Revised: December 26, 2013
Accepted: March 13, 2014
Published online: May 2, 2014
Processing time: 214 Days and 11.7 Hours
Photodynamic therapy (PDT) is a relatively new therapy in dermatology that uses the topical application of a porphyrin derivative to selectively destroy a cutaneous target. The action is implemented by the application of a specific light frequency. The ability of porphyrin to selectively target tumor tissue has been known since the 1960s. In the late 1970s, the underlying mechanism was defined, and Dougherty’s discovery of the first chromophore led to the production and commercialization of Photofrin®. Many other chromophores that can act as photosensitizers have been studied since then, with aminolevulinic acid currently the most commonly used chromophore in clinical practice. PDT is simple, minimally invasive and can be administered on an outpatient basis. The efficacy of PDT has been proven for actinic keratosis, Bowen’s disease and basal cell carcinoma; another of its well-known applications is the treatment of photoaging. Indications for its use are continuously increasing, and promising results are reported for various skin diseases. In this paper we report the mechanism of action of PDT with aminolevulinic acid, the literature concerning the most common diseases treated with PDT and the subsequent level of evidence.
Core tip: Photodynamic therapy (PDT) with aminolevulinic acid is a relatively new therapy in dermatologic practice, and the indications for PDT are increasing continuously. PDT is based on the topical application of a porphyrin derivative followed by exposure of the treated area to a specific wavelength of light to selectively destroy a cutaneous target. A thorough knowledge of the mechanism of action of the treatment and its effects are necessary to provide the patient with an appropriate assessment and indication. In this paper we report the mechanism of action of PDT with aminolevulinic acid, the literature concerning the most common diseases treated with PDT and the subsequent level of evidence, according to the European Guidelines.
- Citation: Negosanti L, Pinto V, Sgarzani R, Negosanti F, Zannetti G, Cipriani R. Photodynamic therapy with topical aminolevulinic acid. World J Dermatol 2014; 3(2): 6-14
- URL: https://www.wjgnet.com/2218-6190/full/v3/i2/6.htm
- DOI: https://dx.doi.org/10.5314/wjd.v3.i2.6
Photodynamic therapy (PDT) with aminolevulinic acid (ALA) is a relatively new therapy in dermatologic practice. PDT is based on the topical application of a porphyrin derivative followed by the exposure to a specific light wavelength to selectively destroy a cutaneous target. The indications for PDT are continuously increasing (Table 1). In this paper, we report the mechanism of action of ALA-PDT, the literature concerning the most common diseases treated with PDT and the subsequent level of evidence, according to the European Guidelines.
| Oncological skin disease |
| Actinic keratoses |
| Bowen’s disease |
| Basal cell carcinoma |
| Squamous cell carcinoma |
| Cutaneous T-cell lymphoma |
| Cutaneous B-cell lymphoma |
| Extra-mammary paget’s disease |
| Non oncological skin disease |
| Acne vulgaris |
| Warts |
| Photorejuvenation |
| Other indications: |
| Cutaneous leishmaniasis |
| Localized scleroderma |
| Lichen sclerosus |
| Perioroal dermatitis |
| Cutaneous mycosis |
The use of light as a therapeutic tool was reported in 1900, when sunlight was used to activate eosin for tumor treatment[1,2]. In 1960, Winkelman[3] used a porphyrin to detect tumor tissue. In the same year, Schwartz isolated an impure hematoporphyrin derivative (HpD)[1], and Lipson suggested that the derivate could be used as a photosensitizer to destroy neoplastic tissue[4]. The mechanism of cellular destruction was recognized as singlet oxygen production in 1976[5].
Dougherty discovered that the HpD had a high singlet oxygen quantum yield, a maximum absorption in the red spectrum and was selectively retained in tumor tissue[6]. The active fraction of the HpD was then isolated and produced as Photofrin®. Dougherty can be considered the inventor of PDT.
Since the introduction of Photofrin® in medicine, many other photosensitizers have been studied, and ALA was recently introduced in PDT for the treatment of superficial skin lesions.
The mechanism of action of PDT can be explained by analyzing the photochemical reaction that generates singlet oxygen[1].
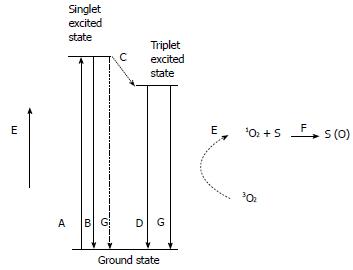
A chromophore exposed to a light with a specific wavelength is excited to a singlet state that is unstable. From the singlet state, the chromophore passes to the ground state, either emitting a fluorescent photon or transforming into the stable triple state (Figure 1). In an aerobic environment, the chromophore transfers energy to ground state oxygen, producing singlet oxygen (1O2). The ability to produce singlet oxygen is one of the most important factors in determining the activity of a chromophore.
Singlet oxygen in an aqueous environment has a lifetime of 2 μs, and its energy is dissipated as heat in a spherical volume of 10 nm in diameter.
In conclusion, PDT can induce oxidative damage that is localized near the point of production of singlet oxygen or in a region larger in diameter than a cell membrane; this induction can only occur in an oxygenated environment.
