Peer-review started: September 10, 2015
First decision: November 7, 2015
Revised: November 23, 2015
Accepted: January 27, 2016
Article in press: January 29, 2016
Published online: May 2, 2016
Processing time: 228 Days and 19.4 Hours
The P2X7 receptor is a trimeric ligand-gated cation channel present on immune and other cells. Activation of this receptor by its natural ligand extracellular adenosine triphosphate results in a variety of downstream responses, including the release of pro-inflammatory mediators and cell death. In normal skin, P2X7 is present on keratinocytes, Langerhans cells and fibroblasts, while the presence of this receptor on other cutaneous cells is mainly inferred from studies of equivalent cell types present in other tissues. Mast cells in normal skin however express negligible amounts of P2X7, which can be upregulated in cutaneous disease. This review discusses the potential significance of P2X7 in skin biology, and the role of this receptor in inflammatory skin disorders such as irritant and chronic dermatitis, psoriasis, graft-versus-host disease, as well is in wound healing, transplantation and skin cancer.
Core tip: The P2X7 receptor is present on immune, stromal and epithelial cells. Activation of this receptor by its natural ligand, extracellular adenosine triphosphate, causes a variety of downstream effects including release of inflammatory mediators and growth factors, as well as cell death. P2X7 has various functions on skin cells, and studies of mouse models of disease and of human cells and tissues highlight emerging roles for this receptor in common skin disorders.
- Citation: Geraghty NJ, Watson D, Adhikary SR, Sluyter R. P2X7 receptor in skin biology and diseases. World J Dermatol 2016; 5(2): 72-83
- URL: https://www.wjgnet.com/2218-6190/full/v5/i2/72.htm
- DOI: https://dx.doi.org/10.5314/wjd.v5.i2.72
The skin fulfils important roles such as barrier protection, thermoregulation, sensation, vitamin D synthesis[1] and immunological protection[2]. Extracellular nucleotides and nucleosides function through a signalling network comprising cell-surface purinergic (P2X, P2Y and adenosine) receptors and ecto-nucleotidases[3]. This network plays important roles in both physiology and pathophysiology, and as such is an emerging therapeutic target to combat many diseases[3]. Evidence indicates that the extracellular nucleotide adenosine triphosphate (ATP) and cell surface purinergic receptors and ecto-nucleotidases play important roles in skin biology[4,5]. Within this context the P2X7 receptor has a major role. This review aims to describe the cellular distribution of P2X7 in skin, and the potential significance of this receptor in skin biology and diseases.
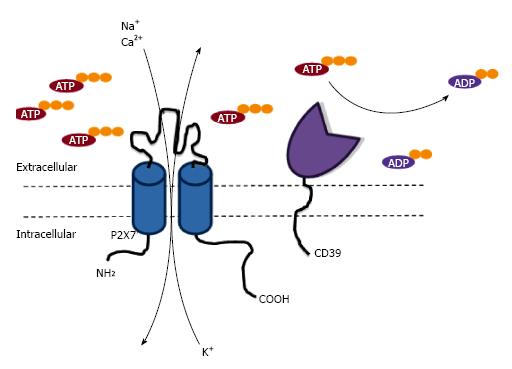
Purinergic signalling comprises a complex network of cell-surface receptors, where activation is mediated by extracellular signalling molecules such as ATP, which can act as a danger associated molecular pattern (DAMP) when released into the extracellular milieu after cell stress, damage or death[6]. Extracellular ATP or other nucleotides can subsequently lead to activation of two purinergic P2 receptor subtypes; P2X and P2Y receptors. P2X receptors are a family of seven trimeric ATP-gated cation channels (P2X1-7); while P2Y receptors are a group of eight G protein-coupled receptors (P2Y1, 2, 4, 6, 11-14). P2 receptors are expressed on numerous cell subtypes, and activation of these receptors by extracellular ATP, or other nucleotides for some receptor subtypes, are important in inflammation and immunity[7]. Activation of P2 receptors by ATP is regulated by the ecto-nucleotidases CD39 and CD73. CD39 degrades ATP into adenosine diphosphate (ADP) and subsequently adenosine monophosphate (AMP) before AMP is converted to adenosine by CD73[8]. Adenosine can then activate P1 receptors; a family of purinergic receptors selective for adenosine[3].
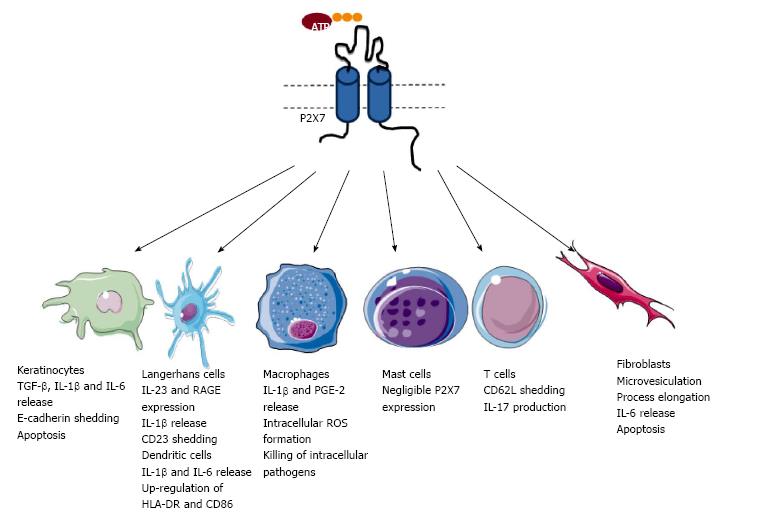
The P2X7 receptor belongs to the family of P2X receptors, which as noted above, are trimeric ATP-gated cation channels. Each P2X7 subunit is composed of intracellular amino and carboxyl termini, as well as two trans-membrane domains connected by a long glycosylated extracellular loop, containing the ATP-binding site[9]. Activation of the P2X7 receptor by extracellular ATP results in K+ efflux, and Na+ and Ca2+ influx, as well as the flux of organic cations and anions including dyes[10]. P2X7 is present on leukocytes, but is also found on other cell types including epithelial cells and fibroblasts[7]. P2X7 activation results in the stimulation of numerous pathways including the release of various pro-inflammatory mediators, modulation of various cell-surface receptors, formation of reactive oxygen and nitrogen species, killing of intracellular pathogens and cell death[11] (Table 1). As a result of various studies in humans and animals, P2X7 is emerging as an important molecule in various biological processes[12] and is attracting considerable interest as a therapeutic target in a wide-range of diseases[13]. Due to this, and the increasing knowledge about the expression and function of P2X7 within the skin (Figure 1), there is a growing interest in the role of P2X7 in skin biology and related disorders.
