (i.e., after 5 and 12-13 wk from the beginning of treatment for relapse) influences prognosis in children with relapsed ALL. Children with MRD levels < 1 × 10-3 or 1 × 10-4 have been shown to carry a lower risk of recurrence than patients with higher levels of MRD[30,31,58,61,62].
Within the COG AALL01P2 study, five-year EFS probabilities differed in patients according to MRD response using flow cytometry-based assays at the end of the first block of chemotherapy (negative < 0.01%; positive ≥ 0.01%)[28]. The absence of MRD at the end of the first month of reinduction therapy portended better outcomes in all patients, and separately in early and late relapse patients. The combination of timing of relapse and MRD appeared to identify three groups of patients. Early relapse patients who were MRD positive had a dismal outcome, while late relapse patients who were MRD negative had an excellent outcome, approaching that seen in newly diagnosed patients. MRD-negative early relapse patients and MRD-positive late relapse patients appeared to form an intermediate group. MRD positivity was also correlated strongly with the duration of initial remission; those patients experiencing relapse at less than 18 mo from initial diagnosis had the highest proportion of MRD positivity[28,29]. In a prospective blinded study, Eckert et al[31] have recently reported that EFS and OS decreased and the cumulative incidence of relapse increased with increasing MRD level (quantified by PCR analysis of antigen receptors) after reinduction chemotherapy in intermediate-risk relapsed ALL patients treated by the ALL-REZ BFM P95/96 protocol. Patients of the lower MRD groups (< 10-4 and < 10-3 to ≥ 10-4) had an acceptable prognosis (EFS at 10 years 80% and 64%, respectively) compared to patients of the higher MRD groups (< 10-3 to ≥ 10-2 and ≥ 10-2) who had EFS at 10 years of 36% and 4.8%, respectively. Multivariate analysis revealed that MRD after the second induction course was the only parameter independently predicting the occurrence of subsequent adverse events[31]. Conflicting results, however, were observed in the Medical Research Council (MRC) UKR3 trial, in which reinduction therapy with mitoxantrone was superior to that with idarubicin, yet no differences in the end of reinduction MRD were observed[30].
In a prospective and blinded study, the ALL-REZ BFM Study Group evaluated the impact of pre-transplantation MRD in children treated according to the ALL-REZ BFM 96 or 2002 protocol who received their transplantation in CR2 or third CR (CR3). MRD proved to be the most important determinant for subsequent relapse and survival after transplantation in univariate and multivariate analysis. The cut-off of less than 10-4 leukemic cells turned out to be a feasible discriminator between patients at high (≥ 10-4 leukemic cells) or low risk (< 10-4 leukemic cells) for subsequent relapse. According to these findings, patients classified as being intermediate risk with conventional clinical parameters could be further classified into a very HR subgroup if MRD proves to persist at a high level until transplantation[61]. In another study, classical risk factors such as immunophenotype, site of relapse, time to relapse, and others were only significant in patients who receive chemotherapy in CR2. These factors lost their relevance in patients undergoing SCT, and MRD remained the only independent prognostic variable in this setting[42]. Thus, MRD of leukemia both during second CR and before transplantation, has been reported to be a very strong prognostic factor for the ultimate outcome[61]. However, the Saint Jude group reported that, although MRD before transplantation was an independent predictor of survival, patients with high levels of MRD (0.1% to < 5.0% leukemia cells) still had a reasonably good chance of survival (43%) after SCT, suggesting that the negative effect of MRD had been partially offset by recent improvements in the transplantation procedure[63].
Given its power as a prognostic factor, quantification of MRD at diagnosis of ALL relapse and regularly during therapy has become an essential tool to characterize the responsiveness of the disease and to allocate the patients to a risk adapted treatment. It is currently being incorporated for relapsed patients into a risk-classification algorithm for the management of relapsed ALL within the COG (Table 2)[28,29].
A similar stratification system was used in the UKALL R3 relapse trial[30], and is currently applied by the International BFM Study Group (I-BFM SG).
Although study designs are incorporating the use of MRD in order to quickly assess responses in patients with relapsed ALL who are treated with novel agents, at present MRD remains an unvalidated surrogate marker for this purpose[28,29]. To this regard, even when a clear superiority from one arm to the other was obtained regarding the primary outcome (i.e., EFS), results from the UK ALLR3 trial failed to demonstrate a difference in MRD level at early assessment between both study arms[30].
There is some debate in the literature on the prognostic factor of the white blood cell (WBC) count and the presence of blasts in the peripheral blood at the time of relapse[25,32,33]. There is some evidence that, among children with relapsed ALL, those who had WBC counts < 50000/μL at initial diagnosis are more likely to have favourable outcomes after relapse[16]. Age at primary diagnosis might influence outcome after relapse. Older age at diagnosis (≥ 10 years), as well as age younger than 1 year, has been associated with significantly inferior post-relapse outcome[16-17,33,35,57]. In a recent analysis of 1150 patients aged 0-18 years registered in four consecutive Austrian ALL-BFM trials, prognosis of relapsed leukaemia was significantly better for younger patients (patients aged 1-15 years at primary diagnosis) than for adolescent (i.e., patients aged between 15 and 18 years at primary diagnosis) even when neither the time point or the site of relapse differed significantly between broth groups[64]. These results suggest that age at initial diagnosis is a prognostic factor in relapsed ALL, just as it is for newly diagnosed disease[29]. Certain unfavourable clonal cytogenetic abnormalities detected at primary diagnosis have been found to portend worse post-relapse survival[22,32]. Philadelphia chromosome-positive (Ph+) and 11q23 abnormalities were associated with early relapse and poorer prognosis[32]. The prognosis of children relapsing after first line treatment for Ph+ ALL, particularly for those relapsing after SCT, is poor[28,65]. The ETV6/RUNX1 fusion gene has been associated with better outcome after relapse[22].
It has been debated whether the intensity of frontline treatment affects the outcome of patients after relapse[29]. Type of first treatment was reported to influence the outcome after relapse, with more recent regimens being associated with improved survival[32]. The Austrian BFM Study Group reported higher post-relapse EFS (but not survival) for 203 children with relapsed ALL who received treatment on the more recent of their frontline studies conducted during the 1980s and 1990s[22]. It might be expected that patients who relapse after receiving an inferior initial treatment regimen would have greater success in retrieval, and greater post-relapse survival than patients who relapse after receiving a superior initial treatment regimen, given that their leukemia clone at relapse should be “less resistant” after being exposed to less effective or intensive prior treatment[17]. However, data from 272 relapsed patients after primary therapy within the COG CCG-1961 Study, demonstrated that there was no difference in 3-year post-relapse survival between two groups of patients having primarily received augmented vs standard intensity post-induction intensification. For subjects initially treated with augmented (n = 109) vs standard-intensity (n = 163) post-induction intensification, the 3-year post-relapse survival was 36.4% vs 39.2%, respectively (P = 0.72). There was no difference in the median time-to-death post-relapse according to initial regimen, (10.5 mo for augmented vs 16.2 mo for standard-intensity, P = 0.27), and no difference in post-relapse survival was seen after adjusting for timing of relapse, site of relapse, age at diagnosis, and lineage of the leukemia[17]. Similarly, in a report of post-relapse survival rates in 1961 children previously enrolled on 10 consecutive CCG clinical trials, according to treatment era at initial diagnosis (trials conducted from 1988-1995 vs 1996-2002), with treatment intensity increasing over time, the post-relapse outcomes were nearly identical[16]. Thus, differing intensity of initial treatment, as reflected in either the cross-regimen setting of single studies (CCG-1952 and CCG-1961) or the trans-era context of sequential CCG/COG studies involving both standard- and high-risk patients, does not alter the generally poor outcome associated with relapsed childhood ALL of any initial risk category[17]. Prognosis seems to be particularly poor for those patients relapsing after SCT[22].
Finally, male sex, African American or Hispanic ethnicity, and central nervous system (CNS) disease at diagnosis were reported to be significant predictors of inferior post-relapse survival in children with newly diagnosed ALL who had been enrolled on COG clinical trials from 1988 to 2002[16].
