Published online Nov 2, 2015. doi: 10.5314/wjd.v4.i4.135
Peer-review started: June 23, 2014
First decision: August 14, 2014
Revised: June 24, 2015
Accepted: July 16, 2015
Article in press: July 17, 2015
Published online: November 2, 2015
Processing time: 498 Days and 11.4 Hours
Mycosis fungoides, the most common primary cutaneous lymphoma, may present with a broad spectrum of clinical features. As both clinical and dermatopathological findings in mycosis fungoides occasionally closely imitate other dermatoses, correct diagnosis may be a challenge both for clinicians as well as dermatopathologists. As a consequence, diagnosis of cutaneous lymphoma may be initially missed and, therefore, prompt and adequate therapeutic measures delayed. Hence, the purpose of our article was to give an overview of hitherto published “mimickers” of mycosis fungoides with a review of its diverse clinical features to alert the clinicians about the wide spectrum of this dissimulating disease. By integrating our own encountered atypical cases of mycosis fungoides we provide a comprehensive illustrated histological and moleculargenetic workup thereof and thereby critically revise the different available diagnostic tools of daily routine. Finally, we derive a practical algorithm to obtain the correct diagnosis even in such ambiguous cases of mycosis fungoides.
Core tip: Mycosis fungoides, the most common cutaneous lymphoma, may imitate diverse diagnoses both on clinical and on histological grounds. Hence, the former “great masquerader” syphilis may be regarded as being outpaced. As diagnosis of such ambiguous, atypical cases of mycosis fungoides may be a challenge for the dermatologist and pathologist and consecutively adequate therapeutic measures may be delayed we herein give a comprehensive overview on previously published cases accomplished by our own data. We conclude that a multi-step diagnostic algorithm including meticulous clinicopathological correlation together with molecular genetic analysis should be applied in such protean cases to obtain the correct diagnosis.
- Citation: Wobser M, Geissinger E, Rosenwald A, Goebeler M. Mycosis fungoides: A mimicker of benign dermatoses. World J Dermatol 2015; 4(4): 135-144
- URL: https://www.wjgnet.com/2218-6190/full/v4/i4/135.htm
- DOI: https://dx.doi.org/10.5314/wjd.v4.i4.135
Traditionally, syphilis has been designated as the “great imitator” within different medical disciplines[1,2]. As the rash of secondary syphilis is highly variable in appearance, cutaneous signs of treponemal infection may be easily mistaken for a wide range of other common dermatological conditions such as psoriasis, tinea corporis, pityriasis rosea, vitiligo and viral exanthems. Of interest, an increasing plethora of case reports and a few recent systematic reviews thereof[1] denote, that a “novel” dermatological mimicker, namely mycosis fungoides, has evolved within this field of clinical masqueraders.
Mycosis fungoides (MF), the most common primary cutaneous lymphoma, is a low-grade lymphoproliferative disorder of skin-homing mature CD45R0+ T-cells[2]. Classically, mycosis fungoides is limited to the skin presenting with erythematous patches or slightly scaling infiltrated plaques. On histology, a band-like, epidermotropic infiltrate of small to medium-sized lymphocytes, some with atypia (“haloed” intraepidermal lymphocytes with hyperchromatic, indented or cerebriform nuclei) is characteristic[3]. In most cases the lymphocytes belong to the CD4+ subtype, and may exhibit variable loss of T-cell antigens such as CD2, CD5 or CD7 and a monoclonal T-cell receptor (TCR) gene rearrangement. During the further clinical course, tumors may develop in a minority of patients, mostly in longstanding, therapy-refractory lesions. Whereas prognosis of early stages is excellent, tumor stage or the rare occurrence of nodal, blood or visceral dissemination is associated with a worse prognosis with 5-year survival rates of less than 30%[4,5].
Especially in early lesions of mycosis fungoides, i.e., patch-stage disease, diagnosis may be difficult due to often non-specific clinical and histological findings and further clinicopathological follow-up is crucial[6]. Beside the rather classical presentation of mycosis fungoides, i.e., patches evolving to plaques and rarely tumors, peculiar variants have been described due to unique clinical signs and/or divergent histopathological findings, thus making diagnosis even more challenging. Two particular variants of mycosis fungoides have already been adopted as separated subtypes in the current WHO-/EORTC-classification of cutaneous lymphoma, namely “granulomatous mycosis fungoides/granulomatous slack skin” and “folliculotropic mycosis fungoides”[7]. To distinguish these subtypes from otherwise conventional mycosis fungoides is of eminent importance as a worse prognosis has been attributed to these variants[8,9] and, therefore, more aggressive therapeutic measures or at the least closer follow-up examinations are warranted.
In addition to these two variants, recent observations have emphasized that cutaneous manifestations of mycosis fungoides can rarely present with clinical features closely imitating various other benign or rarely malignant dermatoses. Its dissimulating capacity often poses a diagnostic dilemma for the clinician, leading to delayed diagnosis and often inappropriate therapeutic measures.
Therefore, the purpose of this article is to provide a comprehensive overview on published cases of mycosis fungoides imitating different diseases primarily on clinical grounds. In addition, we integrate our own atypical cases of mycosis fungoides to exemplarily delineate the diagnostic workup of such ambiguous cases.