| RONS formation |
| Shedding of CD23, CD27, CD62L and E-cadherin |
| Up-regulation of CD80 and CD86 expression |
| PGE-2 synthesis and release |
| IL-1β and IL-18 maturation and release |
| IL-6 release |
| IL-2 and IL-17 synthesis and release |
| VEGF release |
| Killing of intracellular pathogens |
| Cell death |
Keratinocytes comprise the majority of cells within the epidermis to provide a physical and immunological barrier[14]. It is well established that human and rodent keratinocytes express P2X7. Immunohistochemistry reveals that P2X7 is expressed in the upper layer of human and rat skin[15,16] suggesting that this receptor may be involved in the death of terminally differentiated keratinocytes. Consistent with this concept, human keratinocyte P2X7 co-localises with markers of apoptosis[16], while P2X7 activation induces human keratinocyte death in vitro[17] and increases murine keratinocyte death in vivo[18]. P2X7 has been reported to be present on human HaCaT keratinocytes[19] and can mediate ATP-induced death of these cells[20], although the presence of P2X7 in these cells has not been confirmed in all studies[21]. Nevertheless over-expression of protein kinase C alpha (PKCα) can result in increased expression of P2X7 in these cells[19] indicating that this kinase may be involved in the up-regulation of keratinocyte P2X7 in the upper layers of the epidermis. Despite the apparent localisation of keratinocyte P2X7 to the upper layers of the epidermis, functional studies (using ATP-induced dye uptake measurements) show that the majority of human and murine keratinocytes express P2X7[22,23]. Thus, these immunohistochemistry and functional studies combined suggest P2X7 may be present in all layers of the epidermis, with receptor expression increasing with keratinocyte differentiation and its upregulation resulting in the death of terminally differentiated keratinocytes.
In addition to cell death, P2X7 activation can induce interleukin (IL)-6 release from human keratinocytes[24], and can mediate ultraviolet radiation-induced IL-1β release from both human and murine keratinocytes[25,26]. P2X7 activation on HaCaT keratinocytes has also been implicated in the activation of disintegrin-like metalloprotease-mediated shedding of E-cadherin and transforming growth factor alpha (TGF-α) induced by the major bee venom component melittin[27]. Collectively, these results indicate that P2X7 on keratinocytes may also be important in inflammatory and immune functions of these cells.
Langerhans cells (LCs) are professional antigen-presenting cells located in the epidermis, and are important in the establishment of adaptive immunity and the maintenance of peripheral tolerance[28]. P2X7 is present on both human and murine LCs from skin[22,23,29], as well as on migratory LCs [langerin+ dendritic cells (DCs)] from human skin explants[30]. Although functional studies of P2X7 on LCs are largely limited to ATP-induced dye uptake measurements[22,23,29], P2X7 activation of migratory LCs causes increased cell-surface expression of the IL-23 receptor and the alarmin receptor for advanced glycation end products (RAGE)[30]. Further, P2X7 is present on human LCs derived from monocytes in vitro and activation of this receptor results in the rapid shedding of CD23 (the low affinity IgE receptor) from these cells[22]. Finally, P2X7 is present on the murine LC-like line, XS106, and activation of this receptor results in the release of IL-1β from these cells[31]. Collectively, these studies support a role for P2X7 activation on LCs in promoting inflammation and immunity.
The relative amount of P2X7 activity on LCs appears to be negatively modulated by the ecto-nucleotidase CD39 (Figure 2). It has long been known that LCs express high ecto-ATPase and ecto-ADPase activities[32], which is almost completely due to CD39[33]. Comparison of human monocyte-derived LCs and monocyte-derived DCs generated from the same subjects reveals that the relative P2X7 activity is lower on monocyte-derived LCs compared to monocyte-derived DCs despite similar amounts of cell-surface P2X7 expression[22]. This difference in activity between these two cell types is inversely associated with cell-surface CD39 expression[22]. These observations are consistent with the negative regulation of P2X7 activation by CD39 on murine peritoneal macrophages[34] and murine bone marrow-derived mast cells[35] (Figure 2). Notably, CD39 on LCs has been implicated in facilitating a protective or tolerogenic role for these cells in dermatitis[33,36]. Collectively, variations in CD39 activity may play important roles in the regulation of P2X7 activation on LCs, and in determining the relative contribution of these cells in immunity or peripheral tolerance.
Dermal DCs are a heterogeneous population of professional antigen-presenting cells, and like LCs are important in the establishment of adaptive immunity and the maintenance of peripheral tolerance[37]. It is well documented that P2X7 is present on human and murine DCs derived from monocytes[38-41] or within lymphoid tissues[42,43], but direct evidence for P2X7 on dermal DCs is limited. P2X7 is present on foetal skin-derived DCs, where it may be involved in T cell stimulation[44], however direct evidence for DC P2X7 in this process is not well established. Interpretation of these results is complicated by subsequent findings that extracellular ATP can induce human and murine T cell proliferation via P2X7 in an autocrine fashion[45]. Thus, the role of P2X7 in T cell stimulation by dermal DCs remains to be elucidated.
P2X7 is also expressed on migratory DCs from human skin explants[30]. Activation of P2X7 on skin migratory DCs resulted in the release of IL-1β and IL-6, as well as the up-regulation of IL-23 and vascular endothelial growth factor (VEGF) mRNA and cell-surface expression of HLA-DR, and the co-stimulation molecule CD86[30]. Finally, P2X7 activation on these cells promotes the development of T helper 17 (Th17) cell responses[30].
Dermal macrophages are a heterogeneous population of cells important in innate and adaptive immunity, as well as in tissue homeostasis and wound healing[37]. Direct evidence for P2X7 on dermal macrophages is lacking, but it is well established that this receptor is present on human and murine macrophages derived from monocytes[46-48] or isolated from tissues[49,50]. P2X7 activation on human and murine macrophages results in the release of pro-inflammatory mediators such as IL-1β and prostaglandin E2[51], as well as the production of reactive oxygen species[52], and killing of intracellular mycobacteria[53], chlamydia[54] and toxoplasma[55]. Of note, P2X7 activation eliminates Leishmania amazonensis, the causative agent of human cutaneous leishmaniasis[56], within murine peritoneal macrophages[57], supporting the potential importance of macrophage P2X7 in skin biology.
Mast cells are present in the dermis, and play important roles during inflammation and immunity[58]. In contrast to other tissues, mast cells in normal human and murine skin express negligible amounts of P2X7[59,60], and ATP incubation of these cells fails to cause IL-1β release despite inducing IL-1β release from murine bone marrow-derived mast cells[61]. This negligible P2X7 expression on skin mast cells is due to fibroblasts expressing the retinoic acid-degrading enzyme Cyp26b1[61]. Although the exact mechanism by which these fibroblasts prevent P2X7 expression on skin mast cells is not known, exogenous retinoic acid upregulates P2X7 expression on bone marrow-derived mast cells[61]. This suggests that Cyp26b1-expressing fibroblasts in mice regulate retinoic acid concentrations to suppress P2X7 expression on skin mast cells. Whether this same inhibitory mechanism operates for human skin mast cells or limits P2X7 expression on other dermal cell populations remains to be determined.
Granulocytes (neutrophils, eosinophils and basophils) are circulating innate immune cells that infiltrate the skin to promote inflammation and immunity[62]. Small numbers of neutrophils also circulate through normal skin, where they are presumed to function as sentinels[63]. Direct evidence for P2X7 on granulocytes within the skin is lacking, but P2X7 is present on human blood eosinophils[64,65] and murine bone marrow-derived basophils[66]. P2X7 activation on human eosinophils results in cation fluxes, increased expression of the integrin CD11b and reactive oxygen species formation, as well as chemotaxis of these granulocytes[64,65]. P2X7 activation is involved in the IgE-dependent activation of murine bone marrow-derived basophils[66], which may have implications for cutaneous allergic inflammation. Collectively, these results suggest P2X7 may play important roles in the pro-inflammatory actions of these granulocytes.