The BFM cooperative Group developed a relapse score incorporating duration of first complete remission, site of relapse, and immunophenotype to classify patients as standard-, intermediate-, and high-risk, with 6-year post-relapse survival rate reaching 78%, 41% and 19%, respectively for patients receiving more modern treatment. According to this classification, all children with T-cell relapse involving the bone marrow at any time, and children with very early combined and very early or early isolated marrow non-T cell are classified as HR; very early or early isolated extramedullary relapse, irrespective of immunophenotype, as well as early or late combined BM and late isolated marrow BCP ALL relapse, are classified as intermediate risk (IR); while SR category correspond to late isolated extramedullary (both T and non-T cell immunophenotype) (Table 5)[32,42]. Among 1556 patients up to 18 years of age with first relapse of ALL enrolled in trials of ALL-REZ BFM between June 1983 and April 2001, the SR group comprised 5% of patients while 55% and 40% of all patients were allocated to the IR and HR, respectively[42].
| BCP | T-cell | |||||
| Isolated EM | Combined BM | Isolated BM | Isolated EM | Combined BM | Isolated BM | |
| Risk stratification according to the BFM Group classification[42] | ||||||
| Very early1 | Intermediate | High | High | Intermediate | High | High |
| Early1 | Intermediate | Intermediate | High | Intermediate | High | High |
| Late1 | Standard | Intermediate | Intermediate | Standard | High | High |
| Risk stratification according to the United Kingdom ALLR3 Study classification[30] | ||||||
| Very early1 | High | High | High | High | High | High |
| Early1 | Intermediate | Intermediate | High | Intermediate | High | High |
| Late1 | Standard | Intermediate | Intermediate | Standard | High | High |
| Current approach to risk stratification according to I-BFM SG | ||||||
| Very early1 | High | High | High | High | High | High |
| Early1 | Standard | Standard | High | Standard | High | High |
| Late1 | Standard | Standard | Standard | Standard | High | High |
In a retrospective review of 150 relapsed patients form four large pediatric oncology units in the United Kingdom, Roy et al[20] found that children with a very early isolated extramedullary relapse had a significantly poor outcome when compared with the rest of the IR group, and suggested modifying this risk stratification system. Accordingly, within the United Kingdom ALLR3 Study these patients were classified as high risk patients[30]. However, only two risk groups are currently considered by the I-BFM SG. The standard risk group includes patients with: (1) a late or early isolated extramedullary relapse of BCP or T-cell ALL; (2) a late or early combined BM/extramedullary relapse; and (3) a late isolated BM relapse of BCP ALL. The high risk group comprises those with a very early isolated extramedullary relapse of BCP or T-cell ALL; early isolated or very early isolated or combined BCP ALL, and any BM relapse of T-ALL (Table 5).
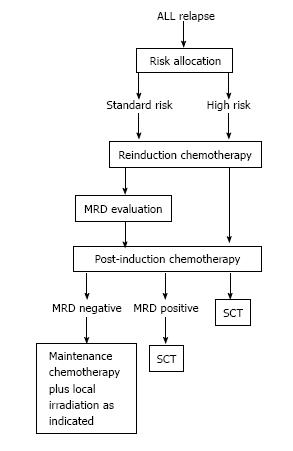
Salvage treatment after ALL relapse involves inducing a CR2 with conventional intensive chemotherapy and apply consolidation, re-intensification and maintenance therapy, or allogeneic stem-cell transplantation (SCT) as further intensification of treatment. As occurs with primary diagnosed ALL, successful treatment of relapse largely relies upon the risk-based treatment allocation of patients in order to maximize response to therapy while minimizing toxicity and adverse effects. Using the prognostic criteria such as first remission duration; site and immunophenotype of relapse; genetic alterations; and initial response to relapse therapy, distinct subgroups of relapsed ALL can be identified that may either be treated with chemoradiotherapy only or by additional allogeneic SCT (Table 6 and Figure 1)[27,29].
| Relapse | Site | Time | MRD | ||
| B-lineage | Marrow | Early | Chemotherapy vs chemotherapy plus novel agents | Negative | SCT |
| Positive | Bridging study before HSCT | ||||
| Late | Chemotherapy vs chemotherapy plus novel agents | Negative | Continuation therapy | ||
| Positive | SCT | ||||
| IEM | Early | Chemotherapy vs chemotherapy plus novel agents | Any | SCT | |
| Late | Chemotherapy vs chemotherapy plus novel agents | Negative | Continuation therapy | ||
| Positive | SCT | ||||
| T-lineage | Marrow | Early | Chemotherapy vs chemotherapy plus novel agents | Negative | SCT |
| Late | Positive | Bridging study before HSCT | |||
| IEM | Early | Chemotherapy vs chemotherapy plus novel agents | |||
| Late | Any | SCT |
Current treatment approaches for relapsed ALL begin with reinduction therapy in an attempt to induce a CR2. Reinduction of patients with relapsed ALL commonly includes conventional agents largely identical to those used at initial diagnosis except with increased dose intensity or alternative schedules with reported rates of toxic deaths around 4%-5%[26,42,47].
Few randomized trials comparing different reinduction regimens in risk-stratified children with relapsed ALL have been conducted[30,39,66], and it remains unclear whether any reinduction combination in use today is significantly superior to any other[18,47]. The Pediatric Oncology Group compared every 2 wk and weekly pegylated asparaginase with vincristine, doxorubicin, and prednisone in a population including both early and late marrow relapse and obtained CR2 rates of 82% and 97%, respectively[66].
The BFM group randomized dose and duration of infusional methotrexate in reinduction, demonstrating similar outcomes between intermediate-dose (1 g/m2 over 36 h) and high-dose (5 g/m2 over 24 h) infusions[39]. In a trial conducted by the United Kingdom Children’s Cancer Group (UKCCG) patients were allocated to receive either idarubicin or mitoxantrone during induction; after 3 blocks of therapy, HR and IR patients with MRD ≥ 10-4 received allogeneic SCT, whereas SR and IR patients with MRD < 10-4 continued chemotherapy. EFS and OS were significantly higher in the mitoxantrone group. The 3-year OS was 69% in the mitoxantrone group (45% in the idarubicin), which overall represented a substantial improvement over preceding trials from the same investigators[30].
The conceptional backbone therapy of the ALL-REZ BFM Group is a series of short (i.e., 5 to 7 d), intensive multiagent chemotherapy courses (block therapy), including most of the same traditional chemotherapy agents with known antileukemic efficacy, with an interval of 2 wk between the blocks to allow for regeneration of bone marrow aplasia, then followed by local irradiation therapy when indicated, and conventional maintenance therapy. This block therapy concept proved to be feasible, effective, and relatively well-tolerated and has been incorporated into many other treatment regimens for relapsed ALL and HR primary ALL worldwide[24,26]. A risk-adapted intensification of treatment by prolonging the intensive treatment phase in an ALL-REZ BFM Study could not prevent a high relapse rate in the high-risk group (early isolated or combined bone marrow relapse)[24]. Other treatment strategies with a more continuous therapy with repetitive application of comparably less intensive chemotherapy has been developed and used by the COG, and the UK ALL Relapse Study Group (MRC UKALLR), achieving comparable results[20,35]. It remains unclear whether short course intensive, or continuous less intensive chemotherapy constitute the most adequate and effective approach in treating childhood relapsed ALL or which subgroups of patients may benefit more from one approach or the other[24].
The COG conducted the AALL01P2 phase II pilot study with the primary objective of developing a safe and active reinduction regimen that could serve as a platform for evaluating the addition of promising new agents in future trials[28]. An objective of this study was to improve the depth of CR2 using three intensive non-overlapping blocks of chemotherapy derived from combinations that were previously shown to be effective in the management of recurrent ALL. With this regimen there was a 40% incidence of febrile neutropenia and a 19% incidence of documented infections[28]. Five toxic deaths occurred among 124 patients (4%) yielding a similar toxic death rate seen with other regimens[20,24,26,28,33,36,66].
The authors concluded that extending the duration of re-induction to three blocks appears to be beneficial for the group of patients with initial favourable morphologic responses and were MRD-negative at the end of the first month of treatment[28].
Reinduction remission rates for patients with a first relapse range from 71% to 95%, depending on the timing and site of relapse. CR2 rates for late bone marrow relapse typically approach 95%, whereas those for early relapse range from 70% to 85% and are frequently < 50% for very early relapses[16,19-22,24-26,28-30,32,34,35,42,66]. T-cell immunophenotype has been related with a lower remission rate[25]. Patients failing to achieve a CR2 after reinduction chemotherapy have a dismal prognosis[25,28,67]. Data from the BFM study group showed that only one third of children treated with curative intention after conventional reinduction failure obtained a CR2 (81% of them only after SCT) with a median survival of 255 d after the diagnosis of reinduction failure[67]. Given that further therapies with curative intent are associated with high treatment-related morbidity, mortality, and minimal survival, children with relapsed ALL and having no response to protocol-therapy should be eligible for innovative, ethically approved phase 1 or 2 trials[18,67].
For patients with HR relapsed ALL, the COG is currently exploring the role of adding novel agents to remission reinduction therapy (Table 6)[29]. The International Cooperative Group on Relapsed ALL conducts 2 randomized trials comparing the classic BFM reinduction therapy with that reported by the UKCCG in standard and intermediate risk patients, and with a novel regimen combining clofarabine, etoposide, and cyclophosphamide in high risk children, respectively[18,37].
For patients with relapsed ALL who attain a second remission, no consensus on optimal therapy exists. All patients who reach a CR2 receive additional chemotherapy, even if SCT is planned. In general, a higher dose intensity is used than in first-line treatment but published data do not show one chemotherapy combination to be better than others[47].
For patients not allocated to SCT, a consolidation phase after induction chemotherapy followed by a prolonged continuation treatment is generally recommended[20,25,42]. According to the ALL-REZ BFM protocols, patients not allocated to SCT receive treatment consisting of alternating courses of polychemotherapy. At the end of intensive systemic chemotherapy, local radiotherapy is applied as indicated followed by conventional maintenance therapy up to 2 years[42].