Among the different chromophores studied as photosensitizers, ∆5-aminolevulinic acid (ALA) is the most commonly used to treat skin disease.
ALA is a precursor of heme[7], and overloading a cell with ALA forces the cell to produce protoporphyrin-IX (PP), which acts as photosensitizer.
Neoplastic cells present a relative reduction of ferrochelatase activity, which is responsible for the conversion of PP into heme. Therefore, PP production is faster than its conversion to heme, and PP accumulates in cells that become photosensitive.
ALA selectively accumulates in tumor cells because of its high reproduction rate[6,8]; the esterification of ALA alters its captation through the membrane from an active to passive mechanism, leading to high ALA penetration into cells.
The association with iron chelation appears to increase porphyrin loading and light action, while the entry of porphyrin into cells is limited by an intact corneal layer.
By illuminating PP with adequate light, we can induce the production of singlet oxygen, which leads to damage that is confined to membranes and subcellular organs.
The light used to activate PP requires a wavelength greater than 600 nm to reduce adsorption from melanin and hemoglobin. The absorption peak of PP is from 630-635 nm.
ALA is offered on the market as a cream with a concentration of 20%.
More recently, methyl aminolevulinate (MAL), a derivative of ALA, was introduced in clinical practice. MAL accumulates in tumor cells with a mechanism similar to ALA and is hydrolyzed in the cytoplasm, releasing ALA into the cytosol. Unlike ALA, MAL is lipophilic.
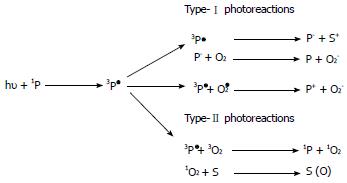
PP is excited by light with an adequate wavelength and, during the subsequent relaxation phase, PP transfers energy to oxygen producing singlet oxygen (type II reaction) and free radicals (type I reaction) (Figure 2). The type II reaction is predominant in PDT[9,10].
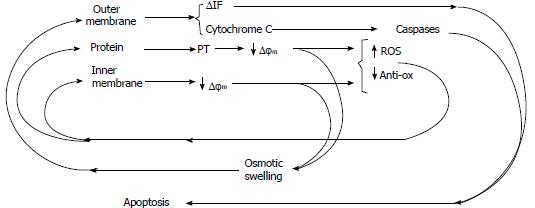
The damage induced in the mitochondrial membrane leads to enzymatic inhibition and a subsequent breakdown of the electron transport chain and cytochrome C release, which is responsible for apoptosis induced by caspase pathway activation[11,12] (Figure 3).
Oxygen free radicals also induce damage in endothelial cells, causing inflammation and platelet aggregation. The consequent thrombosis of intra-tumoral vessels starves neoplastic cells of oxygen and nutrients, promoting necrosis[13,14].
Another effect induced by PDT is linked to indirect immune system activation against the tumor. The damage induced to tumoral tissues releases neoplastic antigens, which are captured by dendritic cells and presented to T lymphocytes, inducing a specific immune action against the tumor[15].
All of the reported mechanisms are responsible for the damage induced by PDT to neoplastic tissues.
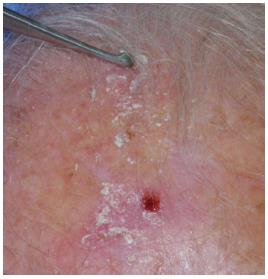
The lesion is adequately treated with a curette to selectively “modify” the corneal layer (Figure 4). No bleeding should be present and hemostasis must be obtained in cases where bleeding occurs (Figure 5).
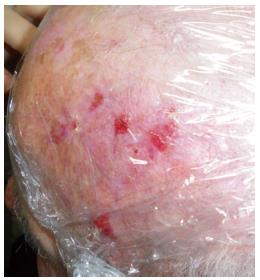
ALA 20% cream is then applied to the lesion using approximately 50 mg/cm2. The lesion is then occlusively dressed (Figure 6), and sun exposure must be avoided for at least 3 h[16].
After 3 h, the dressing is removed, and the lesion is exposed to specific light for 12 min. The eyes must be protected during the exposure.
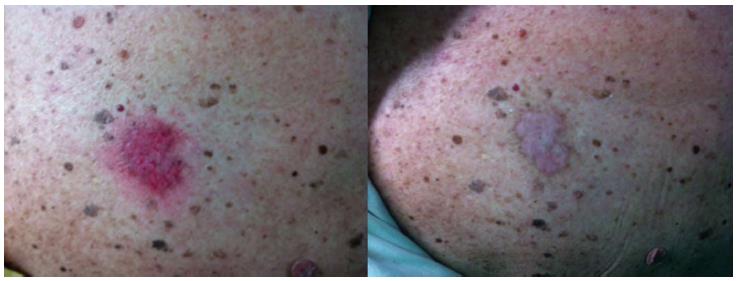
Pain during and/or after the treatment is the most common adverse effect and is most likely due to the direct stimulation of free nerve endings in the epidermis during irradiation and to the inflammatory environment induced by PDT[17,18]. It is particularly noticeable in locations such as the scalp, face and hands and is related to the extension of the treated area. It has been proven that topical anesthesia is not effective in reducing this type of pain[19,20], whereas good results were reported using devices that blow cold air[21]. Erythema and edema are common after PDT; crust formation may also be observed, but complete healing is generally achieved in 2-6 wk (Figure 7).