In contrast to eosinophils and basophils, P2X7 appears to be absent on neutrophils. Repeated evidence demonstrates that P2X7 is not present in human blood neutrophils[67,68]. Neutrophil infiltration however is reduced by P2X7 deficiency in murine models of skin inflammation[69] suggesting that P2X7 may be present on murine neutrophils or that P2X7 activation on other skin cells indirectly promotes neutrophil infiltration. Nonetheless future studies are required to determine if P2X7 is present on murine neutrophils or on neutrophils within skin.
Both human and murine skin contains populations of tissue-resident and recirculating T cells, which are key cellular mediators of adaptive immunity[70]. Direct evidence for P2X7 on these skin T cells is lacking, however it is well known that human and murine T cell subsets from blood and lymphoid tissue express P2X7[71]. P2X7 activation induces the rapid shedding of CD62L (L-selectin) from both human and murine CD4+ and CD8+ T cells[72,73]. This cell adhesion molecule can regulate the migration of certain T cell subsets to sites of skin inflammation[74]. Thus, the possibility remains that P2X7-induced CD62L shedding may regulate T cell migration within the skin. There is also evidence that P2X7 activation promotes Th17 cell development in humans[75] and mice[76]. Thus, a further possible role for P2X7 on skin T cells is in the generation of cutaneous Th17 responses.
Dendritic epidermal T cells (DETCs) are resident T cells found in the epidermis of mice, but not humans, and have important roles in inflammation, immunity and wound healing[77]. Murine DETCs express low amounts of P2X7 mRNA[26] but an earlier study, using an anti-P2X7 monoclonal antibody and ATP-induced dye uptake measurements, failed to observe P2X7 on DETCs, despite the presence of P2X7 on keratinocytes and LCs[23]. Nevertheless ATP, released from keratinocytes, can enhance IL-17 release from CD3-activated DETCs[26]. A direct role for P2X7 activation on DETCs in this process was not established, and these cells express high amounts of mRNA for P2X1, P2X2, P2X3 and P2X5[26], thus it remains to be established if DETCs express functional P2X7. It also remains to be established if P2X7 is present on resident T cells in human skin, which are considered to be the equivalent cell type to murine DETCs[77].
B cells are key cellular mediators of adaptive immunity, but their role in the skin immune system is poorly understood. Emerging evidence indicates the presence of B cells in normal skin, although it is unknown if they are skin-resident or circulating B cells[78]. Further evidence indicates roles for B cells in cutaneous immunity and inflammation, and skin cancer[78]. As for T cells, evidence for P2X7 on skin B cells is lacking, but P2X7 is present on human and murine B cells from blood and spleen[79,80]. P2X7 activation results in the rapid shedding of CD62L from human B cells[79] suggesting that this mechanism may regulate B cell migration within the skin. P2X7 activation also results in the rapid shedding of CD23 from human and murine B cells[80]. Although the functional significance of this process is yet to be established, soluble CD23 can regulate IgE production[81]. Thus, P2X7-mediated release of soluble CD23 may regulate the development or severity of atopic dermatitis.
Fibroblasts are a heterogeneous population of cells located in the dermis with a variety of functions including tissue homeostasis, wound healing and inflammation[82]. Human skin fibroblasts express P2X7[83,84]. In addition to cation fluxes, dye uptake and membrane depolarisation, P2X7 activation in these cells results in microvesiculation, process elongation, IL-6 release and apoptosis[84]. High concentrations of glucose potentiate these P2X7-mediated responses[84]. This effect of glucose is attributed to a redistribution of P2X7 on the cell surface rather than increased expression of this receptor[84]. Of note, skin fibroblasts from type 2 diabetic subjects demonstrate enhanced P2X7-mediated responses compared to skin fibroblasts from normal subjects[85]. This enhanced P2X7 activity is suggested to be an important mechanism in the pathogenesis of vascular damage in diabetic subjects[85], but this concept is yet to be developed. P2X7 may also be expressed on murine skin fibroblasts, but observations are limited to the subcutaneous fibroblast cell line L929[86]. This study demonstrated that P2X7 activation mediates cation fluxes, membrane depolarisation and cytotoxicity in these cells.
Allergic contact dermatitis (ACD) is a type IV delayed-type hypersensitivity (DTH) reaction characterised by a T cell-mediated response to allergens[87]. A role for P2X7 in ACD in humans is supported by the up-regulation of this receptor in the epidermal basal layer of inflamed skin of atopic dermatitis patients compared to normal human skin[88], while other experimental evidence supports a role for P2X7 in murine models of ACD. ACD is commonly studied using animal models of contact hypersensitivity (CHS)[87]. Both pharmacological blockade and genetic deficiency of P2X7 impairs CHS responses in mice[89]. This impaired CHS response is due to the absence of P2X7-mediated IL-1β release from DCs abrogating the sensitising capacity of these cells[89]. Intradermal injection of the hydrolysis-resistant nucleotide, adenosine gamma-thiotriphosphate (ATPγS), can also enhance the CHS response in mice[31] indirectly supporting a role for P2X7 in ACD. However, ATPγS cannot activate murine P2X7 in vitro[90] despite activating other mammalian P2X7[90,91]. This raises the possibility that ATPγS acts on other P2 receptors in this model of murine CHS[31]. Notably, non-metal haptens can induce ATP release from primary human and HaCaT keratinocytes[92] providing a possible source for extracellular ATP in ACD.
Irritant contact dermatitis (ICD) is an inflammatory reaction to chemical irritants involving cells of the innate immune system[93]. Experimental evidence in mice supports a role for P2X7 in ICD. Both pharmacological blockade and genetic deficiency of P2X7 impair oedema, IL-1β production and neutrophil infiltration in croton oil-induced ICD[69]. Furthermore, clodronate-depletion of DCs and macrophages, or pharmacological inhibition of caspase-1 reduced ICD in this model[69] suggesting that P2X7 on DCs and macrophages may contribute to the pathogenesis of ICD through IL-1β production. In addition to a role for P2X7 on DCs and macrophages in ICD, P2X7 on mast cells is involved in retinoid-induced ICD. This form of ICD is mediated by aberrant release of ATP within the skin and increased P2X7 expression on skin mast cells[61]. A role for mast cell P2X7 in chemical-induced ICD remains to be determined.
Consistent with a role for P2X7 in ICD, chemical irritants can induce ATP release from murine and human keratinocytes[33,94,95], and genetic deficiency of CD39 exacerbates croton oil-induced ICD in mice[33,94]. Croton oil also decreases ATPDase activity in mice[20] indicating that chemical irritants may further potentiate P2X7-mediated responses by causing a sustained increase in ATP concentrations during chemical irritant exposure. Of note, zinc deficiency, which is often associated with increased cutaneous inflammation, enhances ICD in mice and augments chemical irritant-induced ATP release from murine keratinocytes and in murine skin[36]. Further, zinc deficiency in murine ICD is associated with loss of LCs[36], which play a protective role in ICD through CD39 expression[33]. This suggests that both increased ATP release from keratinocytes and impaired hydrolysis of ATP by LCs may contribute to the pathogenesis of ICD.