Allogeneic SCT is a curative option for several hematologic malignancies and the current availability of several different stem cell sources has expanded this option for many children. High-dose myeloablative chemotherapy followed by SCT is an alternative to chemotherapy alone for relapsed ALL. Several published retrospective studies suggest some benefit for SCT, particularly for patients with early BM relapse[19,22,42,57]. With improved supportive care and better donor selection, the outcome after unrelated donor and matched sibling SCT for relapsed ALL has become more equal[68]. Therefore, the comparability of several different stem cell sources has expanded this option for many children and SCT has been widely used for patients with relapsed ALL. In the ALL-REZ BFM 87 study, SCT was associated with a superior EFS compared to chemotherapy/radiotherapy as postremission therapy alone, and the performance of SCT (included as time-dependent covariate) was an independent predictor of EFS. Results of autologous transplantation and chemotherapy were the same[24]. In the Austrian BFM Study Group report, patients who received SCT in second CR did significantly better than patients given chemotherapy only (10-year EFS 55% vs 33%) and this was even more obvious following an isolated BM relapse (10-year EFS 58% for SCT in second CR vs 22% for chemotherapy only)[22]. In a report by Eapen et al[57], children with an early BM first relapse of BCP ALL had lower rates of second relapse and higher rates of leukemia-free survival and OS if they received an HLA-matched sibling transplant with a TBI-containing regimen compared with a non-transplant approach. In contrast, for those with a late first relapse and second relapse, leukemia-free and OS rates were similar after chemotherapy alone and transplantation[57]. In a retrospective report from the NOPHO Study group, SCT led to increased long-term survival compared with chemotherapy irrespective of the length of first remission[33]. Matched-pair comparisons across BFM group ALL-REZ trials showed that unrelated donor transplantation achieved significantly better leukemia-free survival than chemotherapy in HR relapse but not in IR relapse. The EFS at 5 years was 17% for the chemotherapy group (0% for HR) and 42% for the SCT group (44% for HR) while rates of treatment related death were 4% and 30%, respectively[42].
Other studies suggest that the type of therapy after relapse does not affect outcome[21], and for a subgroup of patients with relapsed ALL, mainly represented by late BM and extramedullary relapses, combined chemotherapy and radiotherapy may yield durable second remissions[24]. From the BFM group, Borgmann et al[42] reported 39% EFS after transplantation for IR patients compared to 49% for non-transplanted patients. In line with such a statement are the data in a United Kingdom study including 256 patients, who were analyzed on the basis of HLA matched donor availability; no statistical benefit in outcome was seen[69]. Malempati et al[34] found no significant difference in EFS or OS between treatment with SCT or chemotherapy for any site of relapse or duration of the CR1, with a 2-year estimated EFS of 49.5% with SCT compared to 49.1% with chemotherapy for the entire group. For early BM relapse they also found no difference in treatment modality; the 2-year estimated EFS was 43.1% with SCT and 38.0% with chemotherapy; there was also no significant difference in EFS for late BM relapse according to treatment type: 2-year estimated EFS was 56.1% with SCT and 61.5% with chemotherapy. Similarly, 3-year estimated EFS after isolated CNS relapse was equivalent with either SCT or chemotherapy at 45% and 56.1%[34].
The analysis of the ALL REZ BFM 90 Study showed that SCT did not improve EFS for IR patients (represented by late isolated or combined BM and isolated extramedullary regardless of time point of relapse) or for those who received allogeneic HLA-compatible grafts; however, EFS was significantly higher after SCT in HR patients (early BM, very early isolated or combined BM and any relapse of T-lineage) than after the administration of chemo-radiotherapy alone. This group of patients when treated with conventional chemo-radiotherapy had a low chance of cure[26]. In the study by van de Berg et al[38], the benefits of the conditioning and the possible graft-vs-leukemia effect on patients undergoing SCT did not outweigh the benefit of prolonged, rotational chemotherapy for late relapses (including BM relapses); patients treated with chemotherapy only achieved a 65% survival rate[38].
The impact of type of donor (matched related vs unrelated or mismatched related) on outcome has not been demonstrated. Some studies claimed a clear advantage for matched related donor SCT or for matched unrelated donor SCT[42]. Long-term EFS rates from of above 40% have been reported with HLA-matched sibling donor SCT in CR2 after early relapse[57]. By contrast, others found no significant difference in outcome according to type of donor[2,32]. Results with umbilical cord transplantation are comparable to that obtained with unrelated donor SCT[70]. Unrelated donor registries and cord blood banks have increased the donor availability for the majority of patients lacking an HLA matched familial donor but the process of searching for an unrelated donor usually takes several months during which patients in CR2 are at risk of new relapse or even death from treatment related complications[33]. In this regard, reported time to transplant after relapse is commonly around 3 mo[19,26,28,34].
Haploidentical hematopoietic SCT (haplo-SCT) from a mismatched family member donor offers an alternative option for patients who lack a human leucocyte antigen (HLA)-matched donor[71]. The main obstacles are graft rejection, delayed immune reconstitution, graft-vs-host disease (GvHD) and vulnerability to infections[71]. T-cell depletion can prevent overwhelming GvHD allowing the graft to contain large numbers of stem cells. This approach can reduce the risk of graft failure retaining CD34-negative stem cells and most other immune cells, thus allowing expedite immune reconstitution during the early post-transplant period. However, the absence of the T cell-mediated graft-vs-leukemia effect would render the recipients of a T cell-depleted allograft more susceptible to leukemia relapse. In this scenario, donor-vs-recipient NK alloreactivity has emerged as a crucial factor for the outcome of haplo-SCT. Ruggeri et al[72] reported a low relapse risk for patients with acute myeloid leukaemia transplanted from NK-alloreactive donors. This NK-mediated graft-vs-leukemia effect has also been documented in children with ALL[73]. Data from the Pediatric Diseases and the Acute Leukemia Working Parties of the European Blood and Marrow Transplant showed 34% and 22% EFS for children undergoing haplo-SCT in CR2 and CR3, respectively[74]. Therefore, a T cell-depleted haplo-SCT should be included in the treatment algorithm as a valuable option for patients with ALL in need of transplantation and lacking a matched donor, especially if an NK alloreactive relative exists. An unmanipulated HLA-haploidentical SCT has been proposed for those few patients who are unable to locate an HLA-compatible donor, a suitable umbilical cord blood unit, or an NK-alloreactive relative[18]. As stated by Locatelli et al[18], for those patients considered candidates for allo-SCT, the preferred source of stem cells should be a matched sibling donor; for those lacking an HLA-compatible family donor, an unrelated donor, umbilical cord blood or haploidentical family donor are suitable options.
Intensive chemo-radiotherapy has been administered before transplantation to reduce the burden of disease and induce immunosuppression in the host. Total body irradiation (TBI)-containing regimens before SCT from a matched sibling donor proved to be superior to chemotherapy alone and a non-TBI regimen in children with early relapse and BCP ALL who achieve a CR2. Transplantation with a TBI-containing regimen resulted in significantly lower risks of relapse, treatment failure, and overall mortality compared to non-TBI regimens and this was independent of the duration of the first remission[57]. Such conditioning regimens reduce graft rejection but they can cause considerable mortality due to severe toxicity, delayed immune restoration and severe infection, especially in heavily pre-treated patients. Moreover, DFS estimates are not appreciably improved by aggressive chemo-radiotherapy, as recurrent or refractory malignancies have usually become resistant to chemotherapy. These observations have encouraged the reassessment of conditioning strategies for transplantation. Newer strategies aim to minimise toxicity while allowing rapid engraftment and expediting immune reconstitution during the early post-transplant period, thereby protecting the host from infection and perhaps generating a graft-vs-tumour effect against disease relapse[71,75]. In contrast to traditional myeloablative conditioning regimens that use high doses of radiation or chemotherapy or both to suppress host immune responses and eradicate diseases, this approach relies almost exclusively on graft-vs-host effects for eradication of the underlying diseases. Reduced intensity or non-myeloablative conditioning regimens for haplo-SCT reduced mortality and have an acceptable rate of engraftment. However, delayed immune reconstitution, severe GvHD and infection continue to be impediments[71,75]. Elimination of TBI may reduce damage to organs that generate immune cells, while avoidance of anti-thymocyte globulin may prevent complications that include delayed immune reconstitution and Epstein-Barr virus associated lymphoproliferative disease[75]. Using a reduced intensity conditioning regimen (fludarabine, thiotepa, melfalan and OKT3) without TBI and without anti-thymocyte globulin in children with refractory haematological malignancy, a more rapid and robust immune reconstitution when compared to patients transplanted with a myeloablative conditioning regimen was reported. Studies with melphalan-based reduced-intensity conditioning regimens and T/B cell-depleted grafts show high engraftment rates. The risk of acute and chronic GvHD was significantly reduced by graft manipulation procedures (T/B cell depletion) and is comparable to that after matched unrelated donor transplantations[71,75]. Furthermore, the overall incidence of cytomegalovirus, Epstein-Barr virus and adenovirus viremia in the reduced intensity conditioning regimen group was less than that in the myeloablative conditioning regimen group[75]. Transplant related mortality could be effectively reduced by improved T cell recovery and close monitoring of viral loads followed by preemptive therapy[71].
Many factors complicate the analysis of published results comparing outcomes after SCT vs chemotherapy only, including intrinsic selection biases, different lengths of the interval between diagnosis of relapse and transplantation as well as disparate conditioning regimens, supportive strategies and stem cell sources[21,34]. Very often, reported trials assigned HR with matched family donors to allogeneic transplantation and those without donors to chemotherapy or autologous transplantation[47].