PDT is currently licensed for the treatment of actinic keratosis, Bowen’s disease and basal cell carcinoma, but there are several studies on the use of PDT for other skin diseases[22,23], and the possible indications are increasing continuously. The reported results of PDT in various skin diseases are summarized in Table 2. The strength of the recommendation and the level of the evidence for these different indications are reported in Table 3.
| Ref. | Disease | Remission rate | Follow up | n |
| Jeffes et al[24], 1997 | AK | 91% scalp, face 45% trunk | 16 wk | 40 |
| Tschen et al[25], 2006 | AK scalp, face | 78% | 12 mo | 101 |
| Stender et al[26], 1996 | AK lip | 100% | 12 mo | 3 |
| Cairnduff et al[27], 1994 | BD | 89% | 18 mo | 36 |
| López et al[29], 2012 | BD | 90% | 12 mo | 18 |
| Truchuelo et al[30], 2012 | BD | 76% | 17 mo | 47 |
| Calzavara-Pinton et al[31], 2008 | SCC | 57% microinvasive 26% nodular invasive | 2 yr | 55 |
| Christensen et al[32], 2009 | BCC | 68% single session 91% two session | 6 yr | 60 |
| Christensen et al[33], 2012 | BCC | 60% single session 87% two session | 10 yr | 60 |
| Souza et al[34], 2009 | BCC | > 90% | 3 mo | 34 |
| 60% | 5 yr | |||
| Soler et al[35], 2001 | BCC | 89% | 3 yr | 350 |
| Svanberg et al[49], 1994 | TCL | 100% | 6-14 mo | 4 |
| Orenstein et al[50], 2000 | TCL | 100% | 24-27 mo | 2 |
| Mori et al[51], 2006 | BCL | 100% | 8-24 mo | 3 |
| Shieh et al[52], 2002 | PD | 50% | 6 mo | 5 |
| 31% | 10 mo | |||
| Raspagliesi et al[54], 2006 | PD | 57% | 1-5 mo | 7 |
| Stender et al[58], 2000 | Hand-foot W | 76% | 18 wk | 45 |
| Fabbrocini et al[59], 2001 | Plantar W | 75% | 2 mo | 64 |
| Fehr et al[63], 2002 | Vulvar W | 66% | 12 mo | 16 |
| Stefanaki et al[64], 2003 | Male genital W | 73% | 12 mo | 12 |
| Disease | Strength of recommendation | Quality of evidence |
| Actinic keratoses | A | I |
| Bowen’s disease | A | I |
| Invasive squamous cell carcinoma | D | II-iii |
| Superficial basal cell carcinoma | A | I |
| Nodular basal cell carcinoma | A | I |
| NMSC in organ transplant recipients | B | I |
| Prevention of NMSC in organ transplant recipients | B | I |
| Field cancerization | B | I |
| Cutaneous T-cell lymphoma | C | II-iii |
| Extra-mammary Paget’s disease | D | III |
| Infectious and inflammatory dermatoses acne | A | I |
| Hand and foot warts | B | I |
| Genital warts | B | I |
| Cutaneous leishmaniasis | B | I |
| Photorejuvenation | B | I |
Actinic keratosis: Actinic keratosis is a very frequently reported skin disease that can lead to squamous cell carcinoma in 5% to 20% of cases in 10-25 years. Treatment with PDT reported good results, with high remission rates. In 1997, Jeffes et al[24] reported a remission rate of 91% in the treatment of actinic keratosis of the face and scalp using ALA-PDT. Lower success rates (45%) were reported in trunk localizations, most likely due to less penetration of the photosensitizer. A comparison of different ALA concentrations led to Jeffes et al[24] assessment that a 20% concentration is preferable for clinical applications. Tschen et al[25] treated patients affected by actinic keratoses of the face and scalp with up to 2 sessions of ALA-PDT. He reported a clearance rate of 78% at 12 mo, with a recurrence rate of 19%; the cosmetic outcome was satisfactory in all cases, without any hyperpigmentation. Additionally, good results were reported for actinic cheilitis, with a complete clearance after 12 mo of follow up; the final aesthetic outcome was good, even if superficial peeling persisted for several months[26].
ALA-PDT is an efficacious treatment for scalp and facial actinic keratosis, with high clearance rates and satisfactory cosmetic outcomes. The efficacy is higher using an ALA concentration of 20%. Actinic keratosis of the trunk and acral sites presents a lower remission rate in comparison to scalp and facial localization.
Bowen’s disease: PDT is an effective treatment option for Bowen’s disease. Remission rates of 89% were reported by Cairnduff et al[27] in 1994, and in 1996, Morton et al[28] reported higher clearance rates and better cosmetic outcomes with ALA-PDT compared with cryotherapy. More recently, in 2012, 29. López et al[29] reported a complete clearance of 90% in 23 lesions treated by MAL-PDT, with a good aesthetic result. In another study conducted on 47 patients affected by Bowen’s disease, a complete clearance of 76% was reported after 17 mo from 2 sessions of MAL-PDT[30].