Psoriasis is a chronic inflammatory disorder manifesting as plaque or pustular-like lesions of the skin. Psoriasis emerges due to excessive keratinocyte renewal, caused by an innate immune cell response and subsequent engagement of the adaptive immune response, resulting in a feed forward mechanism of inflammation[96]. Although the role of P2X7 has not been investigated in animal models of psoriasis, in vitro studies support a role for P2X7 in psoriasis pathogenesis. Interferon gamma (IFN-γ), a pro-inflammatory cytokine implicated in psoriasis development[96] can upregulate the expression of P2X7 in primary keratinocytes[88]. Moreover, injection of the P2X7 agonist 2’,3’-O-(4-benzoyl)benzoyl ATP (BzATP) into normal human skin explants induces increased expression of cytokines and other molecules commonly associated with psoriasis, including IL-1β, IL-6 and TNF-α[30]. Importantly, these responses could be prevented through pharmacological blockade of P2X7[30]. Of note, P2X7 expression in this model also caused the functional maturation of cutaneous DCs and promoted the development of Th17 responses[30], both of which are important contributors to psoriasis pathogenesis[96].
Graft-vs-host disease (GVHD) is a common complication following bone marrow transplantation used to treat haematological malignancies[97]. Two types of GVHD develop in patients; acute GVHD emerges early after transplantation, while chronic GVHD is a persistent long-lasting inflammation, with both forms causing inflammatory damage to the skin, as well as the gastrointestinal tract, liver and lungs[97]. Pharmacological blockade and genetic deletion of P2X7 attenuates the development of disease in murine models of allogeneic GVHD[98,99]. Additionally, experimental evidence establishes a model whereby ATP released at the site of tissue damage causes upregulation of the co-stimulatory molecules, CD80 and CD86 on DCs to promote T cell responses[98]. P2X7 deficient mice receiving allogeneic bone marrow transplants demonstrated reduced serum concentrations of the pro-inflammatory cytokines IFN-γ, TNF-α, and IL-6[98], which was replicated through blockade of the P2X7 receptor in vivo using the nucleoside reverse transcriptase inhibitor stavudine[99]. Although the effect of P2X7 deficiency or blockade on acute skin GVHD was not directly reported in either study[98,99], skin is a known target organ of GVHD in these models of allogeneic bone marrow transplantation[100]. Of note, P2X7 blockade failed to prevent the development of chronic skin GVHD[98], suggesting P2X7 may not play a role in skin inflammation in chronic GVHD, or longer periods of P2X7 blockade are required for prevention of chronic skin GVHD.
Wound healing is classically defined by the disruption of haemostasis, migration of platelets resulting in blood clotting, followed by inflammation, cell proliferation and tissue remodelling[101]. Studies both in vitro and ex vivo have demonstrated a role for P2X7 in the process of wound healing. P2X7 is important for early cell migration and infiltration of immune cells required for wound healing, with P2X7 deficient cells showing a reduced migratory ability in an in vitro wound repair model suggesting that lack of P2X7 affects chemotaxis[102]. P2X7 also promotes the release of VEGF from primary monocytes, important for control of angiogenesis and wound healing[103]. Conversely, P2X7 is down-regulated on keratinocytes during wound healing[104], suggesting that this reduced expression may be linked with reduced apoptosis of keratinocytes to promote healing of the epidermis. Mast cells also play an important role in wound remodelling and repair[105], but express negligible P2X7 in normal skin[59,60]. It remains to be determined if P2X7 on mast cells is upregulated during wound healing.
Transplantation is an important therapy for many end-stage diseases and rejection of transplants remains a major problem. Studies in transplantation have shown upregulation of P2X7 expression on infiltrating lymphocytes in transplanted hearts in human patients[106]. Pharmacological blockade and genetic deletion of P2X7 in murine models leads to a delay in allograft rejection, which has been demonstrated in several transplant models including models of islet[107], heart[106] and lung[108] transplantation. However, with the exception of one preliminary report[109], there are limited studies investigating P2X7 in skin transplants. In this study[109], ATP is released in allogeneic but not syngeneic skin grafts. This ATP release involved macrophages and the pannexin-1 hemichannel, and was impaired by pharmacological blockade or genetic deletion of P2X7. This inhibition or absence of P2X7 delayed allogeneic skin graft rejection. Collectively, these results support a role for P2X7 in ATP release and tissue rejection in allogeneic skin graft transplantation.
Skin cancers are common cancers within humans and include three main forms: Basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and melanoma[110]. Ultraviolet radiation is the major causative factor of these skin cancers[110]. P2X7 is emerging as an important receptor in many forms of cancers, with various and contradictory roles attributed to this receptor in tumour biology[111]. These include but are not limited to tumour cell proliferation[112], death[113], and invasiveness[114], as well as anti-tumour immunity[115] and cancer pain[116].
The role of P2X7 in skin cancer has been studied most widely in melanoma. Immunohistochemistry reveals expression of P2X7 in human melanoma[117,118] and in various melanoma cell lines[119]. Further, this receptor is expressed at higher quantities in melanoma cells compared to normal melanocytes[119]. Importantly, P2X7 in melanoma and melanoma cell lines is functional[118,119]. Paradoxically, P2X7 activation promotes and suppresses ATP-induced apoptosis in human A375[118] and HT168-M1 melanoma cells[119], respectively. These differences remain to be reconciled, but opposing effects with P2X7 have also been observed in murine models of melanoma. ATP injection impairs the growth of A375 melanoma cells in (athymic) immuno-compromised mice[120] supporting an anti-tumour effect for P2X7 presumably through ATP-induced cell death. Conversely, injection of P2X7 antagonists inhibits the growth of murine B16 melanoma cells (which express P2X7[121]) in immuno-competent mice[121,122]. Additional data from these studies demonstrated that this pro-tumour effect of P2X7 was due to enhanced ATP-induced proliferation of B16 melanoma cells[121,122]. P2X7 on immune cells also plays an important role in preventing melanoma progression by promoting anti-tumour immune responses. B16 melanoma growth and metastasis is increased in P2X7 deficient mice or wild-type chimeric mice transplanted with P2X7-deficient bone marrow compared to control mice[102].