As modern immunohistological and molecular genetic capabilities have significantly improved the diagnosis of cutaneous lymphoma, a vast plethora of clinically atypical variants of cutaneous lymphomas could be unveiled with the aid of these technical facilities. Especially the skin lesions of the most common cutaneous lymphoma subtype, mycosis fungoides, can imitate a wide variety of otherwise benign dermatoses (Table 1), and thus pose major diagnostic obstacles both to the dermatologist as well as the dermatopathologist[10]. Taking together, more than 40 different benign dermatoses - mainly inflammatory dermatoses such as psoriasis or granulomatous diseases - have been described as being clinically imitated by mycosis fungoides. These can be attributed to more than 10 leading clinical signs, mostly with psoriasiform or eczematous characteristics. In such cases, associated dermatopathological findings may also closely imitate its benign counterpart, i.e., psoriasiform epidermal hyperplasia in psoriasis vulgaris-like variants of mycosis fungoides, subepidermal blisters in bullous pemphigoid-like presentations, interstitial histiocytes and giant cells in granuloma annulare-like mycosis fungoides or interface dermatitis in mycosis fungoides with lichen planus-like skin lesions. In some cases, the reactive infiltrate may even overwhelm the malignant lymphoma cells, as it is often the case in granulomatous mycosis fungoides or slack skin syndrome. Especially in these instances, a thorough cytological assessment, extensive immunophenotyping of the infiltrate and, finally, molecular genetic techniques will be crucial diagnostic adjuncts in arriving at the correct diagnosis.
| Leading clinical sign | Differential diagnosis | Ref. |
| Eczematous | Seborrhoeic eczema | Nashan et al[1], Van Doorn et al[31] |
| Rosacea | Sherertz et al[32] | |
| Atopic eczema | Kazakov et al[33] | |
| Palmoplantar eczema | Spieth et al[34], Goldberg et al[35] | |
| Perioral dermatitis | Spieth et al[34] | |
| Other | Spieth et al[34] | |
| Psoriasiform | Psoriasis palmoplantaris | Spieth et al[34], Nashan et al[1] |
| Psoriasis vulgaris | Zackheim et al[36] | |
| Tinea corporis | Chave et al[37] | |
| Chronic discoid lupus erythematodes | Veysey et al[38] | |
| Tinea pedum | Hubert et al[39] | |
| Erysipelas | Brill et al[40] | |
| Erythematous | Annular erythema | Lim et al[41], Cogrel et al[42], Bernardini et al[43] |
| Erythema multiforme | Kazakov et al[33] | |
| Alopecia | Alopecia areata | Burg et al[44] |
| Hypopigmented | Pityriasis versicolor | Kazakov et al[33] |
| Pityriasis alba | Whitmore et al[45] | |
| Vitiligo | Ardigó et al[46] | |
| Leprosy | Kazakov et al[33] | |
| Postinflammatory hypopigmentation | Kazakov et al[33] | |
| Hyperpigmentated | Acanthosis nigricans | Willemze et al[47], Barnhill et al[48] |
| Ashy dermatosis | Kazakov et al[33] | |
| Lichen aureus | Fink-Puches et al[19] | |
| Purpura pigmentosa | Barnhill et al[48] | |
| Bullous | Bullous pemphigoid | Kneitz et al[49] |
| Pemphigus vulgaris | Roenigk et al[50] | |
| Mucositis | Wain et al[51] | |
| Hyperkeratotic | Verruca vulgaris | Goldberg et al[35], Wobser et al[52] |
| Keratosis lichenoides chronica | Bahadoran et al[53] | |
| Ichthyosis | Eisman et al[54], Kütting et al[55], Badawy et al[56] | |
| Follicular hyperkeratosis | Klemke et al[57] | |
| Porokeratosis Mibelli | Breneman et al[58] | |
| Seborrhoeic keratosis | Bazza et al[59] | |
| Bowen´s disease | Yoo et al[60] | |
| Pseudolymphoma | Marzano et al[61] | |
| Papular | Papuloerythroderma Ofuji | Hur et al[12], Pereiro et al[62], Nashan et al[1] |
| Comedones, cysts | Peris et al[63], Lacour et al[64], Oliwiecki et al[65], Vollmer et al[66] | |
| Pustular | Palmoplantar pustulosis | Moreno et al[67] |
| Generalized pustulosis | Camisa et al[68] | |
| Pyoderma gangraenosum | Ho et al[69], Carbia et al[70] | |
| Granulomatous | Granuloma annulare | Jouary et al[25], Goerdt et al[71], Topar et al[72], Kempf et al[7], van Haselen et al[73] |
| Rosacea | Spieth et al[34] | |
| Sarcoidosis | Bessis et al[74] | |
| Necrobiosis | Woollons et al[75] | |
| Others | Gangrene | Goldstein et al[76] |
| other tumors | Machler et al[77], Morcos et al[78] | |
| cutis laxa | Bessis et al[74] | |
| All | 12 major clinical signs | |
| 47 differential diagnoses |
To delineate this diagnostic workup of atypical cases of mycosis fungoides - which in many cases will require an individualized strategy - we herein provide an exemplifying set of own encountered cases on that topic (Table 2). Similar cases of most of these “mimickers” have already been reported in literature. Therefore, most of our cases fit well into one of the categories summarized in Table 1. Selected illustrated cases (case 1, 2, 5) and the clinicopathological workup are shortly reviewed below to better and more vividly illustrate the core message of this review and the complexity of the topic.