Invasive squamous cell carcinoma: Squamous cell carcinoma presented a lower sensitivity to PDT in comparison with basal cell carcinoma. Clearance rates of 57% and 26% have been reported in microinvasive and nodular invasive squamous cell carcinoma, respectively, 2 years after treatment with MAL-PDT[31]. Considering the lower response rate and the metastatic potential, PDT is not the first choice for invasive squamous cell carcinoma treatment.
Basal cell carcinoma: PDT is particularly indicated for basal cell carcinoma treatment, especially in superficial carcinomas and in Gorlin Syndrome.
Several studies reported good results in the treatment of basal cell carcinoma with PDT[32-35], with clearance rates up to 90% at 3 years and 87% at 10 years.
Similar results have been reported in studies comparing PDT to cryotherapy, with PDT obtaining a better aesthetic outcome[36]. Good results were reported in patients affected by Gorlin Syndrome[37], with a reduction in the need for surgical procedures.
Relatively poor results were obtained in invasive carcinoma and in morphoeic basal cell carcinoma[38]; therefore, PDT must be considered not indicated in these cases.
Organ transplant recipients and field cancerization: PDT has been studied for the treatment and prevention of non-melanoma skin cancer (NMSC) in organ transplant recipients. An important factor to emphasize is the reduced response rate in non-immunocompetent patients compared with immunocompetent patients, due to the role of the immune system in the action of PDT[39]. PDT cannot be considered as a first choice in the treatment of skin cancer in organ transplant recipients, nor can it play a significant role in the prevention of NMSC in non-immunocompetent patients[40].
However, in immunocompetent patients with multiple clinical or subclinical cancerous skin lesions, PDT can be used to prevent the lesions from evolving into invasive carcinomas[41-43]. This preventive effect of PDT has been explained by the reduced expression of the proto-oncogene p53 in treated tissues[44].
Cutaneous T-cell and B-cell lymphomas: Dougherty et al[45] and Forbes et al[46] demonstrated the efficacy of PDT in the treatment of cutaneous T-cell lymphoma. The selective uptake of ALA and MAL into CD71+ lymphocytes was observed[47]. Small series with good results are reported in the literature[48-50]. Satisfactory results were also reported by Mori et al[51] in 3 patients affected by cutaneous B-cell lymphoma. The effectiveness of PDT in the treatment of cutaneous lymphomas has not been proven; therefore, PDT should not be considered an indication. Further studies are necessary to verify the application of PDT in this field.
Extra-mammary Paget’s disease: Few reports have shown promising results in the treatment of Paget’s disease[52-55]. A more appropriate role for PDT may be as an adjuvant therapy in association with radiotherapy or surgery[56], as PDT not only induces tissue destruction but also causes a reduction in tumor cells’ adhesive and metastatic capacities.
Acne vulgaris: PDT has been used with good results in the treatment of acne vulgaris.
It was noted that ALA accumulates in sebocytes, which are then destroyed by the photoreaction, reducing sebum excretion. Another positive effect is linked to production of porphyrins by Propionibacterium acnes, which became a target for PDT[57].
Warts: Several studies have reported good results concerning the application of ALA-PDT for the treatment of warts[58-60]. Many comparative studies reported higher clearance rates in patients treated with ALA -PDT versus patients treated with a placebo[58,59] or cryotherapy[60]. Similar results were reported in hand and foot warts and in genital warts[61-64]. In the treatment of genital warts, PDT was reported to have clearance rates similar to other therapies, such as laser CO2, but lower recurrence rates.
It should be noted that studies regarding MAL-PDT in the treatment of warts are limited.
Accurate curettage of lesions is mandatory to reduce typical hyperkeratosis, which limits the penetration of the photosensitizer.
Other indications: Some other indications for PDT are currently under study.
A review of patients affected by cutaneous leishmaniasis reported complete healing in 94%-100% of cases treated with PDT[65]. These results were confirmed by randomized trials[66]. The mechanism was studied in vivo by Kosaka et al[67], who suggested that the clinical outcomes observed with ALA-PDT are the result of unspecific tissue destruction accompanied by the depopulation of macrophages rather than by the direct destruction of parasites, as observed by previous in vitro studies[68].
Several series have reported improvements in PDT-treated patients diagnosed with localized scleroderma[69,70], lichen sclerosus[71], perioral dermatitis[72] and cutaneous mycosis[73,74].
These preliminary results appear to be promising, but further research is necessary to demonstrate the actual efficacy of PDT in the reported diseases.
Photorejuvenation: Many studies have reported good results for skin rejuvenation after the application of PDT[75-77]. PDT is useful for improving dyspigmentation, depigmentation, fine lines and roughness, skin smoothness and for reducing actinic elastosis[78]. Other techniques can be combined with PDT to improve results, acting synergistically to induce self-stimulated collagen biosynthesis (microneedling, ablative fractional lasers) or to improve photosensitizer penetration (curettage, peeling, lasers, microneedling). All of these treatments must be performed before PDT; chemical peeling can be applied immediately before PDT or up to 3 d (maximum) prior to PDT; curettage can be performed 2 wk prior to PDT; and lasers, microneedling and mechanical peeling can be applied immediately before PDT. Other aesthetic procedures can be associated with PDT; fillers can be used 2 wk after PDT, and botulinum toxin can be used 2 wk prior (at the earliest) to PDT. Pre-treatment can increase phototoxic effects, prolonging the downtime of the patient. The expected aesthetic results are usually observed after 3-6 mo, and 2 or 3 sessions conducted every 4 wk are usually necessary. The scalp is more painful than other sites, whereas the hands are usually reported as minimally painful. The neck and décolleté present fewer skin appendages, thereby leading to more prolonged re-epithelization and erythema[79].
