Peer-review started: November 22, 2014
First decision: December 26, 2014
Revised: February 25, 2015
Accepted: March 16, 2015
Article in press: March 18, 2015
Published online: May 2, 2015
Processing time: 158 Days and 16 Hours
Noninvasive in vivo imaging techniques have become an important diagnostic aid for dermatology. Dermoscopy, also known as dermatoscopy, has been shown to increase the clinician’s diagnostic accuracy when evaluating cutaneous neoplasms. Dermoscope, both hand-held and videodermoscope, are nowadays a basic instrument for almost all the dermatologists around the world. Trichoscopy is the term coined for dermoscopic imaging of the scalp and hair. Routinely using dermoscopy and recognizing the structures and patterns of the different types of alopecia will likely improve the observer’s sensitivity for diagnosis and follow up of hair and scalp disorders. Structures which may be visualized by trichoscopy include hair shafts of different types, the number of hairs in one pilosebaceous unit, hair follicle openings (dots), the peri and interfollicular areas and the vasculature. This review summarizes the current knowledge about trichoscopic findings which may aid in the diagnosis of alopecia. Besides diagnosing alopecia, it has the potential for obviating unnecessary biopsies and when a biopsy is still needed it is helpful in choosing an ideal biopsy site. Moreover, trichoscopy can be a valuable tool for evaluating the treatment response photographically at each follow-up. Finally, we have discussed the utility of dermoscopy in inflammatory scalp disorders and infections.
Core tip: Trichoscopy refers to the dermoscopy of the hair and scalp disorders. This is a noninvasive, in office technique that can be performed with a hand-held dermatoscope or a digital videodermatoscopy system. Trichoscopy is useful for the diagnosis and follow-up of hair and scalp disorders. In this article, we have briefly described the most important trichoscopic patterns and structures.
- Citation: Romero JAM, Grimalt R. Trichoscopy: Essentials for the dermatologist. World J Dermatol 2015; 4(2): 63-68
- URL: https://www.wjgnet.com/2218-6190/full/v4/i2/63.htm
- DOI: https://dx.doi.org/10.5314/wjd.v4.i2.63
Non-invasive in vivo imaging techniques have become an important diagnostic aid for evaluating hair and scalp disorders. Trichoscopy is the term coined for dermoscopic imaging of the scalp and hair.
Trichoscopy is a simple and non-invasive technique that can be performed with both handheld dermatoscope and digital videodermatoscopy system[1]. The usual working magnifications with videodermatoscope are 20-fold to 70-fold. While the hand-held dermatoscope with 10-fold magnification may give easy and quick evaluation of hair, it does not precisely measure or document the observed findings[2].
The method allows quick identification of hair and shaft abnormalities without the need of hair sampling for ex vivo evaluation, i.e., optical or scanning electron microscopy. It is also a helpful tool in differential diagnosis of common acquired hair diseases, such as androgenic alopecia or diffuse alopecia areata[2].
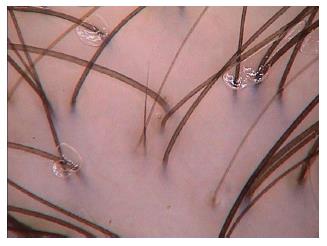
Structures which may be visualized by trichoscopy include hair shafts, hair follicle openings, the perifollicular epidermis and the cutaneous microvessels.
Trichoscopy allows analysing acquired and congenital hair shaft abnormalities.
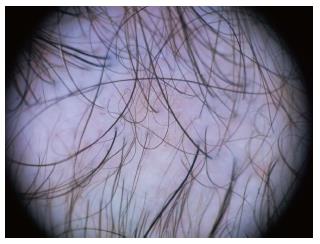
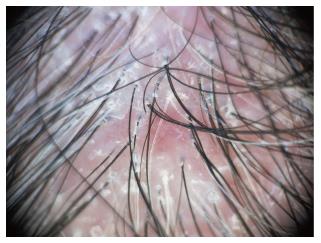
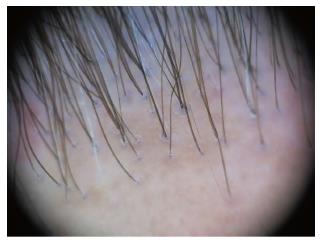
Dermoscopy of the normal scalp shows regularly distributed follicular units, containing 1-4 hair shafts[1]. A normal terminal hair is uniform in thickness and color throughout its length. However, up to 10% of normal scalp hairs are vellus hairs that are lightly pigmented and measure < 3 mm in length and < 30 μm in thickness[1,3].