Another impediment in comparing reports is the more favourable outcomes of non-sibling donors SCT over the years. A matched-pair analysis of unrelated donor SCT vs chemotherapy revealed that only high-risk patients benefited from unrelated donor transplantations[42]. In a prospective randomized trial, Gaynon et al[21] found poor protocol adherence with small numbers of patients recruited to the chemotherapy arm. The authors speculated how this might be related to the known poor outcome of relapsed ALL and “a desire to do everything possible for children for whom aggressive chemotherapy had already failed once” [21,47].
Thus, much debate has centered on optimal postremission therapy including stem cell transplantation[18,33,42]. A recent meta-analysis demonstrated the variability of outcomes and conclusions among studies comparing SCT with chemotherapy for the treatment of ALL in second remission[76]. However, excluding patients with late relapse[38], in no comparison is outcome after transplantation worse than after chemotherapy alone[47]. To this regard, after the induction of the CR2, options for ongoing continuation therapy are frequently risk based in order to allocate patients to treatment regimens with adequate intensity and justifiable toxicity[18,29,31]. There are some patients with an acceptable EFS rate with chemotherapy alone, while other patients need to undergo allogeneic SCT after the 2CR or are even eligible for phase I/II trials with the chance of benefiting from new agents. Both the COG and the BFM as well as the I-BFM groups developed formal criteria for risk stratification for relapsed ALL with the main intention of identifying children for whom SCT might be better than continuation chemotherapy once a second remission is attained (Tables 5 and 6)[2,42].
Children with a very early (< 18 mo after diagnosis) or early (between 18 mo after diagnosis and 6 mo after cessation of frontline chemotherapy) isolated BM relapse, a very early BM/extramedullary combined relapse and all T-cell ALL with BM involvement at relapse diagnosis should be categorized as HR patients and should be allocated to SCT given that nearly all will suffer a subsequent relapse when being treated solely with conventional intensive post-induction chemotherapy. Allogeneic SCT with a matched donor is currently the preferred therapeutic option for these children after the CR2[20,26,29,33,38,42,69].
The outcomes for very early extramedullary recurrences without SCT have been inferior to those of early or late extramedullary relapse, with an EFS of < 50%, and SCT in CR2 has also been considered for these patients[20,40,41,77].
It remains unclear as to what will be the most effective approach for HR patients who continue to have high levels of disease before or after transplantation, as this is associated with a high incidence of relapse post-allogenic SCT[20]. In this scenario, further cytoreductive chemotherapy (clofarabine), immunomodulation, the application of new agents, and/or innovative transplant procedures might be considered.
Children with early or late (> 18 mo from initial diagnosis) isolated extramedullary relapse represent the SR group and, for these patients, outcomes have been very good with chemotherapy and site-directed radiation and there is no indication for SCT. However, intensive systemic therapy is essential for preventing later BM recurrences[20,26,29,31].
The largest group of patients (more than 50%) belongs to the IR group, in which treatment choices are the most difficult[32,42]. This group includes patients with BCP ALL with either late (> 6 mo after cessation of frontline chemotherapy) isolated BM relapse, or with a late or early combined BM/extramedullary relapse as well as early (including T-cell) isolated extramedullary relapses (Table 5)[31]. The optimal post-remission therapy for children with late B-cell precursor BM relapse (either isolated or combined) is controversial[20]. Intensive systemic therapy is essential for preventing later BM recurrences. However, the benefit of SCT for these patients has not been firmly established. SCT is associated with a 10% to 20% risk of peri-transplantation mortality, depending on donor type, and still has a substantial relapse rate[32,57]. While some studies report comparable results with both SCT and chemotherapy[26,57] others argue that the outcome of patients undergoing a transplant is poorer, and that SCT in late relapses is not beneficial[38]. Within the NOPHO study, patients with late BM relapse but with initial HR features and combined BM relapses did not do well on conventional chemotherapy. The authors recommended considering allo-SCT for these subgroups of patients[33]. Data from the DCOG Relapse ALL 98 protocol showed that patients with a late BM relapse undergoing a transplant had poorer outcomes than those undergoing CT only. Although the majority of these patients died from a relapse of leukaemia, the benefits of the conditioning and the possible graft-vs-leukemia effect after SCT did not outweigh the benefit of the prolonged chemotherapy[38]. The COG is currently investigating if outcome for patients with late (≥ 36 mo) B-cell precursor marrow relapse, can be improved by using the same AALL01P2 triple re-induction regimen followed by 2 years of intensive chemotherapy[28,78].
In the IR patients (with EFS rates greater than 40%) additional risk factors, such as the dynamics of treatment response assessed by MRD, would help to identify those patients at a high risk of subsequent relapse who are thus eligible for SCT[26,30]. MRD response is being integrated into risk classification schemes[18,29]. A cut-off point MRD after reinduction of 10-3 (quantified by PCR) was recently proposed by Eckert et al[31] to discriminate between patients with a good or a poor prognosis. Patients belonging to the group with MRD between 10-3 and 10-4 can be categorised as molecular good responders and allogeneic SCT would not be appropriate for these patients. In the subsequent trial ALL-REZ BFM 2002, this level of MRD after induction was applied to decide whether chemotherapy or SCT should be used as consolidation post-induction therapy[31]. A different cut-off MRD of 0.01% (10-4) (measured by flow cytometry) was applied by the COG for prognostic assessment after the first, second and third treatment block of the AALL01P2 study in IR and HR relapses[28]. Within the UK ALLR3 study, patients with MRD ≥ 0.01% (10-4) (quantified by PCR) at the end of induction were eligible for SCT[30]. A different preceding treatment and quantification method within each protocol might explain these differences in MRD cut-off levels as being predictive of outcome.
A major task of ongoing and future trials is to predict subsequent relapses more precisely, thus clarifying which patients benefit from post-remission intensification by allogeneic SCT. In this context, not only the acute mortality and toxicity, but also the long-term sequelae of allogeneic SCT have to be taken into account[24].
Therapeutic irradiation of manifest extramedullary leukemia in addition to systemic chemotherapy for patients experiencing CNS recurrence can be regarded as standard of care, since the disease is protected from chemotherapy by biological blood barriers in extramedullary sanctuary sites such as the CNS and the testes[18]. In accordance with other investigators, for patients with CNS involvement at relapse, we would encourage the use of an Ommaya reservoir during intensification. Intraventricular therapy has several theoretical advantages: a more uniform distribution of chemotherapy throughout the cerebrospinal fluid (CSF), higher ventricular levels than those achieved by lumbar administration, and prolonged concentration over time exposure to cell cycle active chemotherapy[36].
For patients with late isolated CNS relapse (not allocated to SCT), cranio-spinal irradiation is generally postponed until the end of intensive chemotherapy or even after the end of maintenance treatment, in order to avoid intolerability for chemotherapy[38]. The administration of 24 Gy and 15 Gy to cranium and spine, respectively are commonly recommended[38], although the adequate dose (18 vs 24 Gy) and mode of CNS irradiation (cranial vs craniospinal) remains controversial[44]. A 4-year EFS of 78% can be achieved after reduction of the radiation dose to 18 Gy in patients with B-cell precursor ALL whose initial remission lasted > 18 mo while in patients relapsing before 18 mo the EFS was 52%[40]. Further dose reduction (15 Gy) is recommended for patients with prior irradiation[18]. The addition of cranial irradiation, even in patients without obvious CNS-involvement (prophylactic cranial irradiation), was reported to significantly improve the outcome of patients with isolated BM-relapse by the ALL REZ BFM Study Group and was introduced from 1989 onwards[26]. If the CNS was involved at the time of relapse, patients received more intense intrathecal triple chemotherapy with methotrexate, cytarabine and prednisone[26]. In addition, cranial or cranio-spinal radiation was delivered in an age-dependent manner to all patients[22,24,26]. This strategy was adopted by other study groups[21,36]. However, whether protective CNS irradiation is necessary in patients with isolated BM relapse, remains controversial and, given the well documented radiation associated late effects, it is omitted by several groups in favour of intensified intrathecal chemotherapy[25,33].
Most study groups recommend local irradiation of both testes at 24 Gy regardless whether only one or both testes are involved at relapse. Within the BFM studies, orchidectomy has been the treatment of choice for the involved testicle in the case of testicular relapse. In unilateral testicular disease the clinically affected testis is removed and the remaining testis irradiated (15-18 Gy according to the results of biopsy)[22]. In the case of clinical unilateral or bilateral testicular involvement and no resection 24 Gy local irradiation is generally recommended[22,25,26,33,36]. Radiotherapy (24 Gy) for bilateral testicular recurrence is expected to induce infertility and significantly impair hormone production[18]. Within the DCOG Relapse ALL 98 Protocol, patients with late testicular relapses were treated without irradiation and without surgery[38].
A variety of other extramedullary sites may be involved in ALL relapse. Little data are available regarding the prognostic impact of these manifestations and on the necessity of local therapy. Since a blood barrier is not present in these sites, systemic chemotherapy is supposed to be effective. Thus, for an extramedullary relapse other than CNS and testis, no local therapy is generally considered apart from cases where local persistence of the disease occurs after induction/consolidation chemotherapy. In this situation, it is recommended to take a biopsy and to apply local irradiation therapy if vital leukemic cells are still present.