ALA-PDT induces the deposition of collagen in the dermis, normalizes elastotic materials induced by photoaging and may even have a direct effect on the normalization of fibroblast morphology[80]. Marmur et al[81] observed a series of ultrastructural changes leading to clinical improvement. In the epidermis, ALA-PDT induces reorganization because of keratinocyte adhesion recovery. In the dermis, there is a recovery of the dermal extracellular matrix, which is demonstrated by the reappearance of anchoring fibrils, the displacement of elastosis and the superficial remodeling of dermal collagen.
PDT is very useful in dermatology; PDT is minimally invasive, especially compared with surgery, and allows better results than non-surgical treatments, such as lasers and cryotherapy. In our experience, PDT was widely applied in over 250 patients affected by actinic keratoses, basal cell carcinomas and Bowen’s disease, with excellent results. In our opinion, assessing the depth of a lesion to determine the appropriate indication for PDT compared with surgical treatment is important. In deeper lesions, or in particularly sensitive areas such as the face, PDT can be a useful treatment in association with surgery, as described by other authors. Skin cancers, especially if arising on actinic keratosis, often present ill-defined margins. In these cases, PDT may be useful to better define the edges of a lesion before surgical treatment. We have used this method in 14 patients diagnosed with basal cell carcinoma of the face with surrounding photodamaged skin; after 3 PDT sessions, the patients underwent surgery, and the excision margins were found to be free from disease in all of the cases. Additionally, we used PDT for the treatment of photoaging in 57 patients, with excellent results after an average of 3-4 sessions. We believe that the other proposed indications require further research to verify the effectiveness of PDT.
| 1. | Macdonald IJ, Dougherty TJ. Basic principles of photodynamic therapy. J Porphyr Phthalocyanines. 2001;105:105-129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1007] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 2. | Ronchese F. The fluorescence of cancer under the Wood light. Oral Surg Oral Med Oral Pathol. 1954;7:967-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Winkelman J. Intracellular localization of “hematoporphyrin” in a transplanted tumor. J Natl Cancer Inst. 1961;27:1369-1377. [PubMed] |
| 4. | Lipson RL, BALDES EJ, OLSEN AM. The use of a derivative of hematoporhyrin in tumor detection. J Natl Cancer Inst. 1961;26:1-11. [PubMed] |
| 5. | Weishaupt KR, Gomer CJ, Dougherty TJ. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976;36:2326-2329. [PubMed] |
| 6. | Dougherty TJ. A brief history of clinical photodynamic therapy development at Roswell Park Cancer Institute. J Clin Laser Med Surg. 1996;14:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Kennedy JC, Marcus SL, Pottier RH. Photodynamic therapy (PDT) and photodiagnosis (PD) using endogenous photosensitization induced by 5-aminolevulinic acid (ALA): mechanisms and clinical results. J Clin Laser Med Surg. 1996;14:289-304. [PubMed] |
| 8. | Zeitouni NC, Shieh S, Oseroff AR. Laser and photodynamic therapy in the management of cutaneous malignancies. Clin Dermatol. 2001;19:328-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Foote CS. Mechanisms of photooxygenation. Prog Clin Biol Res. 1984;170:3-18. [PubMed] |
| 10. | Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1827] [Cited by in RCA: 1731] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 11. | Kessel D, Luo Y. Photodynamic therapy: a mitochondrial inducer of apoptosis. Cell Death Differ. 1999;6:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 211] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Kessel D, Luo Y. Mitochondrial photodamage and PDT-induced apoptosis. J Photochem Photobiol B. 1998;42:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 228] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Henderson BW, Dougherty TJ, Malone PB. Studies on the mechanism of tumor destruction by photoradiation therapy. Prog Clin Biol Res. 1984;170:601-612. [PubMed] |
| 14. | Henderson BW, Fingar VH. Relationship of tumor hypoxia and response to photodynamic treatment in an experimental mouse tumor. Cancer Res. 1987;47:3110-3114. [PubMed] |
| 15. | Korbelik M. Induction of tumor immunity by photodynamic therapy. J Clin Laser Med Surg. 1996;14:329-334. [PubMed] |
| 16. | Larkö O. Photodynamic therapy. Australas J Dermatol. 2005;46 Suppl 3:S1-S2; discussion S23-S25. [PubMed] |