P2X7 may also play an important role in BCC and SCC. Immunohistochemistry of human samples reveals expression of P2X7 in the necrotic centre of BCCs and within apoptotic cells in both BCCs and SSCs, suggesting that P2X7 activation may mediate killing of malignant cells within these tumours[123]. Evidence for this process in BCC is wanting, but P2X7 can mediate the killing of the human A431 SCC line[123]. Another report however attributed this cytolytic effect to adenosine resulting from ATP hydrolysis rather than ATP directly[124]. Thus, the role of P2X7 in this cell line remains uncertain. As noted above, P2X7 is also present on immortalised HaCaT keratinocytes[19] and mediates ATP-induced death in these cells[20]. Notably, ultraviolet B irradiation down-regulates P2X7 expression in HaCaT keratinocytes, potentially leading to survival of cells with a reduced ability for ATP-induced apoptosis, and allowing for malignant transformation and survival of malignant cells[125]. Consistent with this concept, in BCC patients, more aggressive tumours have lower P2X7 expression, suggesting that loss of P2X7 can act as a marker for increased tumour aggressiveness[123]. Finally, in a murine model of chemically-induced skin papilloma/SCC carcinogenesis, injection of BzATP reduces the frequency and size of papillomas and skin cancers, a response that is absent in P2X7 deficient mice, indicating a role for P2X7 in this process[18]. P2X7 activation in these tumours is associated with apoptosis[18]. Of note, P2X7 expression is reduced in papillomas and skin cancers compared to normal skin[18], suggesting that down-regulation of P2X7 in skin tumours is a possible escape mechanism to avoid ATP-induced apoptosis.
In summary, P2X7 is present on immune, stromal, epithelial and malignant cells in diseased skin, and is up-regulated in some skin disorders. Activation of P2X7 cells and the resulting downstream effects are implicated in numerous skin diseases including allergic and irritant contact dermatitis, psoriasis, cutaneous GVHD, as well as in skin transplantation and skin cancer. In some instances the role of P2X7 in skin disease is supported by mouse models (Table 2) and human studies (Table 3), but for other skin diseases evidence is limited to only one species. Nevertheless, P2X7 represents a potential biomarker and target for treatment of various skin disorders, but further studies are required before the clinical value of P2X7 can be utilised.
| Disease | Observations |
| Allergic contact dermatitis | P2X7 blockade or deficiency impairs CHS[89] |
| Irritant contact dermatitis | P2X7 blockade or deficiency impairs croton oil-induced oedema, IL-1β production and neutrophil infiltration[69] |
| Psoriasis | ND |
| Cutaneous graft-vs-host disease | P2X7 blockade or deficiency increases survival and reduces disease severity, serum concentrations of IFN-γ, TNF-α and IL-6 in allogeneic mouse models[98,99] |
| Wound healing | P2X7 deficient macrophages display reduced migration in an in vitro wound repair model[102] |
| Skin transplantation | P2X7 blockade or deficiency prevents allogeneic skin transplant rejection[109] |
| Melanoma | ATP injection impairs A375 melanoma cell growth in immuno-compromised mice[120] P2X7 blockade inhibits B16 melanoma cell growth in immuno-competent mice[121,122] P2X7 deficiency impairs B16 melanoma cell migration in vitro[102] P2X7 deficiency in host leads to increased B16 melanoma growth and metastasis[102] |
| Basal cell carcinoma | ND |
| Squamous cell carcinoma | P2X7 deficiency in host enhances chemical-induced carcinogenesis[18] BzATP injection led to tumour apoptosis[18] |
| Disease | Observations |
| Allergic contact dermatitis | Increased P2X7 expression in atopic dermatitis lesions[88] |
| Irritant contact dermatitis | ND |
| Psoriasis | Increased P2X7 expression in psoriatic skin lesions[30,88] |
| Cutaneous graft-vs-host disease | ND |
| Wound healing | P2X7 activation promotes VEGF release from monocytes[103] |
| Skin transplantation | ND |
| Melanoma | P2X7 is present on melanoma cells[117,118] and cell lines[119], with increased expression compared to normal melanocytes[119] |
| P2X7 activation induces A375 melanoma[118] but suppresses HT168-M1 melanoma cell death[119] | |
| Basal cell carcinoma | P2X7 is present in necrotic tumour centres and apoptotic tumour cells, and correlates inversely with tumour aggressiveness[123] |
| Squamous cell carcinoma | P2X7 is present in apoptotic tumour cells and its activation causes A431 SCC cell death[123] |
The P2X7 receptor is present on numerous immune and other cell types in the skin including keratinocytes, Langerhans cells, and dermal dendritic cells, and may be present on T and B cells. P2X7 expression is negligible on mast cells, but can be upregulated in skin disease. Activation of P2X7 by ATP results in numerous downstream effects including cytokine release and apoptosis. P2X7 may play a role in homeostatic skin biology and has been implicated in a number of skin disorders, including contact dermatitis, psoriasis, cutaneous GVHD, and is involved in other skin processes including transplantation and wound healing. Thus, P2X7 represents a potential target for therapy of skin diseases.
| 1. | McLafferty E, Hendry C, Alistair F. The integumentary system: anatomy, physiology and function of skin. Nurs Stand. 2012;27:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | SS Tay, Roediger B, Tong PL, Tikoo S, Weninger W. The Skin-Resident Immune Network. Curr Dermatol Rep. 2014;3:13-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Burnstock G. Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. Bioessays. 2012;34:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Holzer AM, Granstein RD. Role of extracellular adenosine triphosphate in human skin. J Cutan Med Surg. 2004;8:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Burnstock G, Knight GE, Greig AV. Purinergic signaling in healthy and diseased skin. J Invest Dermatol. 2012;132:526-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Di Virgilio F, Vuerich M. Purinergic signaling in the immune system. Auton Neurosci. 2015;191:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 598] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 8. | Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 980] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 9. | Jiang LH, Baldwin JM, Roger S, Baldwin SA. Insights into the Molecular Mechanisms Underlying Mammalian P2X7 Receptor Functions and Contributions in Diseases, Revealed by Structural Modeling and Single Nucleotide Polymorphisms. Front Pharmacol. 2013;4:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Alves LA, de Melo Reis RA, de Souza CA, de Freitas MS, Teixeira PC, Neto Moreira Ferreira D, Xavier RF. The P2X7 receptor: shifting from a low- to a high-conductance channel - an enigmatic phenomenon? Biochim Biophys Acta. 2014;1838:2578-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 2011;78:321-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Lenertz LY, Gavala ML, Zhu Y, Bertics PJ. Transcriptional control mechanisms associated with the nucleotide receptor P2X7, a critical regulator of immunologic, osteogenic, and neurologic functions. Immunol Res. 2011;50:22-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Bartlett R, Stokes L, Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev. 2014;66:638-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 341] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 14. | Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1001] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 15. | Gröschel-Stewart U, Bardini M, Robson T, Burnstock G. Localisation of P2X5 and P2X7 receptors by immunohistochemistry in rat stratified squamous epithelia. Cell Tissue Res. 1999;296:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Greig AV, Linge C, Terenghi G, McGrouther DA, Burnstock G. Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes. J Invest Dermatol. 2003;120:1007-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Greig AV, Linge C, Cambrey A, Burnstock G. Purinergic receptors are part of a signaling system for keratinocyte proliferation, differentiation, and apoptosis in human fetal epidermis. J Invest Dermatol. 2003;121:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Fu W, McCormick T, Qi X, Luo L, Zhou L, Li X, Wang BC, Gibbons HE, Abdul-Karim FW, Gorodeski GI. Activation of P2X(7)-mediated apoptosis Inhibits DMBA/TPA-induced formation of skin papillomas and cancer in mice. BMC Cancer. 2009;9:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Gönczi M, Telek A, Czifra G, Balogh A, Blumberg PM, Bíró T, Csernoch L. Altered calcium handling following the recombinant overexpression of protein kinase C isoforms in HaCaT cells. Exp Dermatol. 2008;17:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Zanin RF, da Silva GL, Erig T, Sperotto ND, Leite CE, Coutinho-Silva R, Batastini AM, Morrone FB. Decrease of serum adenine nucleotide hydrolysis in an irritant contact dermatitis mice model: potential P2X7R involvement. Mol Cell Biochem. 2015;404:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Farrell AW, Gadeock S, Pupovac A, Wang B, Jalilian I, Ranson M, Sluyter R. P2X7 receptor activation induces cell death and CD23 shedding in human RPMI 8226 multiple myeloma cells. Biochim Biophys Acta. 2010;1800:1173-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Georgiou JG, Skarratt KK, Fuller SJ, Martin CJ, Christopherson RI, Wiley JS, Sluyter R. Human epidermal and monocyte-derived langerhans cells express functional P2X receptors. J Invest Dermatol. 2005;125:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Tran JN, Pupovac A, Taylor RM, Wiley JS, Byrne SN, Sluyter R. Murine epidermal Langerhans cells and keratinocytes express functional P2X7 receptors. Exp Dermatol. 2010;19:e151-e157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Inoue K, Hosoi J, Denda M. Extracellular ATP has stimulatory effects on the expression and release of IL-6 via purinergic receptors in normal human epidermal keratinocytes. J Invest Dermatol. 2007;127:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Salzer S, Kresse S, Hirai Y, Koglin S, Reinholz M, Ruzicka T, Schauber J. Cathelicidin peptide LL-37 increases UVB-triggered inflammasome activation: possible implications for rosacea. J Dermatol Sci. 2014;76:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | MacLeod AS, Rudolph R, Corriden R, Ye I, Garijo O, Havran WL. Skin-resident T cells sense ultraviolet radiation-induced injury and contribute to DNA repair. J Immunol. 2014;192:5695-5702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Sommer A, Fries A, Cornelsen I, Speck N, Koch-Nolte F, Gimpl G, Andrä J, Bhakdi S, Reiss K. Melittin modulates keratinocyte function through P2 receptor-dependent ADAM activation. J Biol Chem. 2012;287:23678-23689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Chopin M, Nutt SL. Establishing and maintaining the Langerhans cell network. Semin Cell Dev Biol. 2015;41:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Girolomoni G, Santantonio ML, Pastore S, Bergstresser PR, Giannetti A, Cruz PD. Epidermal Langerhans cells are resistant to the permeabilizing effects of extracellular ATP: in vitro evidence supporting a protective role of membrane ATPase. J Invest Dermatol. 1993;100:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Killeen ME, Ferris L, Kupetsky EA, Falo L, Mathers AR. Signaling through purinergic receptors for ATP induces human cutaneous innate and adaptive Th17 responses: implications in the pathogenesis of psoriasis. J Immunol. 2013;190:4324-4336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Granstein RD, Ding W, Huang J, Holzer A, Gallo RL, Di Nardo A, Wagner JA. Augmentation of cutaneous immune responses by ATP gamma S: purinergic agonists define a novel class of immunologic adjuvants. J Immunol. 2005;174:7725-7731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Wolff K, Winkelmann RK. Ultrastructural localization of nucleoside triphosphatase in Langerhans cells. J Invest Dermatol. 1967;48:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Mizumoto N, Kumamoto T, Robson SC, Sévigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 267] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 34. | Lévesque SA, Kukulski F, Enjyoji K, Robson SC, Sévigny J. NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur J Immunol. 2010;40:1473-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Kuhny M, Hochdörfer T, Ayata CK, Idzko M, Huber M. CD39 is a negative regulator of P2X7-mediated inflammatory cell death in mast cells. Cell Commun Signal. 2014;12:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Kawamura T, Ogawa Y, Nakamura Y, Nakamizo S, Ohta Y, Nakano H, Kabashima K, Katayama I, Koizumi S, Kodama T. Severe dermatitis with loss of epidermal Langerhans cells in human and mouse zinc deficiency. J Clin Invest. 2012;122:722-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14:417-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 353] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 38. | Coutinho-Silva R, Persechini PM, Bisaggio RD, Perfettini JL, Neto AC, Kanellopoulos JM, Motta-Ly I, Dautry-Varsat A, Ojcius DM. P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol. 1999;276:C1139-C1147. [PubMed] |
| 39. | Ferrari D, La Sala A, Chiozzi P, Morelli A, Falzoni S, Girolomoni G, Idzko M, Dichmann S, Norgauer J, Di Virgilio F. The P2 purinergic receptors of human dendritic cells: identification and coupling to cytokine release. FASEB J. 2000;14:2466-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Sluyter R, Wiley JS. Extracellular adenosine 5’-triphosphate induces a loss of CD23 from human dendritic cells via activation of P2X7 receptors. Int Immunol. 2002;14:1415-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, Nunez G, Dubyak GR. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J Immunol. 2009;182:5052-5062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 42. | Buell G, Chessell IP, Michel AD, Collo G, Salazzo M, Herren S, Gretener D, Grahames C, Kaur R, Kosco-Vilbois MH. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood. 1998;92:3521-3528. [PubMed] |
| 43. | Nihei OK, de Carvalho AC, Savino W, Alves LA. Pharmacologic properties of P(2Z)/P2X(7 )receptor characterized in murine dendritic cells: role on the induction of apoptosis. Blood. 2000;96:996-1005. [PubMed] |
| 44. | Mutini C, Falzoni S, Ferrari D, Chiozzi P, Morelli A, Baricordi OR, Collo G, Ricciardi-Castagnoli P, Di Virgilio F. Mouse dendritic cells express the P2X7 purinergic receptor: characterization and possible participation in antigen presentation. J Immunol. 1999;163:1958-1965. [PubMed] |
| 45. | Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 46. | Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451-1458. [PubMed] |
| 47. | Eschke D, Wüst M, Hauschildt S, Nieber K. Pharmacological characterization of the P2X(7) receptor on human macrophages using the patch-clamp technique. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 464] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 49. | Coutinho-Silva R, Persechini PM. P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. Am J Physiol. 1997;273:C1793-C1800. [PubMed] |
| 50. | Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 764] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 51. | Barberà-Cremades M, Baroja-Mazo A, Gomez AI, Machado F, Di Virgilio F, Pelegrín P. P2X7 receptor-stimulation causes fever via PGE2 and IL-1β release. FASEB J. 2012;26:2951-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 52. | Pfeiffer ZA, Guerra AN, Hill LM, Gavala ML, Prabhu U, Aga M, Hall DJ, Bertics PJ. Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radic Biol Med. 2007;42:1506-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 309] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 54. | Coutinho-Silva R, Perfettini JL, Persechini PM, Dautry-Varsat A, Ojcius DM. Modulation of P2Z/P2X(7) receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol Cell Physiol. 2001;280:C81-C89. [PubMed] |
| 55. | Lees MP, Fuller SJ, McLeod R, Boulter NR, Miller CM, Zakrzewski AM, Mui EJ, Witola WH, Coyne JJ, Hargrave AC. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J Immunol. 2010;184:7040-7046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 56. | Samady JA, Schwartz RA. Old World cutaneous leishmaniasis. Int J Dermatol. 1997;36:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Chaves SP, Torres-Santos EC, Marques C, Figliuolo VR, Persechini PM, Coutinho-Silva R, Rossi-Bergmann B. Modulation of P2X(7) purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination. Microbes Infect. 2009;11:842-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Kritas SK, Saggini A, Varvara G, Murmura G, Caraffa A, Antinolfi P, Toniato E, Pantalone A, Neri G, Frydas S. Impact of mast cells on the skin. Int J Immunopathol Pharmacol. 2013;26:855-859. [PubMed] |
| 59. | Bradding P, Okayama Y, Kambe N, Saito H. Ion channel gene expression in human lung, skin, and cord blood-derived mast cells. J Leukoc Biol. 2003;73:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Kurashima Y, Amiya T, Nochi T, Fujisawa K, Haraguchi T, Iba H, Tsutsui H, Sato S, Nakajima S, Iijima H. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat Commun. 2012;3:1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 61. | Kurashima Y, Amiya T, Fujisawa K, Shibata N, Suzuki Y, Kogure Y, Hashimoto E, Otsuka A, Kabashima K, Sato S. The enzyme Cyp26b1 mediates inhibition of mast cell activation by fibroblasts to maintain skin-barrier homeostasis. Immunity. 2014;40:530-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 62. | Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol. 2013;34:398-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 63. | Jain R, Weninger W. Shedding light on cutaneous innate immune responses: the intravital microscopy approach. Immunol Cell Biol. 2013;91:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Ferrari D, Idzko M, Dichmann S, Purlis D, Virchow C, Norgauer J, Chiozzi P, Di Virgilio F, Luttmann W. P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett. 2000;486:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Idzko M, Dichmann S, Panther E, Ferrari D, Herouy Y, Virchow C, Luttmann W, Di Virgilio F, Norgauer J. Functional characterization of P2Y and P2X receptors in human eosinophils. J Cell Physiol. 2001;188:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Tsai SH, Kinoshita M, Kusu T, Kayama H, Okumura R, Ikeda K, Shimada Y, Takeda A, Yoshikawa S, Obata-Ninomiya K. The ectoenzyme E-NPP3 negatively regulates ATP-dependent chronic allergic responses by basophils and mast cells. Immunity. 2015;42:279-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Vaughan KR, Stokes L, Prince LR, Marriott HM, Meis S, Kassack MU, Bingle CD, Sabroe I, Surprenant A, Whyte MK. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol. 2007;179:8544-8553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 68. | Martel-Gallegos G, Rosales-Saavedra MT, Reyes JP, Casas-Pruneda G, Toro-Castillo C, Pérez-Cornejo P, Arreola J. Human neutrophils do not express purinergic P2X7 receptors. Purinergic Signal. 2010;6:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | da Silva GL, Sperotto ND, Borges TJ, Bonorino C, Takyia CM, Coutinho-Silva R, Campos MM, Zanin RF, Morrone FB. P2X7 receptor is required for neutrophil accumulation in a mouse model of irritant contact dermatitis. Exp Dermatol. 2013;22:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Mueller SN, Zaid A, Carbone FR. Tissue-resident T cells: dynamic players in skin immunity. Front Immunol. 2014;5:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 71. | Rissiek B, Haag F, Boyer O, Koch-Nolte F, Adriouch S. P2X7 on Mouse T Cells: One Channel, Many Functions. Front Immunol. 2015;6:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (12)] |
| 72. | Aswad F, Dennert G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell Immunol. 2006;243:58-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Sluyter R, Wiley JS. P2X7 receptor activation induces CD62L shedding from human CD4 and CD8 T cells. Inflamm Cell Signal. 2014;1:e92. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Grailer JJ, Kodera M, Steeber DA. L-selectin: role in regulating homeostasis and cutaneous inflammation. J Dermatol Sci. 2009;56:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 75. | Purvis HA, Anderson AE, Young DA, Isaacs JD, Hilkens CM. A negative feedback loop mediated by STAT3 limits human Th17 responses. J Immunol. 2014;193:1142-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, Westendorf AM, Grassi F. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4:ra12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 77. | Macleod AS, Havran WL. Functions of skin-resident γδ T cells. Cell Mol Life Sci. 2011;68:2399-2408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 78. | Egbuniwe IU, Karagiannis SN, Nestle FO, Lacy KE. Revisiting the role of B cells in skin immune surveillance. Trends Immunol. 2015;36:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 79. | Gu BJ, Zhang WY, Bendall LJ, Chessell IP, Buell GN, Wiley JS. Expression of P2X(7) purinoceptors on human lymphocytes and monocytes: evidence for nonfunctional P2X(7) receptors. Am J Physiol Cell Physiol. 2000;279:C1189-C1197. [PubMed] |
| 80. | Pupovac A, Geraghty NJ, Watson D, Sluyter R. Activation of the P2X7 receptor induces the rapid shedding of CD23 from human and murine B cells. Immunol Cell Biol. 2015;93:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Cooper AM, Hobson PS, Jutton MR, Kao MW, Drung B, Schmidt B, Fear DJ, Beavil AJ, McDonnell JM, Sutton BJ. Soluble CD23 controls IgE synthesis and homeostasis in human B cells. J Immunol. 2012;188:3199-3207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015;25:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 298] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 83. | Solini A, Chiozzi P, Morelli A, Fellin R, Di Virgilio F. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. J Cell Sci. 1999;112:297-305. [PubMed] |
| 84. | Solini A, Chiozzi P, Falzoni S, Morelli A, Fellin R, Di Virgilio F. High glucose modulates P2X7 receptor-mediated function in human primary fibroblasts. Diabetologia. 2000;43:1248-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Solini A, Chiozzi P, Morelli A, Adinolfi E, Rizzo R, Baricordi OR, Di Virgilio F. Enhanced P2X7 activity in human fibroblasts from diabetic patients: a possible pathogenetic mechanism for vascular damage in diabetes. Arterioscler Thromb Vasc Biol. 2004;24:1240-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Pizzo P, Murgia M, Zambon A, Zanovello P, Bronte V, Pietrobon D, Di Virgilio F. Role of P2z purinergic receptors in ATP-mediated killing of tumor necrosis factor (TNF)-sensitive and TNF-resistant L929 fibroblasts. J Immunol. 1992;149:3372-3378. [PubMed] |
| 87. | Weintraub GS, Nga Lai I, Kim CN. Review of allergic contact dermatitis: Scratching the surface. World J Dermatol. 2015;4:95-102. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (4)] |
| 88. | Pastore S, Mascia F, Gulinelli S, Forchap S, Dattilo C, Adinolfi E, Girolomoni G, Di Virgilio F, Ferrari D. Stimulation of purinergic receptors modulates chemokine expression in human keratinocytes. J Invest Dermatol. 2007;127:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Weber FC, Esser PR, Müller T, Ganesan J, Pellegatti P, Simon MM, Zeiser R, Idzko M, Jakob T, Martin SF. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J Exp Med. 2010;207:2609-2619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 90. | Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol. 2009;157:1203-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 91. | Spildrejorde M, Bartlett R, Stokes L, Jalilian I, Peranec M, Sluyter V, Curtis BL, Skarratt KK, Skora A, Bakhsh T. R270C polymorphism leads to loss of function of the canine P2X7 receptor. Physiol Genomics. 2014;46:512-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Onami K, Kimura Y, Ito Y, Yamauchi T, Yamasaki K, Aiba S. Nonmetal haptens induce ATP release from keratinocytes through opening of pannexin hemichannels by reactive oxygen species. J Invest Dermatol. 2014;134:1951-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 93. | Sua?rez-Pe?rez JA, Bosch R, Gonza?lez S, Gonza?lez E. Pathogenesis and diagnosis of contact dermatitis: Applications of reflectance confocal microscopy. World J Dermatol. 2014;3:45-49. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (8)] |
| 94. | Mizumoto N, Mummert ME, Shalhevet D, Takashima A. Keratinocyte ATP release assay for testing skin-irritating potentials of structurally diverse chemicals. J Invest Dermatol. 2003;121:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Raoux M, Azorin N, Colomban C, Rivoire S, Merrot T, Delmas P, Crest M. Chemicals inducing acute irritant contact dermatitis mobilize intracellular calcium in human keratinocytes. Toxicol In Vitro. 2013;27:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2361] [Cited by in RCA: 2174] [Article Influence: 127.9] [Reference Citation Analysis (0)] |
| 97. | Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1987] [Cited by in RCA: 1890] [Article Influence: 111.2] [Reference Citation Analysis (7)] |
| 98. | Wilhelm K, Ganesan J, Müller T, Dürr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Jüttner E. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010;16:1434-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 380] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 99. | Fowler BJ, Gelfand BD, Kim Y, Kerur N, Tarallo V, Hirano Y, Amarnath S, Fowler DH, Radwan M, Young MT. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science. 2014;346:1000-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 379] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 100. | Markey KA, MacDonald KP, Hill GR. The biology of graft-versus-host disease: experimental systems instructing clinical practice. Blood. 2014;124:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 101. | Nguyen DT, Orgill DP, Murphy GF. The pathophysiologic basis for wound healing and cutaneous regeneration. Biomaterials for Treating Skin Loss. Cambridge: Woodhead Publishing Limited 2009; 25-57. |
| 102. | Adinolfi E, Capece M, Franceschini A, Falzoni S, Giuliani AL, Rotondo A, Sarti AC, Bonora M, Syberg S, Corigliano D. Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Res. 2015;75:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 103. | Hill LM, Gavala ML, Lenertz LY, Bertics PJ. Extracellular ATP may contribute to tissue repair by rapidly stimulating purinergic receptor X7-dependent vascular endothelial growth factor release from primary human monocytes. J Immunol. 2010;185:3028-3034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 104. | Greig AV, James SE, McGrouther DA, Terenghi G, Burnstock G. Purinergic receptor expression in the regeneration epidermis in a rat model of normal and delayed wound healing. Exp Dermatol. 2003;12:860-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Hebda PA, Collins MA, Tharp MD. Mast cell and myofibroblast in wound healing. Dermatol Clin. 1993;11:685-696. [PubMed] |
| 106. | Vergani A, Tezza S, D’Addio F, Fotino C, Liu K, Niewczas M, Bassi R, Molano RD, Kleffel S, Petrelli A. Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation. 2013;127:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 107. | Vergani A, Fotino C, D’Addio F, Tezza S, Podetta M, Gatti F, Chin M, Bassi R, Molano RD, Corradi D. Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection. Diabetes. 2013;62:1665-1675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 108. | Liu K, Vergani A, Zhao P, Ben Nasr M, Wu X, Iken K, Jiang D, Su X, Fotino C, Fiorina P. Inhibition of the purinergic pathway prolongs mouse lung allograft survival. Am J Respir Cell Mol Biol. 2014;51:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Barbera-Cremades M, Manuel Martinez C, Baroja-Mazo A, Amores-Iniesta J, Pelegrin P. P2X7 receptor controls extracellular ATP during skin graft allogenic rejection. Purinergic Signal. 2014;10:817. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 110. | Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 670] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 111. | Roger S, Jelassi B, Couillin I, Pelegrin P, Besson P, Jiang LH. Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochim Biophys Acta. 2015;1848:2584-2602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 112. | Di Virgilio F, Ferrari D, Adinolfi E. P2X(7): a growth-promoting receptor-implications for cancer. Purinergic Signal. 2009;5:251-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 113. | Adinolfi E, Pizzirani C, Idzko M, Panther E, Norgauer J, Di Virgilio F, Ferrari D. P2X(7) receptor: Death or life? Purinergic Signal. 2005;1:219-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 114. | Roger S, Pelegrin P. P2X7 receptor antagonism in the treatment of cancers. Expert Opin Investig Drugs. 2011;20:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 115. | Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, Smyth MJ, Zitvogel L. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 116. | Franceschini A, Adinolfi E. P2X receptors: New players in cancer pain. World J Biol Chem. 2014;5:429-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 117. | Slater M, Scolyer RA, Gidley-Baird A, Thompson JF, Barden JA. Increased expression of apoptotic markers in melanoma. Melanoma Res. 2003;13:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | White N, Butler PE, Burnstock G. Human melanomas express functional P2 X(7) receptors. Cell Tissue Res. 2005;321:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 119. | Deli T, Varga N, Adám A, Kenessey I, Rásó E, Puskás LG, Tóvári J, Fodor J, Fehér M, Szigeti GP. Functional genomics of calcium channels in human melanoma cells. Int J Cancer. 2007;121:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 120. | White N, Knight GE, Butler PE, Burnstock G. An in vivo model of melanoma: treatment with ATP. Purinergic Signal. 2009;5:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 121. | Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, Di Virgilio F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012;72:2957-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 307] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 122. | Hattori F, Ohshima Y, Seki S, Tsukimoto M, Sato M, Takenouchi T, Suzuki A, Takai E, Kitani H, Harada H. Feasibility study of B16 melanoma therapy using oxidized ATP to target purinergic receptor P2X7. Eur J Pharmacol. 2012;695:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 123. | Greig AV, Linge C, Healy V, Lim P, Clayton E, Rustin MH, McGrouther DA, Burnstock G. Expression of purinergic receptors in non-melanoma skin cancers and their functional roles in A431 cells. J Invest Dermatol. 2003;121:315-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 124. | Völkl T, Ogilvie A, Neuhuber W, Ogilvie A. Cell death induced by uridine 5’-triphosphate (UTP) in contrast to adenosine 5’-triphosphate (ATP) in human epidermoid carcinoma cells (A-431). Cell Physiol Biochem. 2008;22:441-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Ruzsnavszky O, Telek A, Gönczi M, Balogh A, Remenyik E, Csernoch L. UV-B induced alteration in purinergic receptors and signaling on HaCaT keratinocytes. J Photochem Photobiol B. 2011;105:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Cuevas-Covarrubia SA, Husein-ElAhmed H, Kaliyadan F, Negosanti L S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