Most treatment failures after the CR2 are related to subsequent relapses[21]. For 74 patients experiencing a second relapse and enrolled into ALL-REZ BFM trials before 2006, the median duration of the second CR was 7.5 mo (range, 18 d to 4.4 years)[27]. In this situation, a significant decrease in CR rates is expected[21]. A variety of multidrug regimens provide a 40% CR rate in the second and subsequent relapses[28]. Ko et al[19] reported CR rates of 44%, 27%, and 12% for third, fourth, and further therapeutic attempts, respectively. The subsequent CR rate was lower when CR was not achieved or was of short duration after the prior treatment attempt[19]. In contrast, the NOPHO study group reported a third complete remission (CR3) as high as 72% in 274 patients after the second relapse. In this study, those who never achieved 3CR had a shorter first remission, more BM relapses and shorter time intervals between the relapses, indicating a more aggressive disease. However, long-term survival was only 12%[33]. Few other data appear for DFS rates in the CR3 and beyond[19,20,27,33]. According to Ko et al[19], DFS among patients who achieve CR decreased with an increasing number of prior treatment attempts. Two and 5-year DFS for patients achieving CR after third therapeutic attempts was 31% and 15%, respectively. DFS increased with increasing duration of the prior remission[19]. In a report from the Austrian BFM Study Group, the median duration of CR after second relapse was 13 mo, with 10-year EFS rates of only 9% and 6% after the second and third relapses, respectively[22].
Concerning prognostic factors, the length of the CR2 and relapse site are relevant[27,32]. Reismüller et al[27] found that the duration of the second CR seemed to have an influence on EFS: 6% vs 21% for patients with a CR2 duration of less or more than 1.5 years, respectively. In this report, the only other prognostic factors that proved to be statistically significant were site of first and second relapse with isolated extramedullary relapses faring better than isolated and combined BM relapses, and duration of the first CR[27]. Other reported factors associated with survival are NCI risk criteria at initial diagnosis, immunophenotype, presenting leucocyte count and length of first remission[19,32]. Additional extramedullary sites of disease were not significantly associated with DFS[18]. The prognosis for children with BM relapse after SCT, and children with a second relapse of T-cell ALL is dismal. In the latter group, this is mainly due to the lack of ability to achieve a CR3[27]. Patients who relapse after allogeneic SCT often have refractory disease and are particularly susceptible to chemotherapy-related toxicity[79].
Survival after second relapse was reported to vary according to treatment. The role of SCT for patients with a second or third relapse has been debated. Overall survival ranging from 20% to 36% was reported for those undergoing SCT compared with 10% to 15% for those with chemotherapy only[32,33]. Ko and coworkers[19] found increased survival for patients undergoing SCT, regardless of time to relapse or the number of prior relapses.
Given that only a very small group of patients with second ALL relapse has a realistic chance of cure, these patients are ideal candidates for phase I/II trials exploring new innovative drugs, with the consideration of SCT in those achieving a durable remission[18,27,29].
Outcomes for children with relapsed ALL have changed little over time despite efforts by many investigators to intensify therapy with approaches that often include SCT. Although clinical remission can be achieved in most (85%) relapses, the chance to experience a second relapse is still high and long-term survival rates do not exceed 40% to 50%[20-22,24-26,33,35,43]. Results from the CCG 1941 marrow relapse study showed that 50% of patients failed to enter remission, died from toxicity, or relapsed again after achieving a brief second remission[21]. The overall outcomes are dismal for patients who do not achieve a CR2 after an initial attempt[18,28]. In a recent large retrospective review within the Therapeutic Advances in Childhood Leukemia Consortium (TACL), Ko et al[19] found 27% 5-year DFS for patients in CR2. These results are similar to those generally reported by other study groups with DFS rates ranging from 16% to 39% depending on the study, time to end point, and the patient population[16,20-22,24,25,32-34].
Second malignancies such as primary brain tumors and acute myeloid leukemia are another matter of concern in relapsed ALL patients with an estimated actuarial incidence at 15 years from diagnosis of around 11%[32,57].
Unfortunately, retrieval therapy is inadequate in most cases of relapsed ALL and most of these children succumb to their disease. Further intensification of chemotherapy is unlikely to cure additional patients. The failure of intensive chemotherapy to cure most children, as well as its related toxicity, makes it essential to search for new treatment approaches. Approximately one third of relapsed patients can be assigned to a “poor prognosis group” (early BM-relapse or any BM relapse of T-cell ALL), for whom no promising therapy regimen exist[24]. Moreover, the extremely poor survival after relapse underscores the need to focus on improving the outcome of the primary therapy for those patients who are unlikely to be salvaged if they relapse. Promising new therapies should be integrated into trials for subsets of higher risk patients at initial diagnosis[16]. Using analyses of DNA copy number abnormalities, gene expression, DNA methylation and sequencing in matched diagnosis/relapsed ALL BM samples, investigations are under way regarding the evolution of genetic lesions from diagnosis to relapse that lead to drug resistance and disease progression with the aim of identifying new potential biomarkers and therapeutic targets[45,46,48,49,52,53,78]. Further development and the use of targeted therapies or immune modulators may decrease residual disease and may improve the outcome in children with relapsed ALL treated with either intensive chemotherapy or SCT[34]. Offering uniform clinical trials for patients with relapsed ALL while gathering biological data in order to identify new agents not generally used in the treatment of ALL at primary treatment, are the focus of several current collaborative study groups[16].
Because responses to single-agent therapy have been poor, integrating new agents in combination with established chemotherapy platforms in a randomized manner has been adopted as a therapeutic approach by de COG with the aim of exploring improvements in CR2 and MRD rates as a measure to define new agent activity and, potentially, to more efficiently select candidate agents for future study[29,78]. Novel approaches include new formulations of existing chemotherapeutic agents, new antimetabolites and nucleoside analogs, monoclonal antibodies directed against leukemia-associated antigens, adoptive therapy approaches such as chimeric antigen receptor (CAR)-modified T cells, and molecularly targeted drugs such as the proteasome inhibitor bortezomib and JAK kinase, aurora kinase, and mammalian target of rapamycin (mTOR) inhibitors[18,29,37,80].
Intrathecal lyposomal cytarabine may have a role in relapsed ALL with CNS involvement and resistance to conventional therapy[18].
Clofarabine is a second-generation purine analog capable of inhibiting DNA synthesis/repair and inducing cell death[81]. Clofarabine has been granted accelerated approval both in Europe and in the United States for the treatment of pediatric patients with relapsed or refractory ALL who received at least 2 prior regimens of chemotherapy[18]. O’Connor et al[82] reported an overall response rate of 67% in 23 pediatric patients diagnosed with relapsed ALL. Clofarabine was safe and effective when used in combination with cyclophosphamide and etoposide although a high risk of severe infection was noted, including fungal and viral infection. The response rate to treatment with a clofarabine-based regimen was inversely proportional to the number of prior treatment attempts. Durable remissions were achieved, allowing patients the option of hematopoietic stem cell transplantation with the potential of long term cure. Treatment was effective in 3 out of 5 infants with relapsed MLL rearranged ALL[82]. Thus, the use of clofarabine-based regimens should be considered in children with either resistant or second or subsequent BM relapse[18]. Nelarabine is an inhibitor of purine nucleoside phosphorylase. The FDA approved nelarabine in October 2005 for third-line treatment of patients with T-cell ALL/lymphoma[18]. In the COG AALL00P2 trial, patients with T-ALL with a poor early treatment response that predicted poor outcomes in previous trials attained a 5-year EFS rate of 69% with intensive chemotherapy plus nelarabine without increased toxicities. Non HR patients (< 1000/µL peripheral blood blasts on prednisone prephase day 8 and MRD < 1% at induction therapy day 36) who received nelarabine had a 5-year EFS rate of 74%[83].
Monoclonal antibodies directed to cell surface antigens expressed by leukemic blasts (epratuzumab, blinatumomab, inotuzumab ozogamicin, and moxetumomab pasudotox, among others), are ideal candidates. Combinations of monoclonal antibody and cytotoxic therapies may hold particular promise in relapsed ALL[2,29]. Epratuzumab is a humanized monoclonal antibody that binds to the third extracellular domain of CD22. CD22, a B-cell surface antigen, is highly expressed in more than 90% of cases of childhood B-precursor ALL. After binding, the receptor/antigen complex is rapidly internalized and appears to modulate B-cell activation and signaling. Given the high CD22 expression levels in B-precursor ALL, its mechanism of action distinct from cytotoxic agents, and a toxicity profile that could allow for combining it with dose-intensive chemotherapy, epratuzumab became an attractive agent to explore in relapsed ALL. Epratuzumab was the first agent tested by the COG in combination with an established reinduction platform in children and young adults with first, early BM relapses of CD22+ ALL in an effort to improve CR2 rates[84]. Patients received four intravenous doses of epratuzumab, 360 mg/m2 per dose, twice weekly during the 14-d reduction phase, followed by four weekly doses, 360 mg/m2 per dose, administered with block 1 of the AALL01P2 chemotherapy regimen[28,84]. Epratuzumab administration was tolerated with acceptable toxicity, both as a single agent and when combined with chemotherapy. MRD responses in those who achieved remission were significantly more favourable in those who received epratuzumab (42% MRD-negative compared with 25% among historical controls) suggesting that the antibody may enhance response to cytotoxic chemotherapy[29,78,84]. However, the rates of CR2 did not differ compared with a historical control population treated with chemotherapy alone[84]. Based on these results, the COG will not pursue epratuzumab further[78,85].
T-cell engaging antibodies are bispecific antibodies designed to transiently engage primed cytotoxic effector memory T lymphocytes for the lysis of target cells. The T-cell engaging CD19/CD3-bispecific antibody blinatumomab can redirect T lymphocytes against CD19+ ALL blasts, which represents a new approach to the treatment of BCP ALL. Handgretinger et al[79] reported the first clinical experience in three pediatric patients with BCP ALL showing that blinatumomab, administered as a continuous 24 h intravenous infusion at 15 mg/m2 per day for several weeks, was well tolerated and able to rapidly induce MRD-negative complete responses in refractory BCP ALL after multiple relapses and allogeneic HSCT. Blinatumumab is an attractive drug to be explored in the near future for children with second or greater relapsed or refractory ALL[78].
Bortezomib is a proteasome inhibitor, which renders leukemic cells more sensitive to the apoptotic effects of chemotherapy. A phase 1 study conducted by the TACL (TACL study, T2005-003) demonstrated that a standard dose of bortezomib (1.3 mg/m2 given on days 1, 4, 8, and 11) can be safely combined with an intensive 4-drug reinduction regimen in children with relapsed ALL and showed promising activity in relapsed childhood ALL[86]. Within the phase 2 expansion of this combination (TACL study T2005-003) patients were eligible only after they failed 2 or 3 previous treatment regimens. The CR rate was 64% with an additional 9% of CR without platelet recovery for an overall response rate of 73% which was significantly better than in previous trials. BCP ALL patients had an 80% overall response rate while no T-cell ALL patients showed a response. The study reached its predefined early stopping rule for efficacy when 14 complete responses were observed among the first 22 patients enrolled. OS at 24 mo was estimated to be 41%. Lethal bacterial sepsis was the principal toxicity[87]. A study combining bortezomib with a 4-drug reinduction platform (the AALL01P2 triple reinduction regimen) is also in progress within the COG for patients with early BM relapse occurring within 36 mo of diagnosis[29,78]. This approach will also be explored by the I-BFM SG.
In relapse Ph+ ALL, second complete remissions can be obtained with the combination of imatinib and intensive chemotherapy[28,88]. For patients relapsing after treatment with imatinib, the use of scalating doses of imatinib or alternative tyrosine kinase inhibitors may overcome imatinib resistance and help to induce a new remission and a second SCT should be considered in this situation[88,89]. High FLT3 expression identifies MLL-AF4+ ALL patients at very high risk of treatment failure and poor survival, emphasizing the value of ongoing/future clinical trials for FLT3 inhibitors[90]. The COG is conducting a phase I trial (ADVL1011) of the JAK inhibitor ruxolitinib, and plans to develop a trial of ruxolitinib combined with chemotherapy in relapsed ALL patients with JAK mutations. These mutations are present in a proportion of cases of so-called Ph-like ALL overexpressing CRLF2. Similarly, patients with other fusion genes activating ABL1, JAK2, and PDGFRB might be treated with ABL/PDGFRB class tyrosine kinase inhibitors[78].
The occurrence of defective immune recovery after haploidentical SCT was associated with a high risk of severe infections, which heavily affected morbidity and mortality. Post-transplant CD8-depleted donor lymphocyte infusions are feasible and promote immune reconstitution[91]. Further attempts might be directed at increasing the alloreactive potential of the transplantation. Donor lymphocyte infusions have been advocated to convert stable mixed chimerism into full chimerism and have been used successfully in patients with persistent, relapsed, or progressive disease both after conventional and nonmyeloablative SCT to exert graft-vs-tumor effects, most notably in patients with chronic myeloid leukemia[92,93].
Adoptive immuno therapy was investigated mostly in children who have a functional thymus and lower incidence of GvHD compared with adults. Donor-vs-recipient NK alloreactivity has emerged as a crucial factor for the outcome of haplo-SCT[72,73]. Genetic engineering to endow T cells with receptors that bind leukaemia cell surface antigens such as CD19 or CD22, is another promising adoptive therapy approach. Immune cells are genetically modified to express chimeric antigen receptors (CAR) that contain a target recognition domain linked to an intracellular component that activate a signalling cascade[80]. Impressive antileukemic effects have been reported using CD19-CAR constructs in pediatric patients with relapsed/refractory BCP ALL[80,94].
Relapsed ALL remains a significant challenge for pediatric oncologists. According to recent reports regarding genetic and epigenetic signatures, two different biological mechanisms seem to distinguish early vs late ALL relapse. This might partially explain their distinct behaviour, therapy response and outcome. While SCT is generally accepted as the best option as post-induction consolidation therapy for HR patients after CR2, this seems to apply to only a subgroup of patient categorized as IR. Early response evaluation in terms of MRD after reinduction therapy seems to offer the best chance to stratify IR to SCT or conventional chemotherapy and it is currently been applied by several study groups. However, leukemia-free survival remains dismally low for many patients after relapse and, despite efforts by many investigators to intensify therapy with approaches that often include SCT, outcomes for these children have changed little over time. Relapsed ALL represents the focus of considerable pediatric research and alternative treatment options exploring distinct mechanisms of action are being pursued. Given the rarity of the disease, prospective clinical trials need to be coordinated within international cooperative groups.
The author wants to thank Professor Juan Jose Ortega Aramburu for his long and fruitful dedication to childhood leukemia and for encouraging me and many others to get involved in this field.
P- Reviewer: Behfar M, Imashuku S, Ramirez M, Shimoni A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29:551-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 630] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 2. | Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131:579-587. [PubMed] |
| 3. | Teachey DT, Hunger SP. Predicting relapse risk in childhood acute lymphoblastic leukaemia. Br J Haematol. 2013;162:606-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Conter V, Aricò M, Basso G, Biondi A, Barisone E, Messina C, Parasole R, De Rossi G, Locatelli F, Pession A. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Escherich G, Horstmann MA, Zimmermann M, Janka-Schaub GE. Cooperative study group for childhood acute lymphoblastic leukaemia (COALL): long-term results of trials 82,85,89,92 and 97. Leukemia. 2010;24:298-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Kamps WA, van der Pal-de Bruin KM, Veerman AJ, Fiocco M, Bierings M, Pieters R. Long-term results of Dutch Childhood Oncology Group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia. 2010;24:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Liang DC, Yang CP, Lin DT, Hung IJ, Lin KH, Chen JS, Hsiao CC, Chang TT, Peng CT, Lin MT. Long-term results of Taiwan Pediatric Oncology Group studies 1997 and 2002 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Mitchell C, Richards S, Harrison CJ, Eden T. Long-term follow-up of the United Kingdom medical research council protocols for childhood acute lymphoblastic leukaemia, 1980-2001. Leukemia. 2010;24:406-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Möricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, Ludwig WD, Ritter J, Harbott J, Mann G. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 373] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 10. | Pui CH, Pei D, Sandlund JT, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, Campana D, Kun LE, Jeha S. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 11. | Salzer WL, Devidas M, Carroll WL, Winick N, Pullen J, Hunger SP, Camitta BA. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: a report from the children’s oncology group. Leukemia. 2010;24:355-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Schmiegelow K, Forestier E, Hellebostad M, Heyman M, Kristinsson J, Söderhäll S, Taskinen M. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010;24:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 301] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 13. | Silverman LB, Stevenson KE, O’Brien JE, Asselin BL, Barr RD, Clavell L, Cole PD, Kelly KM, Laverdiere C, Michon B. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000). Leukemia. 2010;24:320-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Tsuchida M, Ohara A, Manabe A, Kumagai M, Shimada H, Kikuchi A, Mori T, Saito M, Akiyama M, Fukushima T. Long-term results of Tokyo Children’s Cancer Study Group trials for childhood acute lymphoblastic leukemia, 1984-1999. Leukemia. 2010;24:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Tsurusawa M, Shimomura Y, Asami K, Kikuta A, Watanabe A, Horikoshi Y, Matsushita T, Kanegane H, Ohta S, Iwai A. Long-term results of the Japanese Childhood Cancer and Leukemia Study Group studies 811, 841, 874 and 911 on childhood acute lymphoblastic leukemia. Leukemia. 2010;24:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, Winick NJ, Hunger SP, Gaynon PS, Loh ML. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22:2142-2150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 459] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 17. | Freyer DR, Devidas M, La M, Carroll WL, Gaynon PS, Hunger SP, Seibel NL. Postrelapse survival in childhood acute lymphoblastic leukemia is independent of initial treatment intensity: a report from the Children’s Oncology Group. Blood. 2011;117:3010-3015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Locatelli F, Schrappe M, Bernardo ME, Rutella S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012;120:2807-2816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 19. | Ko RH, Ji L, Barnette P, Bostrom B, Hutchinson R, Raetz E, Seibel NL, Twist CJ, Eckroth E, Sposto R. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010;28:648-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Roy A, Cargill A, Love S, Moorman AV, Stoneham S, Lim A, Darbyshire PJ, Lancaster D, Hann I, Eden T. Outcome after first relapse in childhood acute lymphoblastic leukaemia - lessons from the United Kingdom R2 trial. Br J Haematol. 2005;130:67-75. [PubMed] |
| 21. | Gaynon PS, Harris RE, Altman AJ, Bostrom BC, Breneman JC, Hawks R, Steele D, Zipf T, Stram DO, Villaluna D. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children’s Oncology Group study CCG-1941. J Clin Oncol. 2006;24:3150-3156. [PubMed] |
| 22. | Reismüller B, Attarbaschi A, Peters C, Dworzak MN, Pötschger U, Urban C, Fink FM, Meister B, Schmitt K, Dieckmann K. Long-term outcome of initially homogenously treated and relapsed childhood acute lymphoblastic leukaemia in Austria--a population-based report of the Austrian Berlin-Frankfurt-Münster (BFM) Study Group. Br J Haematol. 2009;144:559-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O’Leary M, Smith FO, Reaman GH. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28:2625-2634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 772] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 24. | Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, Mann G, Hählen K, Göbel U, Klingebiel T. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Münster Group 87. J Clin Oncol. 2005;23:7942-7950. [PubMed] |
| 25. | Rivera GK, Zhou Y, Hancock ML, Gajjar A, Rubnitz J, Ribeiro RC, Sandlund JT, Hudson M, Relling M, Evans WE. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer. 2005;103:368-376. [PubMed] |
| 26. | Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, Ebell W, Escherich G, Schrappe M, Klingebiel T. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 27. | Reismüller B, Peters C, Dworzak MN, Pötschger U, Urban C, Meister B, Schmitt K, Dieckmann K, Gadner H, Attarbaschi A. Outcome of children and adolescents with a second or third relapse of acute lymphoblastic leukemia (ALL): a population-based analysis of the Austrian ALL-BFM (Berlin-Frankfurt-Münster) study group. J Pediatr Hematol Oncol. 2013;35:e200-e204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 28. | Raetz EA, Borowitz MJ, Devidas M, Linda SB, Hunger SP, Winick NJ, Camitta BM, Gaynon PS, Carroll WL. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children’s Oncology Group Study[corrected]. J Clin Oncol. 2008;26:3971-3978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (3)] |
| 30. | Parker C, Waters R, Leighton C, Hancock J, Sutton R, Moorman AV, Ancliff P, Morgan M, Masurekar A, Goulden N. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376:2009-2017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 31. | Eckert C, von Stackelberg A, Seeger K, Groeneveld TW, Peters C, Klingebiel T, Borkhardt A, Schrappe M, Escherich G, Henze G. Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia - long-term results of trial ALL-REZ BFM P95/96. Eur J Cancer. 2013;49:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Chessells JM, Veys P, Kempski H, Henley P, Leiper A, Webb D, Hann IM. Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol. 2003;123:396-405. [PubMed] |
| 33. | Saarinen-Pihkala UM, Heilmann C, Winiarski J, Glomstein A, Abrahamsson J, Arvidson J, Békássy AN, Forestier E, Jonmundsson G, Schroeder H. Pathways through relapses and deaths of children with acute lymphoblastic leukemia: role of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol. 2006;24:5750-5762. [PubMed] |
| 34. | Malempati S, Gaynon PS, Sather H, La MK, Stork LC. Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children’s Oncology Group study CCG-1952. J Clin Oncol. 2007;25:5800-5807. [PubMed] |
| 35. | Lawson SE, Harrison G, Richards S, Oakhill A, Stevens R, Eden OB, Darbyshire PJ. The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: a report on the medical research council UKALLR1 study. Br J Haematol. 2000;108:531-543. [PubMed] |
| 36. | Thomson B, Park JR, Felgenhauer J, Meshinchi S, Holcenberg J, Geyer JR, Avramis V, Douglas JG, Loken MR, Hawkins DS. Toxicity and efficacy of intensive chemotherapy for children with acute lymphoblastic leukemia (ALL) after first bone marrow or extramedullary relapse. Pediatr Blood Cancer. 2004;43:571-579. [PubMed] |
| 37. | Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013;14:e205-e217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 334] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 38. | van den Berg H, de Groot-Kruseman HA, Damen-Korbijn CM, de Bont ES, Schouten-van Meeteren AY, Hoogerbrugge PM. Outcome after first relapse in children with acute lymphoblastic leukemia: a report based on the Dutch Childhood Oncology Group (DCOG) relapse all 98 protocol. Pediatr Blood Cancer. 2011;57:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | von Stackelberg A, Hartmann R, Bührer C, Fengler R, Janka-Schaub G, Reiter A, Mann G, Schmiegelow K, Ratei R, Klingebiel T. High-dose compared with intermediate-dose methotrexate in children with a first relapse of acute lymphoblastic leukemia. Blood. 2008;111:2573-2580. [PubMed] |
| 40. | Barredo JC, Devidas M, Lauer SJ, Billett A, Marymont M, Pullen J, Camitta B, Winick N, Carroll W, Ritchey AK. Isolated CNS relapse of acute lymphoblastic leukemia treated with intensive systemic chemotherapy and delayed CNS radiation: a pediatric oncology group study. J Clin Oncol. 2006;24:3142-3149. [PubMed] |
| 41. | Hagedorn N, Acquaviva C, Fronkova E, von Stackelberg A, Barth A, zur Stadt U, Schrauder A, Trka J, Gaspar N, Seeger K. Submicroscopic bone marrow involvement in isolated extramedullary relapses in childhood acute lymphoblastic leukemia: a more precise definition of “isolated” and its possible clinical implications, a collaborative study of the Resistant Disease Committee of the International BFM study group. Blood. 2007;110:4022-4029. [PubMed] |
| 42. | Borgmann A, von Stackelberg A, Hartmann R, Ebell W, Klingebiel T, Peters C, Henze G. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003;101:3835-3839. [PubMed] |
| 43. | Kolb EA, Steinherz PG. A new multidrug reinduction protocol with topotecan, vinorelbine, thiotepa, dexamethasone, and gemcitabine for relapsed or refractory acute leukemia. Leukemia. 2003;17:1967-1972. [PubMed] |
| 44. | Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:257-268. [PubMed] |
| 45. | Yang JJ, Bhojwani D, Yang W, Cai X, Stocco G, Crews K, Wang J, Morrison D, Devidas M, Hunger SP. Genome-wide copy number profiling reveals molecular evolution from diagnosis to relapse in childhood acute lymphoblastic leukemia. Blood. 2008;112:4178-4183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 46. | Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA, Downing JR. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322:1377-1380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 634] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 47. | Bailey LC, Lange BJ, Rheingold SR, Bunin NJ. Bone-marrow relapse in paediatric acute lymphoblastic leukaemia. Lancet Oncol. 2008;9:873-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Bhojwani D, Kang H, Moskowitz NP, Min DJ, Lee H, Potter JW, Davidson G, Willman CL, Borowitz MJ, Belitskaya-Levy I. Biologic pathways associated with relapse in childhood acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2006;108:711-717. [PubMed] |
| 49. | Hogan LE, Meyer JA, Yang J, Wang J, Wong N, Yang W, Condos G, Hunger SP, Raetz E, Saffery R. Integrated genomic analysis of relapsed childhood acute lymphoblastic leukemia reveals therapeutic strategies. Blood. 2011;118:5218-5226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 50. | Ford AM, Fasching K, Panzer-Grümayer ER, Koenig M, Haas OA, Greaves MF. Origins of “late“ relapse in childhood acute lymphoblastic leukemia with TEL-AML1 fusion genes. Blood. 2001;98:558-564. [PubMed] |
| 51. | Zuna J, Ford AM, Peham M, Patel N, Saha V, Eckert C, Köchling J, Panzer-Grümayer R, Trka J, Greaves M. TEL deletion analysis supports a novel view of relapse in childhood acute lymphoblastic leukemia. Clin Cancer Res. 2004;10:5355-5360. [PubMed] |
| 52. | Staal FJ, de Ridder D, Szczepanski T, Schonewille T, van der Linden EC, van Wering ER, van der Velden VH, van Dongen JJ. Genome-wide expression analysis of paired diagnosis-relapse samples in ALL indicates involvement of pathways related to DNA replication, cell cycle and DNA repair, independent of immune phenotype. Leukemia. 2010;24:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Szczepanski T, van der Velden VH, Waanders E, Kuiper RP, Van Vlierberghe P, Gruhn B, Eckert C, Panzer-Grümayer R, Basso G, Cavé H. Late recurrence of childhood T-cell acute lymphoblastic leukemia frequently represents a second leukemia rather than a relapse: first evidence for genetic predisposition. J Clin Oncol. 2011;29:1643-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 54. | Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, Ma J, Liu W, Cheng C, Schulman BA. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360:470-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1174] [Cited by in RCA: 1110] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 55. | Mullighan CG, Zhang J, Harvey RC, Collins-Underwood JR, Schulman BA, Phillips LA, Tasian SK, Loh ML, Su X, Liu W. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2009;106:9414-9418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 432] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 56. | Harvey RC, Mullighan CG, Chen IM, Wharton W, Mikhail FM, Carroll AJ, Kang H, Liu W, Dobbin KK, Smith MA. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115:5312-5321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 434] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 57. | Eapen M, Raetz E, Zhang MJ, Muehlenbein C, Devidas M, Abshire T, Billett A, Homans A, Camitta B, Carroll WL. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107:4961-4967. [PubMed] |
| 58. | Bader P, Hancock J, Kreyenberg H, Goulden NJ, Niethammer D, Oakhill A, Steward CG, Handgretinger R, Beck JF, Klingebiel T. Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia. 2002;16:1668-1672. [PubMed] |
| 59. | Knechtli CJ, Goulden NJ, Hancock JP, Grandage VL, Harris EL, Garland RJ, Jones CG, Rowbottom AW, Hunt LP, Green AF. Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood. 1998;92:4072-4079. [PubMed] |
| 60. | Coustan-Smith E, Gajjar A, Hijiya N, Razzouk BI, Ribeiro RC, Rivera GK, Rubnitz JE, Sandlund JT, Andreansky M, Hancock ML. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia after first relapse. Leukemia. 2004;18:499-504. [PubMed] |
| 61. | Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, Barth A, Borkhardt A, Peters C, Handgretinger R. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 280] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 62. | Paganin M, Zecca M, Fabbri G, Polato K, Biondi A, Rizzari C, Locatelli F, Basso G. Minimal residual disease is an important predictive factor of outcome in children with relapsed ‘high-risk’ acute lymphoblastic leukemia. Leukemia. 2008;22:2193-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Leung W, Pui CH, Coustan-Smith E, Yang J, Pei D, Gan K, Srinivasan A, Hartford C, Triplett BM, Dallas M. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012;120:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 64. | Pichler H, Reismüller B, Steiner M, Dworzak MN, Pötschger U, Urban C, Meister B, Schmitt K, Panzer-Grümayer R, Haas OA. The inferior prognosis of adolescents with acute lymphoblastic leukaemia (ALL) is caused by a higher rate of treatment-related mortality and not an increased relapse rate--a population-based analysis of 25 years of the Austrian ALL-BFM (Berlin-Frankfurt-Münster) Study Group. Br J Haematol. 2013;161:556-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Aricò M, Schrappe M, Hunger SP, Carroll WL, Conter V, Galimberti S, Manabe A, Saha V, Baruchel A, Vettenranta K. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol. 2010;28:4755-4761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 66. | Abshire TC, Pollock BH, Billett AL, Bradley P, Buchanan GR. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: a Pediatric Oncology Group Study. Blood. 2000;96:1709-1715. [PubMed] |
| 67. | von Stackelberg A, Völzke E, Kühl JS, Seeger K, Schrauder A, Escherich G, Henze G, Tallen G. Outcome of children and adolescents with relapsed acute lymphoblastic leukaemia and non-response to salvage protocol therapy: a retrospective analysis of the ALL-REZ BFM Study Group. Eur J Cancer. 2011;47:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 68. | Dini G, Zecca M, Balduzzi A, Messina C, Masetti R, Fagioli F, Favre C, Rabusin M, Porta F, Biral E. No difference in outcome between children and adolescents transplanted for acute lymphoblastic leukemia in second remission. Blood. 2011;118:6683-6690. [PubMed] |
| 69. | Harrison G, Richards S, Lawson S, Darbyshire P, Pinkerton R, Stevens R, Oakhill A, Eden OB. Comparison of allogeneic transplant versus chemotherapy for relapsed childhood acute lymphoblastic leukaemia in the MRC UKALL R1 trial. MRC Childhood Leukaemia Working Party. Ann Oncol. 2000;11:999-1006. [PubMed] |
| 70. | Smith AR, Baker KS, Defor TE, Verneris MR, Wagner JE, Macmillan ML. Hematopoietic cell transplantation for children with acute lymphoblastic leukemia in second complete remission: similar outcomes in recipients of unrelated marrow and umbilical cord blood versus marrow from HLA matched sibling donors. Biol Blood Marrow Transplant. 2009;15:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Chen X, Hale GA, Barfield R, Benaim E, Leung WH, Knowles J, Horwitz EM, Woodard P, Kasow K, Yusuf U. Rapid immune reconstitution after a reduced-intensity conditioning regimen and a CD3-depleted haploidentical stem cell graft for paediatric refractory haematological malignancies. Br J Haematol. 2006;135:524-532. [PubMed] |
| 72. | Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, Stern M, Pende D, Perruccio K, Burchielli E. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433-440. [PubMed] |
| 73. | Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, Romeo E, Cognet C, Martinetti M, Maccario R. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113:3119-3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 74. | Klingebiel T, Cornish J, Labopin M, Darbyshire P, Handgretinger R, Balduzzi A, Owoc-Lempach J, Fagioli F, Or R, Peters C, Aversa F, Polge E, Dini G, Rocha V; Pediatric Diseases and Acute Leukemia Working Parties of the European Group for Blood and Marrow Transplantation (EBMT). Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood. 2010;115:3437-3446. [RCA] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 75. | Lang P, Handgretinger R. Haploidentical SCT in children: an update and future perspectives. Bone Marrow Transplant. 2008;42 Suppl 2:S54-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Hahn T, Wall D, Camitta B, Davies S, Dillon H, Gaynon P, Larson RA, Parsons S, Seidenfeld J, Weisdorf D. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in children: an evidence-based review. Biol Blood Marrow Transplant. 2005;11:823-861. [PubMed] |
| 77. | Harker-Murray PD, Thomas AJ, Wagner JE, Weisdorf D, Luo X, DeFor TE, Verneris MR, Dusenbery KE, MacMillan ML, Tolar J. Allogeneic hematopoietic cell transplantation in children with relapsed acute lymphoblastic leukemia isolated to the central nervous system. Biol Blood Marrow Transplant. 2008;14:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Hunger SP, Loh ML, Whitlock JA, Winick NJ, Carroll WL, Devidas M, Raetz EA. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 79. | Handgretinger R, Zugmaier G, Henze G, Kreyenberg H, Lang P, von Stackelberg A. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia. 2011;25:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 80. | Fry TJ, Mackall CL. T-cell adoptive immunotherapy for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2013;2013:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Jeha S. Recent progress in the treatment of acute lymphoblastic leukemia: clofarabine. Hematol Oncol Clin North Am. 2009;23:1137-1144, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | O’Connor D, Sibson K, Caswell M, Connor P, Cummins M, Mitchell C, Motwani J, Taj M, Vora A, Wynn R. Early UK experience in the use of clofarabine in the treatment of relapsed and refractory paediatric acute lymphoblastic leukaemia. Br J Haematol. 2011;154:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Dunsmore KP, Devidas M, Linda SB, Borowitz MJ, Winick N, Hunger SP, Carroll WL, Camitta BM. Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2753-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Raetz EA, Cairo MS, Borowitz MJ, Blaney SM, Krailo MD, Leil TA, Reid JM, Goldenberg DM, Wegener WA, Carroll WL. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children’s Oncology Group Pilot Study. J Clin Oncol. 2008;26:3756-3762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 85. | Raetz EA, Cairo MS, Borowitz MJ, Lu XM, Devidas M, Reid JM, Goldenberg DM, Wegener WA, Whitlock JA, Adamson PC, Hunger SP, Carroll WL Reinduction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL) in children, adolescents, and young adults: Results from Children’s Oncology Group (COG) study ADVL04P2. Blood (ASH Annual Meeting Abstracts). 2011;118:573a. |
| 86. | Messinger Y, Gaynon P, Raetz E, Hutchinson R, Dubois S, Glade-Bender J, Sposto R, van der Giessen J, Eckroth E, Bostrom BC. Phase I study of bortezomib combined with chemotherapy in children with relapsed childhood acute lymphoblastic leukemia (ALL): a report from the therapeutic advances in childhood leukemia (TACL) consortium. Pediatr Blood Cancer. 2010;55:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 87. | Messinger YH, Gaynon PS, Sposto R, van der Giessen J, Eckroth E, Malvar J, Bostrom BC. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & amp; Lymphoma (TACL) Study. Blood. 2012;120:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 88. | Fuster JL, Bermúdez M, Galera A, Llinares ME, Calle D, Ortuño FJ. Imatinib mesylate in combination with chemotherapy in four children with de novo and advanced stage Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2007;92:1723-1724. [PubMed] |
| 89. | Millot F, Cividin M, Brizard F, Chomel JC, Méchinaud F, Guilhot F. Successful second allogeneic stem cell transplantation in second remission induced by dasatinib in a child with Philadelphia chromosome positive acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;52:891-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Chillón MC, Gómez-Casares MT, López-Jorge CE, Rodriguez-Medina C, Molines A, Sarasquete ME, Alcoceba M, Miguel JD, Bueno C, Montes R. Prognostic significance of FLT3 mutational status and expression levels in MLL-AF4+ and MLL-germline acute lymphoblastic leukemia. Leukemia. 2012;26:2360-2366. [PubMed] |
| 91. | Dodero A, Carniti C, Raganato A, Vendramin A, Farina L, Spina F, Carlo-Stella C, Di Terlizzi S, Milanesi M, Longoni P. Haploidentical stem cell transplantation after a reduced-intensity conditioning regimen for the treatment of advanced hematologic malignancies: posttransplantation CD8-depleted donor lymphocyte infusions contribute to improve T-cell recovery. Blood. 2009;113:4771-4779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 92. | Bethge WA, Hegenbart U, Stuart MJ, Storer BE, Maris MB, Flowers ME, Maloney DG, Chauncey T, Bruno B, Agura E. Adoptive immunotherapy with donor lymphocyte infusions after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Blood. 2004;103:790-795. [PubMed] |
| 93. | Peggs KS, Mackinnon S. Cellular therapy: donor lymphocyte infusion. Curr Opin Hematol. 2001;8:349-354. [PubMed] |
| 94. | Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2818] [Article Influence: 216.8] [Reference Citation Analysis (0)] |