| 17. | Grapengiesser S, Ericson M, Gudmundsson F, Larkö O, Rosén A, Wennberg AM. Pain caused by photodynamic therapy of skin cancer. Clin Exp Dermatol. 2002;27:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Sandberg C, Stenquist B, Rosdahl I, Ros AM, Synnerstad I, Karlsson M, Gudmundson F, Ericson MB, Larkö O, Wennberg AM. Important factors for pain during photodynamic therapy for actinic keratosis. Acta Derm Venereol. 2006;86:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Langan SM, Collins P. Randomized, double-blind, placebo-controlled prospective study of the efficacy of topical anaesthesia with a eutetic mixture of lignocaine 2.5% and prilocaine 2.5% for topical 5-aminolaevulinic acid-photodynamic therapy for extensive scalp actinic keratoses. Br J Dermatol. 2006;154:146-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Skiveren J, Haedersdal M, Philipsen PA, Wiegell SR, Wulf HC. Morphine gel 0.3% does not relieve pain during topical photodynamic therapy: a randomized, double-blind, placebo-controlled study. Acta Derm Venereol. 2006;86:409-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Pagliaro J, Elliott T, Bulsara M, King C, Vinciullo C. Cold air analgesia in photodynamic therapy of basal cell carcinomas and Bowen’s disease: an effective addition to treatment: a pilot study. Dermatol Surg. 2004;30:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Morton CA, Szeimies RM, Sidoroff A, Braathen LR. European guidelines for topical photodynamic therapy part 1: treatment delivery and current indications - actinic keratoses, Bowen’s disease, basal cell carcinoma. J Eur Acad Dermatol Venereol. 2013;27:536-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 23. | Morton CA, Szeimies RM, Sidoroff A, Braathen LR. European guidelines for topical photodynamic therapy part 2: emerging indications--field cancerization, photorejuvenation and inflammatory/infective dermatoses. J Eur Acad Dermatol Venereol. 2013;27:672-679. [RCA] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Jeffes EW, McCullough JL, Weinstein GD, Fergin PE, Nelson JS, Shull TF, Simpson KR, Bukaty LM, Hoffman WL, Fong NL. Photodynamic therapy of actinic keratosis with topical 5-aminolevulinic acid. A pilot dose-ranging study. Arch Dermatol. 1997;133:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Tschen EH, Wong DS, Pariser DM, Dunlap FE, Houlihan A, Ferdon MB. Photodynamic therapy using aminolaevulinic acid for patients with nonhyperkeratotic actinic keratoses of the face and scalp: phase IV multicentre clinical trial with 12-month follow up. Br J Dermatol. 2006;155:1262-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Stender IM, Wulf HC. Photodynamic therapy with 5-aminolevulinic acid in the treatment of actinic cheilitis. Br J Dermatol. 1996;135:454-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Cairnduff F, Stringer MR, Hudson EJ, Ash DV, Brown SB. Superficial photodynamic therapy with topical 5-aminolaevulinic acid for superficial primary and secondary skin cancer. Br J Cancer. 1994;69:605-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 267] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Morton CA, Whitehurst C, Moseley H, McColl JH, Moore JV, Mackie RM. Comparison of photodynamic therapy with cryotherapy in the treatment of Bowen’s disease. Br J Dermatol. 1996;135:766-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | López N, Meyer-Gonzalez T, Herrera-Acosta E, Bosch R, Castillo R, Herrera E. Photodynamic therapy in the treatment of extensive Bowen’s disease. J Dermatolog Treat. 2012;23:428-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Truchuelo M, Fernández-Guarino M, Fleta B, Alcántara J, Jaén P. Effectiveness of photodynamic therapy in Bowen’s disease: an observational and descriptive study in 51 lesions. J Eur Acad Dermatol Venereol. 2012;26:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Calzavara-Pinton PG, Venturini M, Sala R, Capezzera R, Parrinello G, Specchia C, Zane C. Methylaminolaevulinate-based photodynamic therapy of Bowen’s disease and squamous cell carcinoma. Br J Dermatol. 2008;159:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Christensen E, Skogvoll E, Viset T, Warloe T, Sundstrøm S. Photodynamic therapy with 5-aminolaevulinic acid, dimethylsulfoxide and curettage in basal cell carcinoma: a 6-year clinical and histological follow-up. J Eur Acad Dermatol Venereol. 2009;23:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Christensen E, Mørk C, Skogvoll E. High and sustained efficacy after two sessions of topical 5-aminolaevulinic acid photodynamic therapy for basal cell carcinoma: a prospective, clinical and histological 10-year follow-up study. Br J Dermatol. 2012;166:1342-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Souza CS, Felicio LB, Ferreira J, Kurachi C, Bentley MV, Tedesco AC, Bagnato VS. Long-term follow-up of topical 5-aminolaevulinic acid photodynamic therapy diode laser single session for non-melanoma skin cancer. Photodiagnosis Photodyn Ther. 2009;6:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Soler AM, Warloe T, Berner A, Giercksky KE. A follow-up study of recurrence and cosmesis in completely responding superficial and nodular basal cell carcinomas treated with methyl 5-aminolaevulinate-based photodynamic therapy alone and with prior curettage. Br J Dermatol. 2001;145:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 147] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Basset-Seguin N, Ibbotson SH, Emtestam L, Tarstedt M, Morton C, Maroti M, Calzavara-Pinton P, Varma S, Roelandts R, Wolf P. Topical methyl aminolaevulinate photodynamic therapy versus cryotherapy for superficial basal cell carcinoma: a 5 year randomized trial. Eur J Dermatol. 2008;18:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 37. | Pauwels C, Mazereeuw-Hautier J, Basset-Seguin N, Livideanu C, Viraben R, Paul C, Meyer N. Topical methyl aminolevulinate photodynamic therapy for management of basal cell carcinomas in patients with basal cell nevus syndrome improves patient’s satisfaction and reduces the need for surgical procedures. J Eur Acad Dermatol Venereol. 