Peer-review started: August 24, 2016
First decision: October 8, 2016
Revised: November 16, 2016
Accepted: January 20, 2017
Article in press: January 21, 2017
Published online: May 2, 2017
Processing time: 244 Days and 15.9 Hours
To evaluate the efficacy of Nexobrid® in the initial management of burns and lessons learned with the procedure.
From January 27th 2015 until January 25th 2016, 25 patients aged between 18-94 years old with deep partial and full thickness burns were treated with Nexobrid® covering 1%-30% of their total body surface area (TBSA). The debridement was applied in the first 96 h post-injury following the protocol suggested for Nexobrid®. In patients with burns of more than 15% TBSA a second application of Nexobrid® was performed. After the removal of the product - 4 h post application and after a 2 h period of wet dressing - we used several products to cover the wound like Suprathel®, Biobrane®, Mepitel® with wet dressing, silver sulphadiazine 1% cream, and in some cases even autografts. We treated patients with inhalation injury as well. All the procedures were done under deep sedation, regional blocks in extremities or general anaesthesia in the intensive care unit room or in the operating theatre.
After these first 25 cases, we have observed that patients with partial thickness burns treated with Nexobrid®, experienced great benefits in the reduction of the need for autografting compared with the standard of care. This is because after selective enzymatic debriding of the burn scar we can distinguish different wound beds, which can coexist in the same patient, and we also managed to associate each one to its ability to epithelize. In major burns, besides the improvement in wound healing, we observed an important improvement in their general state. This may be because SIRS significantly improved through a bloodless debridement of necrotic tissue, decreasing the requirements of vasoactive drugs and fluid resuscitation. Circumferential burns also benefited from enzymatic debridement, observing a decrease in the number of compartment syndromes and the need for escharotomies. At present, we have not observed a positive effect in the evolution and outcome of major burns with inhalation injury.
The introduction of Nexobrid® shows significant improvement in burn treatment. Cumulative experiences are necessary to adapt its application in all Burns Centres.
Core tip: Burns continue to be a common injury in western countries. It is difficult to assess the severity of burns, but knowing its mechanisms as well as its characteristics can be of help. An alternative to surgical debridement is the enzymatic or chemical debridement, although past reports that used it with patients show that its efficiency is limited. Nexobrid® is a new enzymatic debridement agent. We show our learning curve with it.
- Citation: Palao R, Aguilera-Sáez J, Serracanta J, Collado JM, Dos Santos BP, Barret JP. Use of a selective enzymatic debridement agent (Nexobrid®) for wound management: Learning curve. World J Dermatol 2017; 6(2): 32-41
- URL: https://www.wjgnet.com/2218-6190/full/v6/i2/32.htm
- DOI: https://dx.doi.org/10.5314/wjd.v6.i2.32
When a thermic, electric or chemical aggression acts in the skin for the necessary time and with enough intensity, then a local injury known as burn occurs. Nowadays, burns continue to be a common injury in western countries. Many of these burns will need urgent medical attention, hospital admission, and even surgery[1]. It is difficult to assess the severity of burns, but knowing its mechanisms as well as its characteristics can be of help. Nonetheless, the majority of burns present with different degrees in all it’s extension through the skin. Consequently, it can be extremely complicated to differentiate between a superficial partial thickness burn and a deep partial thickness one[2,3]. The diagnosis of burn depth is really important in order to plan the treatment. While the epidermal and superficial partial thickness burns will heal spontaneously, the deep partial thickness as well as the full thickness burns will need surgery[4]. For the latter, the tangential debridement followed by the coverage with skin autograft within the first five days after suffering the injury, is considered to be the current standard of care[5-7]. An alternative to such surgical debridement is the enzymatic or chemical debridement, although past reports that used it with patients show that its efficiency is limited, it is a slow process and increases the infection risk due to maceration of necrotic tissue[8]. Since 2004 many articles have been published on the use, efficiency and security that NexoBrid® (enzymatic debridement) provides. NexoBrid® consists of a mixture of proteolytic enzymes enriched in bromelain, which is extracted from the stem of the pineapple plant[9-13]. The purpose of this manuscript is to share the experience acquired during the learning process with this enzymatic debridement used in the Burns Unit of Vall d’Hebron University Hospital at Barcelona.
The Burns Unit of Vall d’Hebron University Hospital at Barcelona is the top reference center for Catalonia, Balearic Islands (Spain) and Andorra. Every year we receive approximately between 400 and 500 burns related entries. We also receive, but to a lesser extent, other entries due to exfoliative skin illnesses.