Trichoscopy also allows diagnosing most genetic hair shaft dystrophies such as monilethrix, trichorrhexis nodosa, trichorrhexis invaginata, pili torti or pili annulati[4] (Table 1).
| > 20% of vellus hairs | AGA, long lasting AA |
| Exclamation mark hairs | AA, trichotillomania, chemotherapy-induced alopecia |
| Pohl-Pinkus constrictions | AA, chemotherapy-induced alopecia, blood loss, malnutrition, chronic intoxication |
| Comma hairs | Tinea capitis |
| Corkscrew hairs | Tinea capitis |
| Coiled hairs | Trichotillomania |
| Flame hairs | Trichotillomania |
| Tulip hairs | Trichotillomania, AA |
| Regrowing pigtail hairs | AA, cicatricial alopecia |
| Zig-zag-shaped hairs | Tinea capitis |
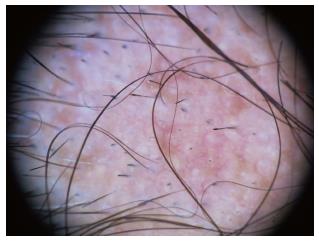
The term “dots” refers to the small, round hair follicle openings seen on trichoscopy. Trichoscopy may distinguish whether hair follicle openings are normal, empty, fibrotic or containing biological material, such as hyperkeratotic plugs or hair residues.
Black dots (cadaverized hairs) represent pigmented hairs broken or destroyed at scalp level.
Yellow dots are follicular infundibula with keratotic material and/or sebum. They vary in color, shape and size[4].
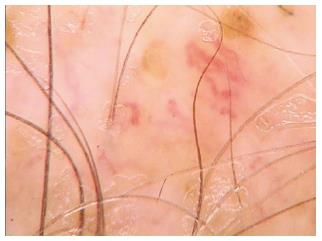
White dots may appear as fibrotic white dots or pinpoint white dots. The classic, big, irregular white dots represent areas of perifollicular fibrosis, observed in primary, folliculocentric cicatricial alopecias, and most commonly in lichen planopilaris[3]. The pinpoint white dots are small and regular, with occasional peripheral hyperpigmentation. They correspond to empty hair follicles or to the eccrine sweat ducts openings. They are observed in sun exposed areas and in dark skin phototypes.
Red dots have been described in discoid lupus erythematosus and in patients with vitiligo.
Pink-grey and grey dots have been observed in the eyebrow area of patients with frontal fibrosing alopecia[3]. This finding is believed to be a favourable prognostic factor for eyebrow regrowth (Table 2).
| Black dots | Active AA, dissecting cellulitis, tinea capitis, chemotherapy-induced alopecia, trichotillomania, after laser depilation, after trichogram, incidental finding in other diseases |
| Yellow dots | AA: Marker of disease severity Discoid lupus erythematosus: Large, dark yellow to brownish-yellow dots Androgenic alopecia: "Oily" appearance and predominance in frontal area Dissecting cellulitis, trichotillomania: Imposed over dark dystrophic hairs |
| White dots | Primary folliculocentric alopecias, lichen planopilaris: Fibrotic white dots Dark skin, sun exposed areas: Pinpoint white dots |
| Red dots | Discoid lupus erythematosus, vitiligo |
| Pink-grey/grey dots | Frontal fibrosing alopecia (eyebrows) |
The classification of peri- and interfollicular skin surface abnormalities in trichoscopy is based on features related to scaling, color, discharge and surface structure[3] (Table 3).
| Epidermal scaling | Diffuse scaling: Healthy individuals, psoriasis, seborrheic dermatitis Perifollicular scaling and tubular scaling structures: Lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans |
| Hyperpigmentation | Honeycomb: Sun exposed areas, dark skin phototypes Perifollicular: Androgenetic alopecia Scattered interfollicular: Discoid lupus erythematosus |
| Yellow or yellow-red discharge | Folliculitis decalvans, bacterial infections, dissecting cellulitis, tinea capitis |
| Structural changes in the skin surface | Starburst pattern hyperplasia in folliculitis decalvans |
The interest for trichoscopy had greatly increased in the last years. There is an extensive literature on the different types of alopecia. Nowadays it has become an indispensable technique in the evaluation of the hair loss patient.
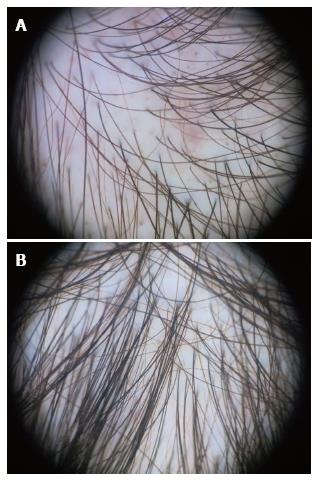
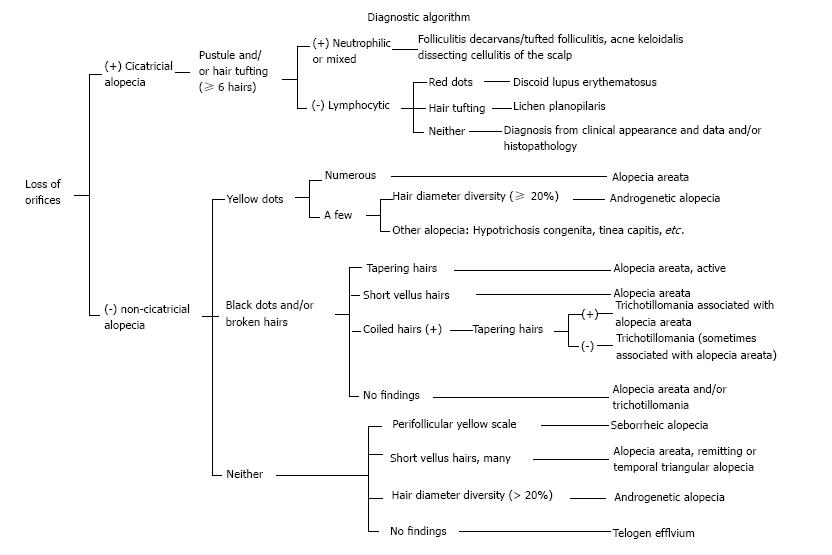
We have summarized the main trichoscopic findings in the most common types of non cicatricial (Table 4) (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5) and cicatricial alopecia (Table 5, Figure 6, Figure 7, Figure 8). In addition, there are some diagnostic algorithms in use for alopecia using trichoscopy (Figure 9).
| AGA | AA | Telogen effluvium | Trichotillomania |
| Hair shaft thickness heterogeneity | Exclamation mark hairs | Empty follicles | Simultaneous, chaotic coexistence of multiple hair shaft abnormalities |
| Increased proportion of vellus hairs | Broken hairs | Increased proportion of single-hair follicular units | Hairs broken at different lengths |
| > 10% thin hairs in the frontal area | Clustered short vellus hairs | Short regrowing hairs | Short hairs with trichoptilosis (split ends) |
| Increased proportion of single-hair follicular units | Pigtail hairs | < 20% hair diameter diversity | Coiled hairs |
| Yellow dots | Black dots | Brown perifollicular discoloration | Exclamation mark hairs |
| Perifollicular discoloration (peripilar sign) | Numerous yellow dots | No significant difference between frontal and occipital areas | Black dots |
| All the trichoscopic features appear most prominently in the frontal scalp area compared to the occipital area | Others: flame hairs, V-sign, hook hairs, hair powder and tulip hairs |
| LPP | FFA | DLE |
| Intense perifollicular scaling, tubular structures | Minor perifollicular scaling | Large yellow dots (follicular keratotic plugs) |
| Violaceous inter or perifollicular areas | Perifollicular erythema | Mottled dyschromia |
| Fibrotic white dots | Strong predominance of single-hair follicular units at the hair-bearing margin | Thin and radial arborizing vessels that emerge from yellow dots (“red spider in yellow dot”) |
| Blue grey dots | Absence of vellus hairs | Thick arborizing vessels at the perifery ot the lesion |
| Small hair tufts | Pink-grey and grey dots in the lateral eyebrow area | Follicular red dots |
Standard methods used to diagnose hair disorders are clinical inspection, pattern of hair loss, pull test, trichogram, biopsy, chronology of preceding events, and screening blood test. They vary in sensitivity, reproducibility, and invasiveness. There is accumulated evidence that the use of trichoscopy in the clinical evaluation of hair disorders improves diagnostic capability beyond simple clinical inspection[5-7]. Trichoscopy have the advantages of being a quick and non-invasive, semiquantitative method. Trichoscopy allows to evaluate larger areas than other invasive techniques like biopsy or trichogram.
A recent study have demonstrated superiority of trichoscopy as compared to the trichogram, in the diagnosis of female androgenetic alopecia, especially in early cases[8].
An additional advantage of trichoscopy is that it allows digital surveillance and monitoring of the patients. Photographic evaluation at each visit is very appreciated by patients[9].
By the other hand, the use of trichoscopy can result in lower diagnostic accuracy if the physician does not recognize or correctly interpret the significance of structures. Moreover, trichoscopy can lead to lower diagnostic accuracy when patients are diagnosed using dermoscopy alone, without clinical context.
Psoriasis and seborrheic dermatitis: Dermatoscopy of both cases shows diffuse or localized scales, which tend to be more yellowish in seborrheic dermatitis and more withish in psoriasis.
The major difference in this cases is the vascular pattern. Psoriasis shows red dots, globules and glomerular vessels. In seborrheic dermatitis the most common findings are arborizing and atypical red vessels and the absence of red dots and globules[1].
Pediculosis capitis: Trichoscopy is useful to observe the adult parasites ant to evaluate if the nits are empty or not.
Piedra and tinea capitis: Many features have been described in mycotic infections and dermatoscopy is very useful in these cases.
Trichoscopy may be used to select the best area from which to obtain a biopsy specimen[3].
This includes possible application of trichoscopy in identifying follicular spicules in multiple myeloma, follicular mucinosis in lymphoproliferative disorders, scalp lesions in Langerhans histiocytosis or altered interfollicular microvessels in dermatomyositis and scleroderma[4].
Ex vivo assessment of scalp biopsies by dermatoscopy can identify the correct plane of transversal bisection and it is useful to control the tissue processing[10].
It has been proposed the use of dermoscopy when performing a trichogram, instead of using the optical microscope, as most of dermatologist nowadays have a dermoscope[1].
| 1. | Torres F, Tosti A. Trichoscopy: an update. G Ital Dermatol Venereol. 2014;149:83-91. [PubMed] |
| 2. | Olszewska M, Rudnicka L, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy. Arch Dermatol. 2008;144:1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Mubki T, Rudnicka L, Olszewska M, Shapiro J. Evaluation and diagnosis of the hair loss patient: part II. Trichoscopic and laboratory evaluations. J Am Acad Dermatol. 2014;71:431.e1-431.e11. [PubMed] |
| 4. | Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J Dermatol Case Rep. 2011;5:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (2)] |
| 5. | Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 191] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 6. | Tosti A, Whiting D, Iorizzo M, Pazzaglia M, Misciali C, Vincenzi C, Micali G. The role of scalp dermoscopy in the diagnosis of alopecia areata incognita. J Am Acad Dermatol. 2008;59:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 7. | Toncić RJ, Lipozencić J, Pastar Z. Videodermoscopy in the evaluation of hair and scalp disorders. Acta Dermatovenerol Croat. 2007;15:116-118. [PubMed] |
| 8. | Galliker NA, Trüeb RM. Value of trichoscopy versus trichogram for diagnosis of female androgenetic alopecia. Int J Trichology. 2012;4:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Tosti A. Tricoscopia. Tricología. Enfermedades del folículo pilosebáceo. Madrid: Editorial Aula Medica Ed 2013; 183. |
| 10. | Miteva M, Lanuti E, Tosti A. Ex vivo dermatoscopy of scalp specimens and slides. J Eur Acad Dermatol Venereol. 2014;28:1214-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
P- Reviewer: Cuevas-Covarrubias SA, González-López MA S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/