2011;25:861-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 469] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 39. | Dragieva G, Hafner J, Dummer R, Schmid-Grendelmeier P, Roos M, Prinz BM, Burg G, Binswanger U, Kempf W. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen’s disease in transplant recipients. Transplantation. 2004;77:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Willey A, Mehta S, Lee PK. Reduction in the incidence of squamous cell carcinoma in solid organ transplant recipients treated with cyclic photodynamic therapy. Dermatol Surg. 2010;36:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Braathen LR, Morton CA, Basset-Seguin N, Bissonnette R, Gerritsen MJ, Gilaberte Y, Calzavara-Pinton P, Sidoroff A, Wulf HC, Szeimies RM. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Basset-Seguin N, Baumann Conzett K, Gerritsen MJ, Gonzalez H, Haedersdal M, Hofbauer GF, Aguado L, Kerob D, Lear JT, Piaserico S. Photodynamic therapy for actinic keratosis in organ transplant patients. J Eur Acad Dermatol Venereol. 2013;27:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Apalla Z, Sotiriou E, Chovarda E, Lefaki I, Devliotou-Panagiotidou D, Ioannides D. Skin cancer: preventive photodynamic therapy in patients with face and scalp cancerization. A randomized placebo-controlled study. Br J Dermatol. 2010;162:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Bagazgoitia L, Cuevas Santos J, Juarranz A, Jaén P. Photodynamic therapy reduces the histological features of actinic damage and the expression of early oncogenic markers. Br J Dermatol. 2011;165:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635. [PubMed] |
| 46. | Forbes IJ, Cowled PA, Leong AS, Ward AD, Black RB, Blake AJ, Jacka FJ. Phototherapy of human tumours using haematoporphyrin derivative. Med J Aust. 1980;2:489-493. [PubMed] |
| 47. | Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 48. | Wolf P, Fink-Puches R, Cerroni L, Kerl H. Photodynamic therapy for mycosis fungoides after topical photosensitization with 5-aminolevulinic acid. J Am Acad Dermatol. 1994;31:678-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Svanberg K, Andersson T, Killander D, Wang I, Stenram U, Andersson-Engels S, Berg R, Johansson J, Svanberg S. Photodynamic therapy of non-melanoma malignant tumours of the skin using topical delta-amino levulinic acid sensitization and laser irradiation. Br J Dermatol. 1994;130:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 280] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 50. | Orenstein A, Haik J, Tamir J, Winkler E, Trau H, Malik Z, Kostenich G. Photodynamic therapy of cutaneous lymphoma using 5-aminolevulinic acid topical application. Dermatol Surg. 2000;26:765-769; discussion 765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Mori M, Campolmi P, Mavilia L, Rossi R, Cappugi P, Pimpinelli N. Topical photodynamic therapy for primary cutaneous B-cell lymphoma: a pilot study. J Am Acad Dermatol. 2006;54:524-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Shieh S, Dee AS, Cheney RT, Frawley NP, Zeitouni NC, Oseroff AR. Photodynamic therapy for the treatment of extramammary Paget’s disease. Br J Dermatol. 2002;146:1000-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Mikasa K, Watanabe D, Kondo C, Kobayashi M, Nakaseko H, Yokoo K, Tamada Y, Matsumoto Y. 5-Aminolevulinic acid-based photodynamic therapy for the treatment of two patients with extramammary Paget’s disease. J Dermatol. 2005;32:97-101. [PubMed] |
| 54. | Raspagliesi F, Fontanelli R, Rossi G, Ditto A, Solima E, Hanozet F, Kusamura S. Photodynamic therapy using a methyl ester of 5-aminolevulinic acid in recurrent Paget’s disease of the vulva: a pilot study. Gynecol Oncol. 2006;103:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Zawislak AA, McCarron PA, McCluggage WG, Price JH, Donnelly RF, McClelland HR, Dobbs SP, Woolfson AD. Successful photodynamic therapy of vulval Paget’s disease using a novel patch-based delivery system containing 5-aminolevulinic acid. BJOG. 2004;111:1143-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Henta T, Itoh Y, Kobayashi M, Ninomiya Y, Ishibashi A. Photodynamic therapy for inoperable vulval Paget’s disease using delta-aminolaevulinic acid: successful management of a large skin lesion. Br J Dermatol. 1999;141:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Hongcharu W, Taylor CR, Chang Y, Aghassi D, Suthamjariya K, Anderson RR. Topical ALA-photodynamic therapy for the treatment of acne vulgaris. J Invest Dermatol. 2000;115:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 344] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 58. | Stender IM, Na R, Fogh H, Gluud C, Wulf HC. Photodynamic therapy with 5-aminolaevulinic acid or placebo for recalcitrant foot and hand warts: randomised double-blind trial. Lancet. 