| Otherwise classical MF lesions | |||||||
| Patient | Leadingclinical sign | Differential diagnosis | Otherwiseatypical lesions | At timeof presentation | Duringclinical course | Histologicalfindings | Moleculargenetic findings |
| 1 | Erythematous | Erythema exsudativum multiforme | Yes | Yes | Yes | Classical MF | Not done |
| Psoriasiform | Tinea corporis | Classical MF | |||||
| Granulomatous | Granuloma anulare | Classical MF | |||||
| 2 | Papular | Papuloerythroderma Ofuji | No | No | No | MF/SS with folliculotropism, only slight epidermotropism | Monoclonal |
| 3 | Psoriasiform | Psoriasis | No | Yes | Yes | Classical MF | Monoclonal |
| 4 | Granulomatous | Granuloma anulare | No | Yes | Yes | Transformed MF with folliculotropism and giant cells | Biclonal |
| 5 | Hyperpigmented | Urticaria pigmentosa | No | No | Yes | Purpuric CD8+ MF | Monoclonal |
| 6 | Hypopigmented | Vitiligo | No | Yes | Yes | Purpuric CD8+ MF | Monoclonal |
| 7 | Hyperkeratotic | Plantar eczema | No | No | No | Classical MF | Monoclonal |
| 8 | Ulcerative | venous ulcer | No | Yes | Yes | Tumor stage MF | Not done |
| 9 | Eczematous | Rosacea | Yes | No | No | Folliculotropic MF | Polyclonal |
| Granulomatous | Anetoderma | MF with elastolysis | |||||
| 10 | Bullous | Bullous pemphigoid | No | Yes | Yes | CD8+ and CD30+ MF, subepidermal blistering | Monoclonal |
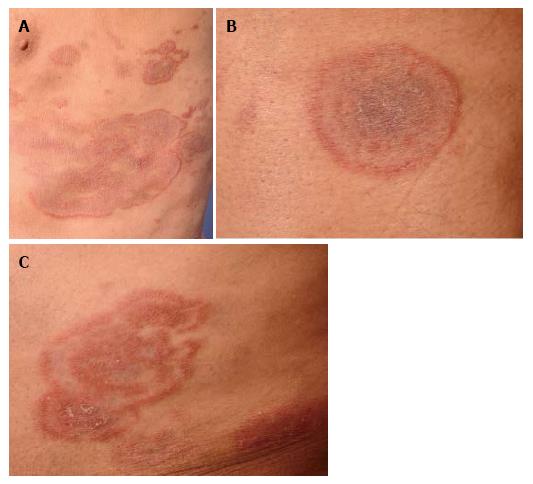
A 75-year-old patient presented with extensive patches and plaques, some of them with unusual configuration being clinically undistinguishable from tinea corporis, granuloma annulare or erythema exsudativum multiforme (Figure 1). Repetitive mycological examinations (KOH, fungal cultures) were negative. Corresponding to the simultaneous presence of more classical patches and plaques on whole-body examination, biopsies of different lesions revealed classical histological features consistent with CD4+ mycosis fungoides. Vacuolar interface reaction or granulomatous features - as would be suggestive of erythema exsudativum multiforme or granuloma annulare - were absent. Due to this histopathologically clear-cut diagnosis of mycosis fungoides, we refrained from further molecular genetic testing of the biopsy specimens. The patient responded to oral PUVA therapy in combination with bexarotene. This case shall provide an example that thorough clinical examination (revealing classical patches or plaques of mycosis fungoides) may provide the major contribution to the diagnostic workup of atypical cases in certain instances.
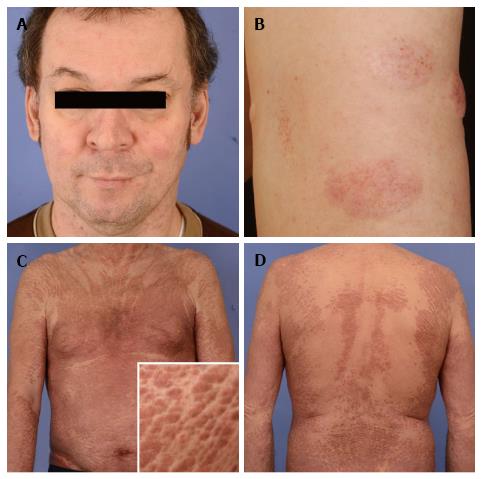
This patient was a 71-year-old patient presenting with slightly itchy skin lesions showing slow progression under topical steroids and UV-light therapy during several years. To note, a characteristic sparing of skin folds resembling Ofuji’s papuloerythroderma was noted (Figure 2C and D). After 3 years of an otherwise eventless course, follow-up examinations revealed an elevated leukocyte count with pathological CD4+ CD7- T-cell population in the peripheral blood corresponding to circulating Sézary-cells. Clonally identical neoplastic T-cells could also be detected in enlarged lymph nodes and bone marrow, so that stage 4 mycosis fungoides was diagnosed. The patient is currently scheduled under bexarotene in combination with extracorporal photophoresis. Under this regimen, stabilization of all lymphoma manifestations could be achieved. This case highlights that the matter of “mimikry” is even more complex. On the one hand, primarily benign dermatoses such as papuloerythroderma Ofuji may antedate overt lymphoma, thus being designated as putative precursor dermatoses[11]. On the other hand, however, mycosis fungoides and its leukemic counterpart may disguise as Ofuji´s papuloerythroderma and thus may delay the correct diagnosis of malignant disease[12].
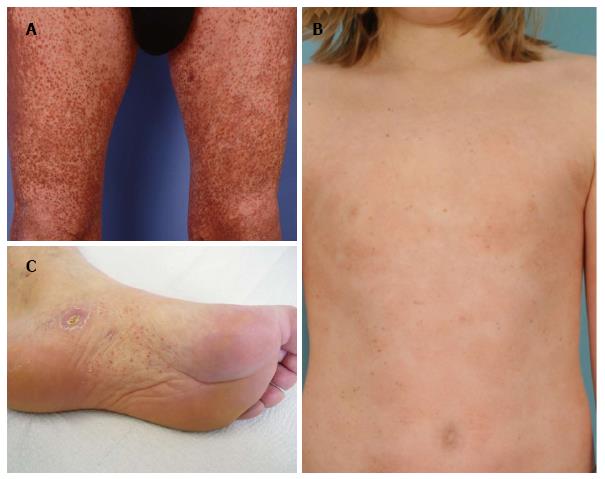
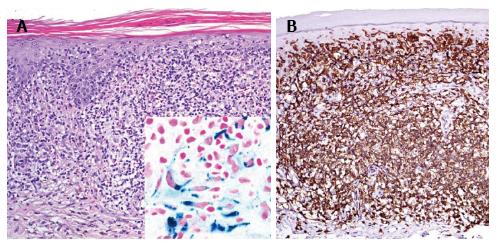
A 46-year-old patient presented with slowly progressive hyperpigmented brown to red papules and disseminated small macules on trunk and extremities highly resembling cutaneous mastocytosis/urticaria pigmentosa (Figure 3A). However, Darier’s sign was negative and histological examination of two representative lesions revealed an atypical CD8+ epidermotropic infiltrate with extensive purpura, interface dermatitis, melanophages and haemosiderophages without increased mast cell count (Figure 4C, inlet). At both biopsy sites the same T-cell clone was identified. Hence, purpuric/hyperpigmented CD8+ mycosis fungoides with atypical clinical manifestation was diagnosed. During further follow-up over the subsequent months blood involvement by clonally identical CD8+ T-cells was unrevealed and enlarged, suspicious lymph nodes were histologically proven to be involved by lymphoma. This peculiar imitator of case 5, mimicking urticaria pigmentosa/cutaneous mastocytosis, respectively, has not been previously described in literature. However, cases of mycosis fungoides with clinical features of pigmented purpuric eruptions including lichen aureus are well known[13-20]. Hyperpigmented or purpuric lesions of mycosis fungoides often show an otherwise uncommon CD8+ and cytotoxic phenotype of the neoplastic lymphocytes. Their cytotoxic effects on keratinocytes may result in prominent interface changes with resulting pigment incontinence. Moreover, capillary damage conveyed by perivascular lymphocytes releasing cytotoxic granules may lead to the observed purpuric changes. TCRγ+ and CD8+ cytotoxic cases of cutaneous lymphomas are usually associated with a poor therapeutic response even to multimodal aggressive treatment and therefore a dismal prognosis with 5-year survival rates below 30%[21-23]. Our patient initially exhibited rather rapid progression with peripheral blood and lymph node involvement of CD8+ lymphoma, however, achieved stabilization of lymphoma at time of submission of the manuscript under monochemotherapy with liposomal doxorubicin during a follow-up of 3 mo.
These exmplifying cases together with the prior integrating review of literature clearly delineates that, first, thorough clinical examination remains the mainstay in diagnosing ambiguous cases of mycosis fungoides. Thus, most of our patients showed one ore more further classical lesions of mycosis fungoides, either at time of initial presentation (6 out of 10) or later on during clinical follow-up (7 out of 10), so that diagnosis could be readily suspected based on thorough clinical whole-body examination and close follow-up examinations. Of note, some of our cases showed more than one atypical, imitating feature (case 1 and 9).
In a next step, we further correlated the corresponding histological findings as well as the results of the PCR-analysis of the TCR gene rearrangement in such clinically ambiguous cases.
All of those patients, who did not show any leading clinical signs suggestive of cutaneous lymphoma (4 out of 10) additionally demonstrated peculiar histological findings, such as uncommon immunophenotype (e.g., positive staining for CD8 or cytotoxic molecules), interface dermatitis, nearly absent epidermotropism, extensive folliculotropism or granulomatous changes. Especially CD8+ variants of mycosis fungoides tend to show distinct histological features. These include a subtle to prominent interface dermatitis with vacuolar degeneration of keratinocytes, subepidermal blistering/papillary edema in the absence of antiepidermal antibodies, or purpuric changes. As mentioned above, some of these characteristics may be due to the coexpression of cytotoxic molecules (perforin, granzyme B) by the lymphoma cells with putative consecutive proapoptotic properties on nearby stroma cells. Distinction from otherwise aggressive cutaneous lymphoma subtypes such as the provisional category of CD8+ primary cutaneous aggressive epidermotropic T-cell is of outstanding importance in patient management. Clinical signs (rapid onset of multiple disseminated ulcerated plaques, papules and tumors without prior patches and plaques, mucosal involvement) and symptoms (reduced performance state, fever, weight loss) and histological features (striking pagetoid epidermotropism of highly proliferating, atypical CD8+ lymphoma cells, epidermal necrosis and extensive ulceration) hint to this aggressive subtype[24]. Prompt and adequate, mostly more aggressive therapeutic measures are warranted for this fatal lymphoma subtype with an often dismal prognosis. Granulomatous features of mycosis fungoides may mimic palisading or necrobiotic granuloma reminiscent of granuloma annulare[25] or a more interstital pattern is present reminescent of palisading and granulomatous disease. Elastolysis and elastophagocytosis observed in the distinct and rare lymphoma subtype granulomatous slack skin syndrome[26] may dissimulate elastolytic giant cell granuloma on histological grounds. Stains for microorganism or culture are to be included within the diagnostic workup if infectious processes (e.g., mycobacterial or deep fungal infections) are suspected.
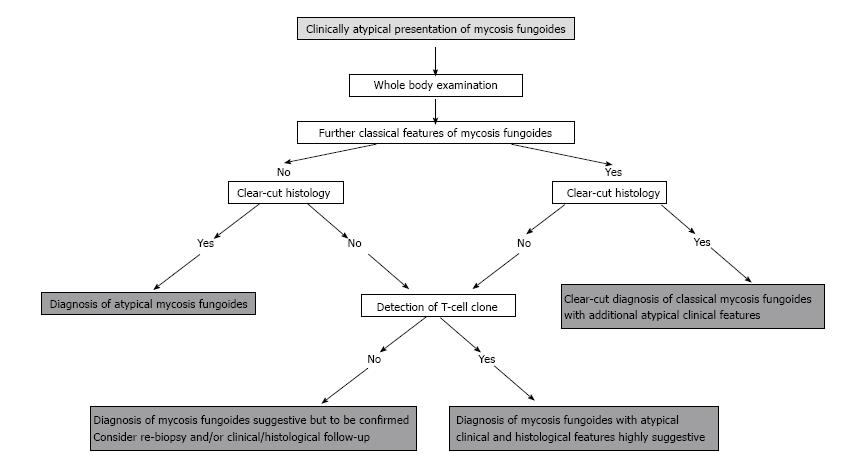
Usually, such lymphoid infiltrates with an ambiguous clinical picture as well as a challenging, arbitrary histology at time of initial presentation reveal a dominant T-cell clone on molecular genetic testing, thus providing the final decisive and pivotal step for correct diagnosis. This was also the case in our patient cohort. In this context, it has recently been shown that molecular genetic analysis is especially important in granulomatous lymphoma variants, a special subtype in which clinical manifestations may be atypical and on histology only the minority of the cellular infiltrate may actually be built up by the lymphoma cells itself[7,27,28]. Recently, it was shown that the proof of a dominant T-cell clone represents an important diagnostic tool to distinguish ambiguous cases of granulomatous lymphoma from reactive granulomatous disorders such as sarcoidosis, granuloma annulare or necrobiosis lipoidica, all presenting with overlapping clinical and histological features. Whereas in granulomatous lymphoma a TCR rearrangement was present in > 90% of cases, a monoclonal T-cell population was present in only 13% of the cases of granuloma annulare and could not be detected in sarcoidosis[27]. A persistent T cell clone is present in most cutaneous T-cell lymphomas, especially in advanced stages, most likely representing the disease causing tumour clone. Repetitive biopsies with tracking and comparative analysis of the lesional T-cell clones over the time course clearly improve diagnostic accuracy (“dual clonality”)[29]. This is also true for the differentiation from benign granulomatous diseases[28]. In addition, recent data have delineated that further, transiently appearing T cell clones frequently occur during the course of disease. The biological relevance of these additional clones is still unclear and has to be determined. Nevertheless, it may of interest to track additional T cell clones for diagnostic analyses[28]. On the contrary, however, molecular genetic analysis may also come to its limits as clone detection may be susceptible to faults such as it depends upon the applied technique, the density of the lymphoma infiltrate within the histological specimen and the quality of the investigated tissue. The detection rate may be enhanced by laser capture microdissection in sparse infiltrate, which, however, is not practical in every-day routine[30]. Hence, the application of molecular genetic techniques with analysis of the TCR rearrangement may in certain circumstances turn out to be the ultimate, pivotal diagnostic step in ambiguous cases. This point not for the first time again to its substantial, indispensable value in the diagnostic algorithm of cutaneous lymphoma (Figure 5).
To summarize, the comprehensive review of previously published data accomplished by our own cases underline that in ambiguous cases of mycosis fungoides a meticulous clinicopathological correlation including follow-up examination together with molecular genetic analysis provide decisive diagnostic adjuncts in finally obtaining the correct diagnosis. The intention of our article was to provide a comprehensive, illustrated overview of the fascinating and versatile nature of cutaneous lymphoma. By this, we intended to alert the clinician and dermatopathologist of rare and protean variants of mycosis fungoides, hence, not to miss the correct diagnosis and unnecessarily delay appropriate therapeutic measures.
| 1. | Nashan D, Faulhaber D, Ständer S, Luger TA, Stadler R. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;156:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 2. | Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 271] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 3. | Ferrara G, Di Blasi A, Zalaudek I, Argenziano G, Cerroni L. Regarding the algorithm for the diagnosis of early mycosis fungoides proposed by the International Society for Cutaneous Lymphomas: suggestions from routine histopathology practice. J Cutan Pathol. 2008;35:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Burg G, Kempf W, Cozzio A, Feit J, Willemze R, S Jaffe E, Dummer R, Berti E, Cerroni L, Chimenti S. WHO/EORTC classification of cutaneous lymphomas 2005: histological and molecular aspects. J Cutan Pathol. 2005;32:647-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 5. | Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2714] [Cited by in RCA: 2610] [Article Influence: 124.3] [Reference Citation Analysis (2)] |
| 6. | Cho-Vega JH, Tschen JA, Duvic M, Vega F. Early-stage mycosis fungoides variants: case-based review. Ann Diagn Pathol. 2010;14:369-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Kempf W, Ostheeren-Michaelis S, Paulli M, Lucioni M, Wechsler J, Audring H, Assaf C, Rüdiger T, Willemze R, Meijer CJ. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization For Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Benton EC, Crichton S, Talpur R, Agar NS, Fields PA, Wedgeworth E, Mitchell TJ, Cox M, Ferreira S, Liu P. A cutaneous lymphoma international prognostic index (CLIPi) for mycosis fungoides and Sezary syndrome. Eur J Cancer. 2013;49:2859-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Mantaka P, Helsing P, Gjersvik P, Bassarova A, Clausen OP, Delabie J. Clinical and histopathological features of folliculotropic mycosis fungoides: a Norwegian patient series. Acta Derm Venereol. 2013;93:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Sarantopoulos GP, Palla B, Said J, Kinney MC, Swerdlow SM, Willemze R, Binder SW. Mimics of cutaneous lymphoma: report of the 2011 Society for Hematopathology/European Association for Haematopathology workshop. Am J Clin Pathol. 2013;139:536-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Martínez-Barranca ML, Muñoz-Pérez MA, García-Morales I, Fernández-Crehuet JL, Segura J, Camacho F. Ofuji papuloerythroderma evolving to cutaneous T-cell lymphoma. J Eur Acad Dermatol Venereol. 2005;19:104-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Hur J, Seong JY, Choi TS, Jang JG, Jang MS, Suh KS, Kim ST. Mycosis fungoides presenting as Ofuji’s papuloerythroderma. J Eur Acad Dermatol Venereol. 2002;16:393-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Georgala S, Katoulis AC, Symeonidou S, Georgala C, Vayopoulos G. Persistent pigmented purpuric eruption associated with mycosis fungoides: a case report and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Lor P, Krueger U, Kempf W, Burg G, Nestle FO. Monoclonal rearrangement of the T cell receptor gamma-chain in lichenoid pigmented purpuric dermatitis of gougerot-blum responding to topical corticosteroid therapy. Dermatology. 2002;205:191-193. [PubMed] |
| 15. | Lipsker D. The pigmented and purpuric dermatitis and the many faces of mycosis fungoides. Dermatology. 2003;207:246-247. [PubMed] |
| 16. | Ugajin T, Satoh T, Yokozeki H, Nishioka K. Mycosis fungoides presenting as pigmented purpuric eruption. Eur J Dermatol. 2005;15:489-491. [PubMed] |
| 17. | Hanna S, Walsh N, D’Intino Y, Langley RG. Mycosis fungoides presenting as pigmented purpuric dermatitis. Pediatr Dermatol. 2006;23:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Magro CM, Schaefer JT, Crowson AN, Li J, Morrison C. Pigmented purpuric dermatosis: classification by phenotypic and molecular profiles. Am J Clin Pathol. 2007;128:218-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Fink-Puches R, Wolf P, Kerl H, Cerroni L. Lichen aureus: clinicopathologic features, natural history, and relationship to mycosis fungoides. Arch Dermatol. 2008;144:1169-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Toro JR, Sander CA, LeBoit PE. Persistent pigmented purpuric dermatitis and mycosis fungoides: simulant, precursor, or both? A study by light microscopy and molecular methods. Am J Dermatopathol. 1997;19:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 84] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Rodríguez-Pinilla SM, Ortiz-Romero PL, Monsalvez V, Tomás IE, Almagro M, Sevilla A, Camacho G, Longo MI, Pulpillo Á, Diaz-Pérez JA. TCR-γ expression in primary cutaneous T-cell lymphomas. Am J Surg Pathol. 2013;37:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Guitart J, Weisenburger DD, Subtil A, Kim E, Wood G, Duvic M, Olsen E, Junkins-Hopkins J, Rosen S, Sundram U. Cutaneous γδ T-cell lymphomas: a spectrum of presentations with overlap with other cytotoxic lymphomas. Am J Surg Pathol. 2012;36:1656-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Toro JR, Liewehr DJ, Pabby N, Sorbara L, Raffeld M, Steinberg SM, Jaffe ES. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003;101:3407-3412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Robson A, Assaf C, Bagot M, Burg G, Calonje J, Castillo C, Cerroni L, Chimenti N, Dechelotte P, Franck F. Aggressive epidermotropic cutaneous CD8(+) lymphoma: a cutaneous lymphoma with distinct clinical and pathological features. Report of an EORTC Cutaneous Lymphoma Task Force Workshop. Histopathology. 2014;Jan 18; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Jouary T, Beylot-Barry M, Vergier B, Paroissien J, Doutre MS, Beylot C. Mycosis fungoides mimicking granuloma annulare. Br J Dermatol. 2002;146:1102-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | LeBoit PE. Granulomatous slack skin. Dermatol Clin. 1994;12:375-389. [PubMed] |
| 27. | Pfaltz K, Kerl K, Palmedo G, Kutzner H, Kempf W. Clonality in sarcoidosis, granuloma annulare, and granulomatous mycosis fungoides. Am J Dermatopathol. 2011;33:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Dabiri S, Morales A, Ma L, Sundram U, Kim YH, Arber DA, Kim J. The frequency of dual TCR-PCR clonality in granulomatous disorders. J Cutan Pathol. 2011;38:704-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Thurber SE, Zhang B, Kim YH, Schrijver I, Zehnder J, Kohler S. T-cell clonality analysis in biopsy specimens from two different skin sites shows high specificity in the diagnosis of patients with suggested mycosis fungoides. J Am Acad Dermatol. 2007;57:782-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Yang H, Xu C, Tang Y, Wan C, Liu W, Wang L. The significance of multiplex PCR/heteroduplex analysis-based TCR-γ gene rearrangement combined with laser-capture microdissection in the diagnosis of early mycosis fungoides. J Cutan Pathol. 2012;39:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides, a distinct disease entity with or without associated follicular mucinosis: a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Sherertz EF, Westwick TJ, Flowers FP. Sarcoidal reaction to lymphoma presenting as granulomatous rosacea. Arch Dermatol. 1986;122:1303-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Kazakov DV, Burg G, Kempf W. Clinicopathological spectrum of mycosis fungoides. J Eur Acad Dermatol Venereol. 2004;18:397-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Spieth K, Grundmann-Kollmann M, Runne U, Staib G, Fellbaum C, Wolter M, Kaufmann R, Gille J. Mycosis-fungoides-type cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. [PubMed] |
| 35. | Goldberg DJ, Stampien TM, Schwartz RA. Mycosis fungoides palmaris et plantaris: successful treatment with the carbon dioxide laser. Br J Dermatol. 1997;136:617-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Zackheim HS, McCalmont TH. Mycosis fungoides: the great imitator. J Am Acad Dermatol. 2002;47:914-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Chave TA, Graham-Brown RA. Mycosis fungoides masquerading as tinea of the axilla. Clin Exp Dermatol. 2002;27:66-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Veysey EC, Wilkinson JD. Mycosis fungoides masquerading as cutaneous lupus erythematosus and associated with antiphospholipid syndrome. Clin Exp Dermatol. 2008;33:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Hubert JN, Callen JP. Recalcitrant tinea corporis as the presenting manifestation of patch-stage mycosis fungoides. Cutis. 2003;71:59-61. [PubMed] |
| 40. | Brill TJ, Ludwig RJ, Wolter M, Oeschger S, Thaçi D, Boehncke WH, Kaufmann R. Complicated mycosis fungoides mimicking facial erysipelas. Br J Dermatol. 2005;152:1381-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Lim DS, Murphy GM, Egan CA. Mycosis fungoides presenting as annular erythema. Br J Dermatol. 2003;148:591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Cogrel O, Boralevi F, Lepreux S, Vergier B, Merlio JP, Taieb A, Léauté-Labrèze C. Lymphomatoid annular erythema: a new form of juvenile mycosis fungoides. Br J Dermatol. 2005;152:565-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Bernardini ML, Brandozzi G, Campanati A, Giangiacomi M, Offidani A. Bullous-vesicular variant of mycosis fungoides presenting as erythema annulare centrifugum: a case report. J Eur Acad Dermatol Venereol. 2009;23:839-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Burg G, Schmöckel C. Syringolymphoid hyperplasia with alopecia--a syringotropic cutaneous T-cell lymphoma? Dermatology. 1992;184:306-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 45. | Whitmore SE, Simmons-O‘Brien E, Rotter FS. Hypopigmented mycosis fungoides. Arch Dermatol. 1994;130:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Ardigó M, Borroni G, Muscardin L, Kerl H, Cerroni L. Hypopigmented mycosis fungoides in Caucasian patients: a clinicopathologic study of 7 cases. J Am Acad Dermatol. 2003;49:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Willemze R, Scheffer E, Van Vloten WA. Mycosis fungoides simulating acanthosis nigricans. Am J Dermatopathol. 1985;7:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Barnhill RL, Braverman IM. Progression of pigmented purpura-like eruptions to mycosis fungoides: report of three cases. J Am Acad Dermatol. 1988;19:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 69] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Kneitz H, Bröcker EB, Becker JC. Mycosis fungoides bullosa: a case report and review of the literature. J Med Case Rep. 2010;4:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Roenigk HH. Pemphigus vulgaris. Arch Dermatol. 1971;104:105-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 51. | Wain EM, Setterfield J, Judge MR, Harper JI, Pemberton MN, Russell-Jones R. Mycosis fungoides involving the oral mucosa in a child. Clin Exp Dermatol. 2003;28:499-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Wobser M, Petrella T, Kneitz H, Kerstan A, Goebeler M, Rosenwald A, Geissinger E. Extrafacial indolent CD8-positive cutaneous lymphoid proliferation with unusual symmetrical presentation involving both feet. J Cutan Pathol. 2013;40:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Bahadoran P, Wechsler J, Delfau-Larue MH, Gabison G, Revuz J, Bagot M. Mycosis fungoides presenting as keratosis lichenoides chronica. Br J Dermatol. 1998;138:1067-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Eisman S, O’Toole EA, Jones A, Whittaker SJ. Granulomatous mycosis fungoides presenting as an acquired ichthyosis. Clin Exp Dermatol. 2003;28:174-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 55. | Kütting B, Metze D, Luger TA, Bonsmann G. Mycosis fungoides presenting as an acquired ichthyosis. J Am Acad Dermatol. 1996;34:887-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Badawy E, D‘Incan M, El Majjaoui S, Franck F, Fabricio L, Dereure O, Souteyrand P, Guillot B. Ichthyosiform mycosis fungoides. Eur J Dermatol. 2002;12:594-596. [PubMed] |
| 57. | Klemke CD, Dippel E, Assaf C, Hummel M, Stein H, Goerdt S, Orfanos CE. Follicular mycosis fungoides. Br J Dermatol. 1999;141:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Breneman DL, Breneman JC. Cutaneous T-cell lymphoma mimicking porokeratosis of Mibelli. J Am Acad Dermatol. 1993;29:1046-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Bazza MA, Ryatt KS, Dharmagunawardena PV. Mycosis fungoides masquerading as seborrhoeic keratosis. Br J Dermatol. 2002;147:1264-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Yoo SS, Viglione M, Moresi M, Vonderheid E. Unilesional mycosis fungoides mimicking Bowen‘s disease. J Dermatol. 2003;30:417-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Marzano AV, Berti E, Lupica L, Alessi E. Unilesional follicular mycosis fungoides. Dermatology. 1999;199:174-176. [PubMed] |
| 62. | Pereiro M, Sánchez-Aguilar D, Pereiro Ferreirós MM, Amrouni B, Toribio J. Cutaneous T-cell lymphoma: an expression of papuloerythroderma of Ofuji. J Eur Acad Dermatol Venereol. 2003;17:240-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Peris K, Chimenti S, Sacerdoti G, Muscardin L, Fazio M. Pilotropic mycosis fungoides. Dermatology. 1999;199:192-194. [PubMed] |
| 64. | Lacour JP, Castanet J, Perrin C, Ortonne JP. Follicular mycosis fungoides. A clinical and histologic variant of cutaneous T-cell lymphoma: report of two cases. J Am Acad Dermatol. 1993;29:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Oliwiecki S, Ashworth J. Mycosis fungoides with a widespread follicular eruption, comedones and cysts. Br J Dermatol. 1992;127:54-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Vollmer RT. Mycosis fungoides and follicular mucinosis. Arch Dermatol. 2002;138:1613-1614; author reply 1614-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 67. | Moreno JC, Ortega M, Conejo-Mir JS, Sanchez-Pedreño P. Palmoplantar pustulosis as a manifestation of cutaneous T cell lymphoma (mycosis fungoides). J Am Acad Dermatol. 1990;23:758-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Ho KK, Browne A, Fitzgibbons J, Carney D, Powell FC. Mycosis fungoides bullosa simulating pyoderma gangrenosum. Br J Dermatol. 2000;142:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Carbia SG, Hochman A, Chaín M, Dei-Cas I, Lagodín C, Devés A, Woscoff A. Mycosis fungoides presenting with extensive pyoderma gangrenosum-like ulcers. J Eur Acad Dermatol Venereol. 2002;16:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Goerdt S, Trautmann C, Kütting B, Ramaker J, Schmuth M, Thiel E, Luger T, Stein H, Orfanos CE. [Rare variants of cutaneous T-cell lymphomas]. Hautarzt. 1996;47:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 72. | Topar G, Zelger B, Schmuth M, Romani N, Thaler J, Sepp N. Granulomatous slack skin: a distinct disorder or a variant of mycosis fungoides? Acta Derm Venereol. 2001;81:42-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 73. | van Haselen CW, Toonstra J, van der Putte SJ, van Dongen JJ, van Hees CL, van Vloten WA. Granulomatous slack skin. Report of three patients with an updated review of the literature. Dermatology. 1998;196:382-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Bessis D, Sotto A, Farcet JP, Barnéon G, Guilhou JJ. Granulomatous mycosis fungoides presenting as sarcoidosis. Dermatology. 1996;193:330-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Woollons A, Darvay A, Khorshid SM, Whittaker S, Jones RR. Necrobiotic cutaneous T-cell lymphoma. J Am Acad Dermatol. 1999;41:815-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 76. | Goldstein LJ, Williams JD, Zackheim HS, Helfend LK. Mycosis fungoides masquerading as an ischemic foot. Ann Vasc Surg. 1999;13:305-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 77. | Machler BC, Elgart GW, Kerdel FA. Extracutaneous mycosis fungoides of the gastrocnemius muscle mimicking sarcoma. J Am Acad Dermatol. 1994;31:673-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 78. | Morcos SM, Girardi M, Subtil A, Wilson LD, Cowper SE. Mycosis fungoides exhibiting features of a dermatofibroma: a case report and review of the literature. J Cutan Pathol. 2012;39:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
P- Reviewer: Chen GS, Mesquita RA, Stanojevic GZ S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/