We considered initial entries of the learning curve the first 25 cases, which received the enzymatic debridement treatment. These were done between January of 2015 and January of 2016. We worked with the enzymatic debridement in the circumstances where there was at least an intermediate partial thickness burn with an extension not above 15%, according to the product indication, unless we organized the therapy in two separated days if the patient achieved a total enzymatic debridement of 30% of its total body surface area. Based on our experience we now have our own recommendations regarding its indications based on our learning and when not to use it. As it is a painful process pain management is indicated. We do this particularly at the extremities in which we can perform locoregional nerve block, or we can also do it either in the extremities or trunk if the patient has been intubated with sedoanalgesia for whatever reason.
The technique is always performed in a sterile environment, either the theater or the same room for major burns, removing devitalized tissue. Then, we will proceed to mix both components of the product, creating the Nexobrid® gel. After that, we will delimit the area to treat with a trail of Vaseline in order to avoid spillage of the product out of the treatment area. Once we have done this, we will proceed to use the cream - creating a uniform fine layer of 1.5-3 mm thick - over the area of the body that we want to debride. Afterwards, we will cover the extremity with a transparent plastic wrap so that the lotion will not move during the 4 h that the product needs in order to work. Always remember that we must try to maintain a uniform layer of gel when we cover the extremity with the plastic wrap. After the 4 h we remove the product as well as the plastic wrap, and the rest of necrotic tissue, vaseline, exudate and bleeding. We do this with an intense brushing or scratching with the scraper. Then, we apply a wet dressing with normal saline solution 0.9% that we will leave until the next day. Once we remove the wet dressing we must apply a new one that varies according to the appearance of the treated wound bed. In the Discussion we elaborate on what we use in each step.
Between January 2015 and January 2016 we treated 25 patients (Table 1), 19 of them were men, 6 women. Their mean age was 52.6 years old (ranging from 19 to 94 years old). The etiology of their burns was flame in 11 cases, deflagration in 5 cases, scald in 5 cases, electrical flash in 1 case, chemical burn in another one, abrasion in 1 case, and finally one case of contact burn. The mean burned body surface area was 14.4% (ranging from 1% to 65% TBSA).
| Patient | Age | Sex | Etiology | Extension(%) | Depth | Time to escarectomy (d) | Treated area | Predebridement dressing | Postdebridement dressing | Surgery | Time to complete healing (d) |
| 1 | 24 | W | Scald | 2 | DPT | 4 | Foot | SSD | Mepitel one | Yes | 35 |
| 2 | 31 | M | Flame | 20 | SPT and DPT | 1 | Hands and lower limbs | SSD | Mepitel one | No | 21 |
| 3 | 33 | M | Flame | 60 | DPT and FT | 0 | Trunk and upper limbs and foot | SSD | Mepitel one | Yes | 15 |
| 4 | 46 | M | Chemical | 6 | DPT and FT | 4 | Trunk | SSD | Biobrane | Yes | 19 |
| 5 | 90 | M | Flame | 4 | DPT and FT | 2 | Forearm and foot | SSD | Suprathel | Yes | 33 |
| 6 | 58 | M | Flame | 53 | DPT and FT | 0 | Hands and lower limbs | SSD | Biobrane | Yes | Exitus |
| 7 | 18 | W | Scald | 2 | SPT and DPT | 1 | Lower limbs | WD | Suprathel | Yes | 30 |
| 8 | 64 | M | Scald | 10 | MPT | 3 | Lower limbs | SSD | Suprathel | No | 14 |
| 9 | 58 | M | Contact | 1 | SPT and DPT | 3 | Foot | WD | Suprathel | Yes | 20 |
| 10 | 44 | M | Deflagration | 15 | SPT and DPT | 1 | Upper limbs | WD | Suprathel | No | 27 |
| 11 | 69 | M | Deflagration | 8 | SPT and DPT | 4 | Upper limbs | WD | Suprathel | No | 21 |
| 12 | 51 | M | Scald | 2 | SPT and DPT | 2 | Upper limbs | Nitrofurazone | Biobrane | Yes | 51 |
| 13 | 21 | M | Flame | 10 | SPT and DPT | 1 | Upper limbs | WD | Aquacel Ag | No | 16 |
| 14 | 56 | M | Flash | 5 | MPT and DPT | 4 | Hands | WD | Suprathel | No | 19 |
| 15 | 40 | M | Deflagration | 6 | SPT and DPT | 2 | Upper limbs | WD | SSD | No | 22 |
| 16 | 94 | W | Flame | 10 | DPT and FT | 4 | Trunk and upper limbs | WD | SSD | Yes | 21 |
| 17 | 81 | M | Flame | 2 | SPT and FT | 2 | Lower limb | WD | WD | Yes | 31 |
| 19 | 72 | W | Deflagration | 14 | MPT | 1 | Lower limbs | WD | Suprathel | Yes | 27 |
| 20 | 38 | M | Deflagration | 7 | DPT | 1 | Hands | WD | Biobrane | Yes | 38 |
| 21 | 63 | M | Flame | 65 | MPT and FT | 0 | Lower limbs | SSD change to WD before 24 h | Mepitel and Nitrofurazone | Yes | 23 |
| 22 | 46 | W | Scald | 4 | MPT | 2 | Lower limb | WD | Suprathel | No | 37 |
| 23 | 62 | M | Flame | 18 | SPT and DPT | 1 | Upper and lower limbs | WD | Aquacel Ag | Yes | 36 |
| 24 | 56 | M | Flame | 28 | SPT and DPT | 2 | Lower limbs | WD | SSD | Yes | EXITUS |
| 25 | 38 | W | Abrasion | 1 | DPT | 2 | Hand | WD | Aquacel Ag | No | 17 |
In all cases patients received enzymatic debridement treatment in their extremities, and 3 cases also required enzymatic debridement treatment of their anterior trunk. As mentioned previously, this was done within 4 d of injury. It is important to highlight that in most cases the treatment was successful (it didn’t go well in 5 cases). The reason was 4 of the cases had received local silver sulphadiazine 1% cream during more than 24 h before being treated with the enzymatic debridement. This caused the consolidation of the characteristic pseudo-eschar that this cream forms. This explains why it was unsuccessful. In the fifth case, we believe that the treatment didn’t work due to the use of an insufficient amount of the product.
Pre-Nexobrid® treatment, local dressing employed was silver sulphadiazine 1% cream in 9 cases. As mentioned, in 4 of those this was used for more than 24 h which lead to incomplete eschar debridement. In the rest of patients the enzymatic debridement was performed within the first 24 h (in 3 of them), or wound dressing was replaced with wet dressing also within the first 24 h (2 patients). In 15 cases a wet dressing of normal saline solution and chlorhexidine was used. In one case, initial dressing was silicone sheets and nitrofurazone cream. The mean burned area treated with enzymatic debridement was 6.5% (ranging from 1% to 30%).
We essayed with a wide range of possibilities regarding local dressings once we have already performed the enzymatic debridement, and also after placing wet dressing: Suprathel® (9 cases), Biobrane® (4 cases), silver sulphadiazine (3 cases), Aquacel Ag® (3 cases), Mepitel one® (3 cases), Mepitel® and nitrofuazone cream (1 case), wet dressing (1 case).
Fifteen patients underwent surgery to cover the debrided areas with skin grafts. The other 10 patients showed spontaneous epithelialization. Those patients who needed surgery (apart from the 4 patients that died) healed within an average of 30.45 d, whereas those who didn’t need surgery within 22.30 d.
Each patient has been followed-up until today, so they are still receiving the same post-burn care than the patients that have been treated with the standard treatment (tangential debridement plus skin graft coverage). The post-burn care means: Use of silicone patches, pressure therapy, massages, hydration, rehabilitation, and so on. As mentioned, 4 patients died, 3 of them because of a bad systemic evolution that caused a multiple organ failure, while the other death was due to a respiratory insufficiency in the context of smoke inhalation that caused burn in the air way.
When a new technique is first introduced we always need a period of time in order to learn its different new aspects. Once we verified the debriding capacity of the product, and with the knowledge of the publications on it, then we had some doubts about which where the most suitable circumstances in which we should use the product, how to assess the wound bed after the procedure or which local dressings to apply on it, that’s what we want to discuss here from our experience.
In our opinion, this product should be used to treat circumferential burns that can lead to a compartment syndrome; intermediate and deep partial thickness burns as well as burns where there are different degrees, full thickness burns. We consider that children, elderly people and major burns victims, could specially benefit from this procedure for their specific circumstances. Now we will proceed to elaborate on the benefits of using the product in each of the circumstances mentioned above.
Compartment syndrome: When there is a suspicion that the patient is suffering a compartment syndrome in any of the extremities it is, in our opinion, one of the clearest cases in which we should use the product. It has been proved that the first 30 min after employing the product the compartment pressure descends below 30 mmHg. This descent will stand even after removing the product[10]. Eliminating the secondary rigid eschar from the circumferential burn will allow the expansion of the muscles, which consequently will eliminate the possibility of suffering compartment syndrome[12]. None of the 25 patients that we debrided with such technique required surgical escharotomy. It is true that the surgical escharotomy effects are immediate in relation to the compartment pressure fall, however we also need to highlight that an expert surgeon as well as basic surgical staff are needed. Sometimes the expert surgeon can’t treat victims fast enough, which subsequently will cause the victims to suffer irreversible pain. Furthermore, surgical escharotomy is not exempted from possible complications such as haemorrhages, loss of fluids, subcutaneous tissue infection, and so on[10].
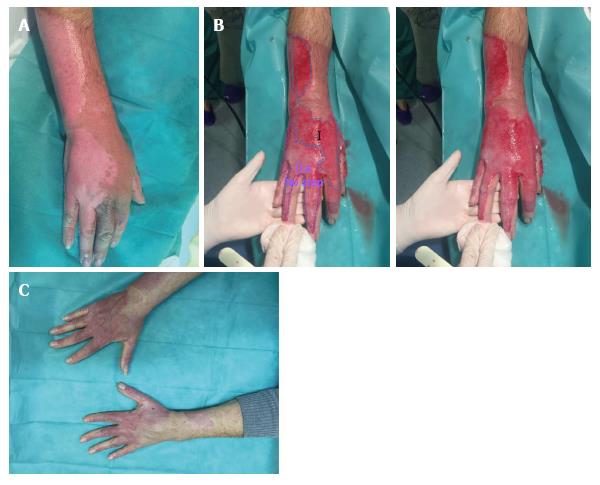
Partial and full thickness burns: NexoBrid® can help us diagnose intermediate partial thickness burns. These burns are the ones that due to their appearance are difficult to classify upon initial presentation. This also occurs with superficial partial thickness burns and the deep partial thickness ones. The clinical assessment performed by an expert surgeon on the depth of a burn is only accurate in two-thirds of the examined cases[14]. There are some equipment that help us to differentiate burn depth, such as Laser Doppler Imaging, which allows to clearly differentiate depth, because it informs about the status of the dermic vascularization with an positive predictive value around 90%[14,15]. However, NexoBrid® has a considerable advantage against this equipment, as it shows the wound bed itself thus facilitating diagnosis, at the same time we are doing the escharectomy. Another advantage of the product is that it reduces the time elapsed between the burn injury and complete burn eschar removal[13]. Later we will talk about how to assess the wound bed after enzymatic debridement. Nowadays there are no publications that exactly delineate the interpretation of the wound bed in intermediate partial thickness burns.
Children and elderly people: In children and elderly people we tend to be more conservative in the surgical aspects as they are more delicate patients than others. Both types of patients have less epithelial reserve, while only elderly people have commonly associated comorbidities[16,17]. We believe that Nexobrid® is an ideal medical tool. First of all, it allows us to predict if the burn will need to be treated in a conservative manner or a surgical debridement is necessary, and it also let us know how to handle such burn (conservative or surgical treatment). What is more, the extension of the area treated will be smaller in comparison to standard therapy[12,13]. If we limit the surgical areas, we will consequently limit the extension of the donor site, where we obtain the skin graft that will cover the debrided part of the body[13]. Moreover, if we manage to avoid surgical debridement bleeding will also be avoided[13]. However, we must say that no child has participated in this research in our Unit.
Major burn victims: In relation to major burn patients, we think that an early enzymatic debridement can contribute many benefits to them. Firstly, we consider that by reducing the time until the complete escharotomy, we are also reducing the amount of time in which the body is producing cytokines and inflammatory mediators as a systemic response to the burn[18,19]. So, we believe that the syndrome caused by the systemic inflammatory response will be minor. Additionally, early removal of the necrotic tissue can lead to a reduced incidence of sepsis. There are no publications that support our arguments, but still we consider that it is a reasonable argument that needs to be studied in the future. Finally, it is also important to say that as these patients tend to show poor overall state, we believe that by limiting surgical aggression from tangential debridement this will be beneficial to them.
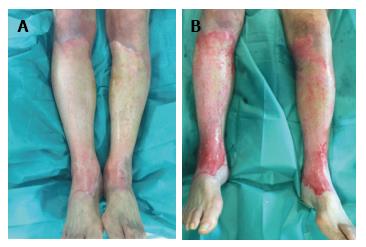
On the other hand, we will not follow this principle in superficial partial thickness burns, as it doesn’t result in any benefit. We also will not use it in electric burns because they have a complex injury mechanism and employing it would be useless. It also won’t be used in small burns where the cost outweights the benefits. We will not employ it in burns that had been treated during more than 24 h with silver sulphadizine cream or dressings with silver as the pseudo-eschar formed over the burn limits the debriding action of the product, as is shown in Figure 1. We can support this information with the other experiences present in the bibliography[9]. However, if the silver sulphadiazine has been working less than 24 h, then we will proceed to remove the pseudo-eschar through an intense scratching (with a surgical brush or scraper), and after that we will use Nexobrid®.
After 24 h the pseudo-eschar cannot be completely removed. This is the reason why we apply a wet dressing with normal saline 0.9% to the patients that we initially believe can benefit from enzymatic debridement (Nexobrid®), while we wait.
As previously stated, we believe Nexobrid® is a great tool for proper diagnosis of burn depth. Actually, the selective debridement allows us to perform a precise diagnosis of the total depth of the burn, through a direct view of the skin structure and vascular patterns[12].
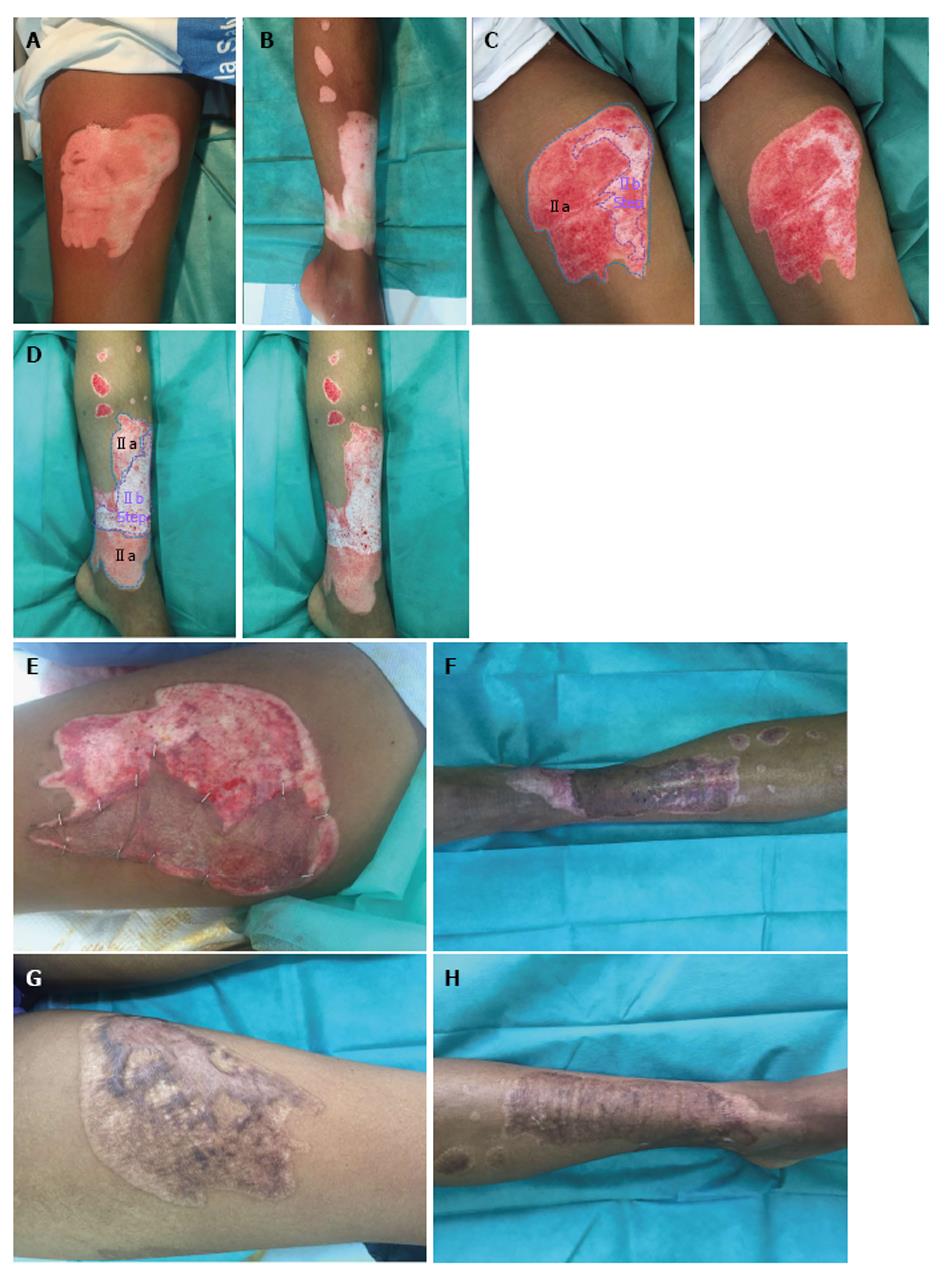
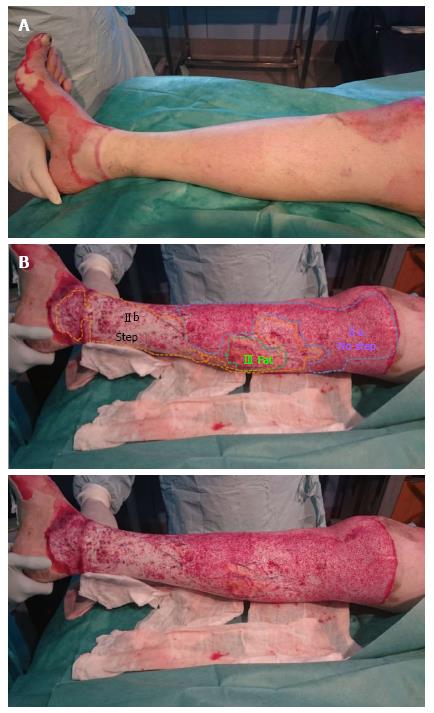
The current publications recommend grafting as soon as possible those wound beds that do not present valid dermic remainders; while those valid dermic remainders will be protected with an allograft or with a dressing that will consequently allow the epithelisation during 3 wk, the time in which the areas that remain denuded will be then grafted[12]. This has been one of the most difficult aspects to understand, as until now we associated a white wound bed without much bleeding to a deep dermic burn. But there is nothing in relation to the pre-debridement inspection with the post Nexobrid® debridement one. However, when we were reviewing our clinical cases we managed to distinguish different dermic wound beds after performing enzymatic debridement with Nexobrid®, and we also managed to associate each one to its ability to epithelize. So, after using Nexobrid® we can face four different wound beds, which obviously can coexist in the same patient, because a burn can have different depths. Now we are going to analyse the different wound beds (Table 2).
| Wound bed | Appearance | Depression | Type of burn |
| Type I | Abundance of small diameter pin-point bleeders (uniform shades of red to pink) | No | Superficial PT |
| Type IIa | Sparse pattern of larger diameter bleeders (irregular shades of pink to white) | No | Mid/deep PT |
| Type IIb | Sparse pattern of larger diameter bleeders (no shades, white colour) | Yes | Deep PT |
| Type III | Fatty | Yes | PT |
Type I: Dermic wound bed with abundance of small diameter pin-point bleeders (uniform shades of red to pink). This bed corresponds to a superficial dermic burn (Figure 2).
Type IIa: Dermic wound bed with a sparse pattern of larger diameter bleeders (irregular shades of pink to white) that it is not depressed in relation to the surrounding healthy skin, or the own bed surface skin burn. It corresponds to an intermediate/deep dermal burn, but the loss of tissue thickness cannot be macroscopically observed (Figures 2 and 3).
Type IIb: Dermic wound bed with a sparse pattern of larger diameter bleeders (no shades, white colour) that it is depressed in relation to the surrounding healthy skin, or the own bed surface skin burn. It corresponds to a deep dermal burn where the loss of its tissue thickness can be macroscopically observed (Figures 3 and 4).
Type III: Fatty wound bed. It corresponds to a full thickness burn (Figure 4).
The importance of all this, in our experience, is that the typesI and IIa will heal spontaneously within a maximum period of 30 d; whereas the types IIb and III won’t heal spontaneously over a period of 30 d, so we will consider covering the burns with skin autografts. Despite 30 d for burns, at risk of hypertrophic scarring, is more time than the classically accepted for a spontaneous epithelialization (3 wk), it has been seen that in a long-term outcome the burn scars treated with Nexobrid® are not inferior than the ones who are subjected to the standard treatment[13]. We think that two factors can have an influence on this. On the one hand, the spontaneous epithelialization in a inflammatory bed is different from the one produced in a clean dermal base, and secondly, the effectiveness of the postoperative care of these scars.
We believe that the difference between types IIa and IIb is the amount of epithelial reserve available for wound healing by reepithelialisation. When we talk about epithelial reserve, we are referring to the presence of cells capable of generating a new healthy epithelial tissue. In this case it would be the basal cells of the epidermis, which are damaged in both types (IIa as well as IIb). However, these basal cells of the epidermis are in continuity with the basal sebaceous gland cells and the cells of the outer hair shaft, as both share embryologic development. These cells have the ability to form new keratinocytes that migrate into the wound[9]. In addition, the cells of the protuberance, structure located in the hair follicle below the sebaceous glands, are pluripotential cells that can reconstitute all the epithelial lineages after suffering an insult, including the interfolicular epidermis, albeit they are not involved in the normal process of epidermal renewal. Furthermore, it has been proved that no less than 25% of the new cells that have repopulated a wound derive from this structure[20].
It’s obvious to think that in deep dermal burns healing by regeneration of a healthy epithelium will depend on the number of these structures that have survived the injury. According to our observations the beds type IIa maintain a sufficient epithelial reserve for healing, while the beds type IIb don’t (if we are macroscopically able to see a depression it is logical to think that the remaining dermis corresponds to deeper layers, and therefore is poorer in these areas).
This explanation should be taken carefully in areas of thin skin, as well as in children and elderly people who already have a thin skin. In a minor skin thickness it can be assumed that an injury that doesn’t produce this macroscopic dermal depression, if it is able to destroy the epithelial reserve so that the injury cannot heal by regeneration. Therefore, we should also be cautious in areas without hair or with patients that have suffered a hair removal treatment that removes the hair follicle. These areas will have less epithelial reserve.
Once we have done the initial assessment of the wound bed after practicing the enzymatic debridement, we must be aware that there are some factors that can have an influence in relation to the evolution of the wound healing. Factors that may lead to the stasis zone or even cause irreversible damage. These factors are infection, edema or prolonged hypotension[21]. The latter can occur to major burns patients for reasons such as distributive shock and the use of vasoactive drugs which may difficult the healing due to bad peripheral perfusion.
To sum up, once we have already performed the enzymatic debridement treatment and while we are waiting for the spontaneous healing or for the coverage with skin autografts, it is very important to remember that we must keep the wound bed wet in order to prevent it from drying out and therefore deepening, even more important than with a regular wound. As we previously stated, we will use different creams and dressings that we can easily find in our Unit: If we believe the wound bed to be type IIb or III, then we will have to do surgery, but first it is really important to previously apply to the patient some dressings to keep the wound bed hydrated. For example, dressings such as an hydrocolloid type that must be changed every 48 h until the moment that the general conditions of the patients and the availability of the operating rooms allow us to perform the surgery. If on the other hand we consider the wound bed is type I or IIa, then the injury will heal spontaneously, so that we will only have to apply a dressing that does not interfere and promotes spontaneous epithelization. At this moment, our first choice to cover the wound bed is Suprathel® and nitrofurazone cream with Mepitel®. On Suprathel® we place a non-adherent meshed dressing and finally, dry gauzes to finish the dressing. We keep this for 10 d (despite the fact that at the fifth day we should have a look and check that there haven’t been setbacks. Depending on the state of the affected area, after the 10th day we continue using the same treatment or change it. With nitrofurazone cream with Mepitel® we change the dressing every 48-72 h.
When we introduced the enzymatic debridement with Nexobrid® in our armamentarium we could only use the nitrofurazone cream in one case, because the Producer stopped the production for some months. However, now it is available again, so we have introduced its use after Nexobrid® treatment of new patients (25 patients more since January 2016, 50 patients totally), combined with a silicone meshed non-adherent dressing (Mepitel®).
Our choice, to use one or the other, depends on either if the patient is going to be treated as in-patient or as out patient. In in-patients, we prefer to apply the nitrofurazone cream combined with Mepitel®, because it favours their comfort. We change the dressings every 2-3 d, which consequently allow their cleaning. On the other hand, in out patients we prefer to apply Suprathel® because it reduces the amount of dressing changes and controls.
It is important to highlight that we have used silver sulphadiazine 1% cream, but the pseudoeschar formation promotes delayed healing and the appearance of hypertrophic tissue granulation.
When we decide that autografting is necessary in type IIB and III wound bed, we usually wait at least 3-4 d before doing it, because in our first patients some skin grafts failed when the surgery was performed in the first two days after the debridement. Our opinion is that some of the eschar and product still remain on the wound bed for several days after debridement. If we wait these days before surgery, we have removed them and then, the skin grafts intake is much better.
The introduction of the new enzymatic debridement agent Nexobrid® in our Burn Centre has shown significant improvement in wound healing in general and in the overall state of major burn patients. Cumulative experiences are necessary to adapt its utilisation to other Burn Centres.
When a thermic, electric or chemical aggression acts in the skin for the necessary time and with enough intensity, then a local injury known as burn occurs. Nowadays, burns continue to be a common injury in Western countries. Many of these burns will need urgent medical attention, hospital admission, and even surgery. The majority of burns present with different degrees in all its extension through the skin. Consequently, it can be extremely complicated to differentiate between a superficial partial thickness burn and a deep partial thickness one. The diagnosis of burn depth is really important in order to plan the treatment. While the epidermal and superficial partial thickness burns will heal spontaneously, the deep partial thickness as well as the full thickness burns will need surgery. For the latter, the tangential debridement followed by coverage with skin autograft is considered to be the current standard of care. An alternative to surgical debridement is enzymatic or chemical debridement, although past reports that used it on patients show that its efficiency is limited, it is a slow process and increases the infection risk due to maceration of necrotic tissue. The introduction of a new selective enzymatic debridement agent (Nexobrid®) in armamentarium for burn wound management has provided us some new concepts in the initial management and wound healing as well. In this study, the authors show learning curve of the procedure, lessons learned, advantages and disadvantages compared to the standard of care.
Nexobrid® is a selective enzymatic debridement agent for burn wound management. The results of this study contribute to a better understanding the procedure and to avoid mistakes in its use.
The efficacy of Nexobrid® for burn wound management has been previously well established. In this study, the authors show learning curve of the procedure and give some advises for a better understanding of the remaining wound bed after debridement, which can help in the diagnosis of burn depth and the plan of treatment.
This study suggests that Nexobrid® is useful for burn wound management, specially the mid-partial, deep-partial and full thickness burns which can benefit in the reduction of the need for autografting compared with the standard of care. In major burns, besides the improvement in wound healing, the authors observed an important improvement in their general state.
The manuscript is an interesting and well written study.
| 1. | Hettiaratchy S, Dziewulski P. ABC of burns. Introduction. BMJ. 2004;328:1366-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Hlava P, Moserová J, Königová R. Validity of clinical assessment of the depth of a thermal injury. Acta Chir Plast. 1983;25:202-208. [PubMed] |
| 3. | Palao R. Quemados: Valoración y criterios de actuación. Barcelona: Marge Médica Books 2011; 19-52. |
| 4. | Hettiaratchy S, Papini R. Initial management of a major burn: II--assessment and resuscitation. BMJ. 2004;329:101-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Papini R. Management of burn injuries of various depths. BMJ. 2004;329:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Gómez PA, Palao R. Tratamiento de las quemaduras en el siglo XXI desde la cirugía. Cir Plast Iberlatinamer. 2002;28:69. |
| 7. | Heimbach DM. Early burn excision and grafting. Surg Clin North Am. 1987;67:93-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Klasen HJ. A review on the nonoperative removal of necrotic tissue from burn wounds. Burns. 2000;26:207-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Rosenberg L, Lapid O, Bogdanov-Berezovsky A, Glesinger R, Krieger Y, Silberstein E, Sagi A, Judkins K, Singer AJ. Safety and efficacy of a proteolytic enzyme for enzymatic burn debridement: a preliminary report. Burns. 2004;30:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Krieger Y, Rosenberg L, Lapid O, Glesinger R, Bogdanov-Berezovsky A, Silberstein E, Sagi A, Judkins K. Escharotomy using an enzymatic debridement agent for treating experimental burn-induced compartment syndrome in an animal model. J Trauma. 2005;58:1259-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Rosenberg L, Krieger Y, Silberstein E, Arnon O, Sinelnikov IA, Bogdanov-Berezovsky A, Singer AJ. Selectivity of a bromelain based enzymatic debridement agent: a porcine study. Burns. 2012;38:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Krieger Y, Bogdanov-Berezovsky A, Gurfinkel R, Silberstein E, Sagi A, Rosenberg L. Efficacy of enzymatic debridement of deeply burned hands. Burns. 2012;38:108-112. [PubMed] [DOI] [Full Text] |
| 13. | Rosenberg L, Krieger Y, Bogdanov-Berezovski A, Silberstein E, Shoham Y, Singer AJ. A novel rapid and selective enzymatic debridement agent for burn wound management: a multi-center RCT. Burns. 2014;40:466-474. [PubMed] [DOI] [Full Text] |
| 14. | Monstrey S, Hoeksema H, Verbelen J, Pirayesh A, Blondeel P. Assessment of burn depth and burn wound healing potential. Burns. 2008;34:761-769. [PubMed] [DOI] [Full Text] |
| 15. | Khatib M, Jabir S, Fitzgerald O’Connor E, Philp B. A systematic review of the evolution of laser Doppler techniques in burn depth assessment. Plast Surg Int. 2014;2014:621792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Sheridan R, Remensnyder J, Prelack K, Petras L, Lydon M. Treatment of the seriously burned infant. J Burn Care Rehabil. 1998;19:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Keck M, Lumenta DB, Andel H, Kamolz LP, Frey M. Burn treatment in the elderly. Burns. 2009;35:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Wilmore DW, Long JM, Mason AD, Skreen RW, Pruitt BA. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180:653-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 510] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Yamada Y, Endo S, Inada K. Plasma cytokine levels in patients with severe burn injury--with reference to the relationship between infection and prognosis. Burns. 1996;22:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 405] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 21. | Hettiaratchy S, Dziewulski P. ABC of burns: pathophysiology and types of burns. BMJ. 2004;328:1427-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Dermatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aksoy B, Negosanti L S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