2000;355:963-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 159] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Fabbrocini G, Di Costanzo MP, Riccardo AM, Quarto M, Colasanti A, Roberti G, Monfrecola G. Photodynamic therapy with topical delta-aminolaevulinic acid for the treatment of plantar warts. J Photochem Photobiol B. 2001;61:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Stender IM, Lock-Andersen J, Wulf HC. Recalcitrant hand and foot warts successfully treated with photodynamic therapy with topical 5-aminolaevulinic acid: a pilot study. Clin Exp Dermatol. 1999;24:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Wang XL, Wang HW, Wang HS, Xu SZ, Liao KH, Hillemanns P. Topical 5-aminolaevulinic acid-photodynamic therapy for the treatment of urethral condylomata acuminata. Br J Dermatol. 2004;151:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Liang J, Lu XN, Tang H, Zhang Z, Fan J, Xu JH. Evaluation of photodynamic therapy using topical aminolevulinic acid hydrochloride in the treatment of condylomata acuminata: a comparative, randomized clinical trial. Photodermatol Photoimmunol Photomed. 2009;25:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Fehr MK, Hornung R, Degen A, Schwarz VA, Fink D, Haller U, Wyss P. Photodynamic therapy of vulvar and vaginal condyloma and intraepithelial neoplasia using topically applied 5-aminolevulinic acid. Lasers Surg Med. 2002;30:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Stefanaki IM, Georgiou S, Themelis GC, Vazgiouraki EM, Tosca AD. In vivo fluorescence kinetics and photodynamic therapy in condylomata acuminata. Br J Dermatol. 2003;149:972-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | van der Snoek EM, Robinson DJ, van Hellemond JJ, Neumann HA. A review of photodynamic therapy in cutaneous leishmaniasis. J Eur Acad Dermatol Venereol. 2008;22:918-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Asilian A, Davami M. Comparison between the efficacy of photodynamic therapy and topical paromomycin in the treatment of Old World cutaneous leishmaniasis: a placebo-controlled, randomized clinical trial. Clin Exp Dermatol. 2006;31:634-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 67. | Kosaka S, Akilov OE, O’Riordan K, Hasan T. A mechanistic study of delta-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis. J Invest Dermatol. 2007;127:1546-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Abok K, Cadenas E, Brunk U. An experimental model system for leishmaniasis. Effects of porphyrin-compounds and menadione on Leishmania parasites engulfed by cultured macrophages. APMIS. 1988;96:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Karrer S, Abels C, Landthaler M, Szeimies RM. Topical photodynamic therapy for localized scleroderma. Acta Derm Venereol. 2000;80:26-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Karrer S, Bosserhoff AK, Weiderer P, Landthaler M, Szeimies RM. Influence of 5-aminolevulinic acid and red light on collagen metabolism of human dermal fibroblasts. J Invest Dermatol. 2003;120:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Hillemanns P, Untch M, Pröve F, Baumgartner R, Hillemanns M, Korell M. Photodynamic therapy of vulvar lichen sclerosus with 5-aminolevulinic acid. Obstet Gynecol. 1999;93:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Richey DF, Hopson B. Photodynamic therapy for perioral dermatitis. J Drugs Dermatol. 2006;5:12-16. [PubMed] |
| 73. | Sotiriou E, Koussidou T, Patsatsi A, Apalla Z, Ioannides D. 5-Aminolevulinic acid-photodynamic treatment for dermatophytic tinea pedis of interdigital type: a small clinical study. J Eur Acad Dermatol Venereol. 2009;23:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 74. | Sotiriou E, Panagiotidou D, Ioannides D. 5-Aminolevulininic acid photodynamic therapy treatment for tinea cruris caused by Trichophyton rubrum: report of 10 cases. J Eur Acad Dermatol Venereol. 2009;23:341-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Kohl E, Torezan LA, Landthaler M, Szeimies RM. Aesthetic effects of topical photodynamic therapy. J Eur Acad Dermatol Venereol. 2010;24:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Dover JS, Bhatia AC, Stewart B, Arndt KA. Topical 5-aminolevulinic acid combined with intense pulsed light in the treatment of photoaging. Arch Dermatol. 2005;141:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 77. | Ruiz-Rodríguez R, López L, Candelas D, Pedraz J. Photorejuvenation using topical 5-methyl aminolevulinate and red light. J Drugs Dermatol. 2008;7:633-637. [PubMed] |
| 78. | Szeimies RM, Lischner S, Philipp-Dormston W, Walker T, Hiepe-Wegener D, Feise K, Podda M, Prager W, Kohl E, Karrer S. Photodynamic therapy for skin rejuvenation: treatment options - results of a consensus conference of an expert group for aesthetic photodynamic therapy. J Dtsch Dermatol Ges. 2013;11:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Lehmann P. Side effects of topical photodynamic therapy. Hautarzt. 2007;58:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 80. | Park JY, Jang YH, Kim YS, Sohn S, Kim YC. Ultrastructural changes in photorejuvenation induced by photodynamic therapy in a photoaged mouse model. Eur J Dermatol. 2013;23:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Marmur ES, Phelps R, Goldberg DJ. Ultrastructural changes seen after ALA-IPL photorejuvenation: a pilot study. J Cosmet Laser Ther. 2005;7:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
P- Reviewers: Hu SCS, Kaliyadan F, Katiyar SK, Vasconcellos C S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL