Peer-review started: October 14, 2022
First decision: November 4, 2022
Revised: November 10, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 16, 2023
Processing time: 93 Days and 16 Hours
Prurigo nodularis (PN) is a chronic condition characterized by a papulonodular pruriginous eruption of unknown aetiology. Currently, there are no medications for PN that the United States Food and Drug Administration has approved, which leads to very variable practices in the prescription of off-label treatments. Treatment of PN is based on clinical experience rather than controlled trials. We present our case of generalized PN, in which we had a dramatic response with dupilumab.
A 58-year-old female patient was admitted to our clinic with severe itchy, erythematous nodular lesions that were widespread all over her body, especially on the legs and back. It was learned that the patient's complaints started 4 years ago, and there was a significant increase in the lesions in the last period. Dermatological examination revealed diffuse firm erythematous excoriated nodular lesions all over the body. In the blood tests of the patient, serum Immunoglobulin E (IgE) was measured at 9330 IU/mL. The patient was diagnosed with generalized prurigo nodularis together with clinical and histopathological findings. Due to severe clinical findings and the presence of comorbidities, dupilumab treatment was planned for the patient. In the follow-up 4 mo later, it was observed that all nodular lesions healed with postinflammatory hypopigmentation. The IgE value decreased to 1500 IU/mL after 4 mo of dupilumab treatment.
Dupilumab treatment stands out as an effective and safe systemic treatment agent among existing systemic treatments.
Core Tip: Prurigo nodularis (PN) is a difficult disease to treat and causes frustration to both the patient and the treating doctor. Treatment of PN is based on clinical experience rather than controlled trials. PN is a disease that negatively affects the quality of life of patients due to severe itching. Patients often receive limited benefit from first-line treatments and require systemic therapy. Dupilumab treatment stands out as an effective and safe systemic treatment agent among existing systemic treatments. In this case, we show how effective and well tolerated treatment with dupilumab is in the treatment of recalcitrant PN.
- Citation: Boyvadoglu C, Inaloz HS. Generalized prurigo nodularis with dramatic response to dupilumab treatment: A case report. World J Dermatol 2023; 11(1): 1-6
- URL: https://www.wjgnet.com/2218-6190/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.5314/wjd.v11.i1.1
Prurigo nodularis (PN) is a chronic condition characterized by a papulonodular pruriginous eruption of unknown aetiology. PN is a difficult disease to treat and causes frustration for both the physician and the patient. Previously, it was reported that there is an association between various systemic diseases and PN. The classic lesion in PN is a firm pruritic nodule that is hyperkeratotic, numbers from a few to hundreds, and ranges from several millimetres to 2 cm in diameter[1]. PN most frequently affects middle-aged adults and tends to be observed more often in women compared with men. PN is related to psychiatric, cardiovascular, renal, and endocrine disorders, besides malignancy and the human immunodeficiency virus (HIV). The burden of systemic comorbidities in PN frequently exceeds that of other inflammatory skin disorders (i.e., psoriasis or atopic dermatitis)[2].
Immune and neural dysregulation are important in the pathogenesis of PN. Neuropeptides and immune cells are implicated in cutaneous inflammation. Interleukin (IL)-31, tryptase, eosinophil cationic protein, histamine, prostaglandins, and neuropeptides are only a few of the mediators that immune cells in the skin release to cause a significant inflammatory reaction and severe itching. That immune reaction is critical to the pathogenesis of PN. Additionally, eosinophils play an important role in the cutaneous inflammation and itching related to PN. Eosinophil infiltration is observed in the dermis of PN patients' lesional skin. It is believed that the pathophysiology of PN is a cutaneous reaction pattern brought on by recurrent cycles of chronic itching and scratching[3].
Therapy for PN is based on topical, intralesional, and systemic neuroimmune modulatory treatments to split a short-circuited itch-scratch cycle. A personalized therapy plan, centered on the comorbidities, patient’s age, disease severity, and side effect profile of treatments, is needed[3].
Dupilumab is a human monoclonal antibody; it blocks interleukin-4 and interleukin-13. Dupilumab has shown efficacy in asthma patients with high eosinophil levels. The blockade by dupilumab of these key drivers of type 2 helper T-cell (Th2)–mediated inflammation could benefit the therapy of Th2-associated diseases, including atopic dermatitis[4]. Guttman-Yassky et al[5] showed that dupilumab rapidly and effectively inhibited cellular and molecular cutaneous mediators of inflammation, reversed related epidermal abnormalities, and improved disease severity scores and symptoms in patients with AD. Dupilumab remarkably inhibited systemic type 2 inflammatory mediators, including the chemokines, periostin, and total and allergen-specific Immunoglobulin E (IgE).
Dupilumab treatment has been demonstrated to be an efficacious therapy for PN. Compared to atopic dermatitis, the treatment response to dupilumab therapy initiates later. Two months of treatment are needed until the pruritus is relieved. Complete remission is uncommonly observed before 4 mo of treatment. Atopic dermatitis-related PN patients need longer therapy than non-atopic dermatitis-related PN patients[6].
Our patient had persistent and severe generalized PN. In our patient, for whom we started dupilumab treatment, a complete response was obtained in a short time. We present this case to emphasize that dupilumab therapy should be an important treatment agent that should be considered in the treatment of severe generalized PN.
A 58-year-old female patient was admitted to our clinic with severe itchy, erythematous nodular lesions that were widespread all over her body, especially on her legs and back.
It was learned that the patient's complaints started 4 years ago, and there was a significant increase in the lesions in the last period. She had previously used systemic corticosteroid and oral antihistamine treatments for these itchy lesions, but there was no improvement. Then she was treated with omalizumab 300 mg every 4 wk for 19 mo, but did not benefit and there was a significant increase in nodular lesions, especially in the last period.
She had diabetes, hypertension, and coronary artery disease. She was using sitagliptin, metformin, insulin, telmisartan, acetylsalicylic acid, and trimetazidine for these diseases.
The patient and her family had a known history of atopy.
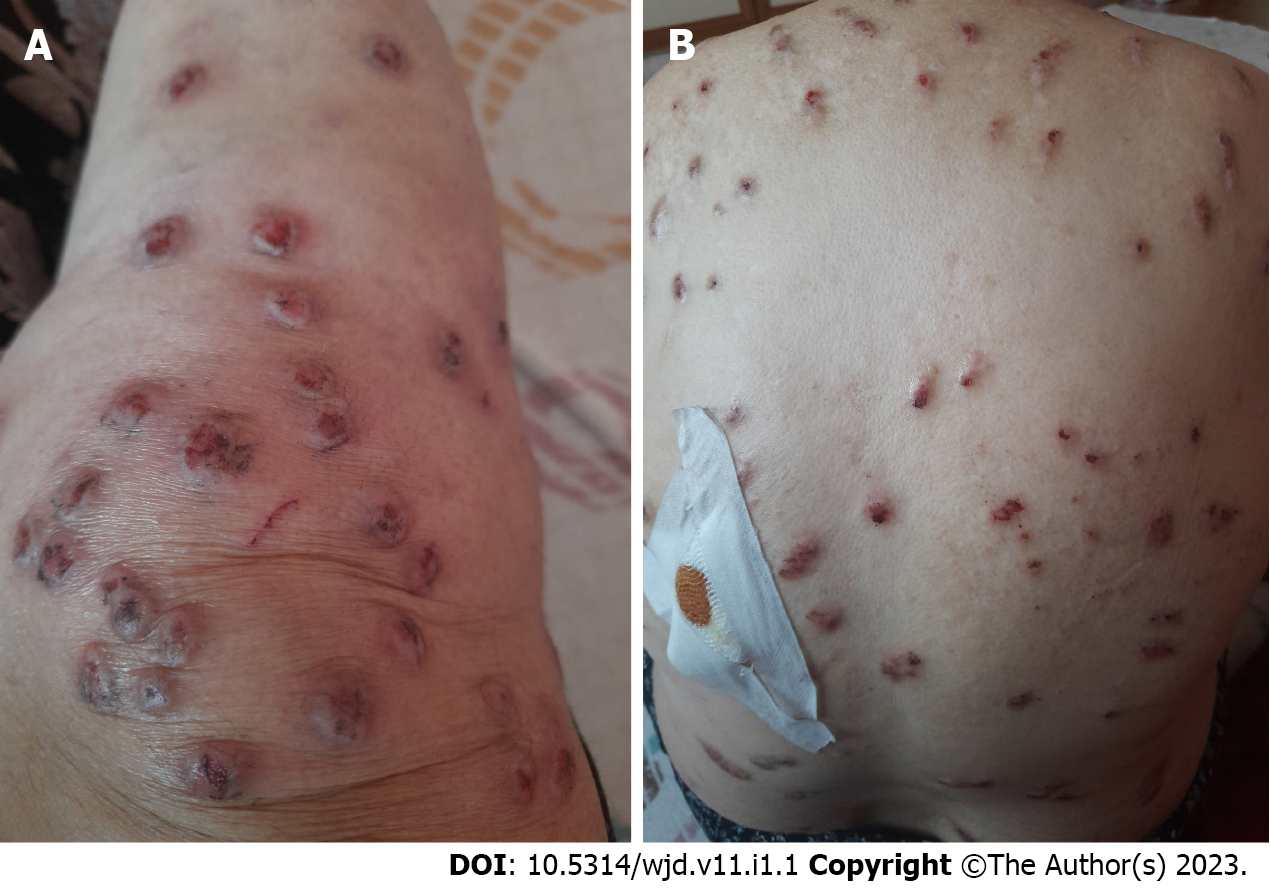
Dermatological examination revealed diffuse firm erythematous excoriated nodular lesions all over the body, especially on the anterior surfaces of the tibia (Figure 1A) and back (Figure 1B).
In the blood tests of the patient, serum IgE 9330 IU/mL (normal range: 0-100 IU/mL), white blood cell 14.8 × 103/μL (normal range: 3.39-8.86 × 103/μL), eosinophil 7.6% (1.13 × 103/μL (normal range: 0.03-0.27 × 103/μL)), lymphocyte 16.10% (2.38 × 103/μL), neutrophil 69.30% (10.25 × 103/μL (normal range: 1.5-5 × 103/μL)), hemoglobin 10.2 g/dL (normal range: 11.1-14.7 g/dL), hematocrit 32.4% (normal range: 36.9%-49.1%) , mean corpuscular volume 74.5 fL (normal range: 87-102.2 fL), platelet 542 × 103/μL (normal range: 158-374 × 103/μL), iron 17 ug/dL (normal range: 70-180 ug/dL), total iron binding capacity 421 ug/dL, C-reactive protein 7 mg/L (normal range: 0-5 mg/L), erythrocyte sedimentation rate 67 mm/h (normal range: 1-30 mm/h), glucose 151 mg/dL (normal range: 74-106 mg/dL), vitamin B12 83 ng/L (normal range: 180-914 ng/L), anti-HIV (-), anti- hepatitis C virus (-), hepatitis B surface antigen (-) were measured.
Biopsy taken from the nodular lesion on the patient's back: "Focal keratotic, prominent granular layer, and large area of the epidermis with parakeratotic and psoriasiform hyperplasia containing fibrin and neutrophils was observed; increased vascularity in the upper dermis; infiltration consisting of perivascular condensed lymphocytes, plasmacytes, and eosinophil polymorphs." reported as. It was observed as “IgG, IgA, IgM, complement C3, and complement component 1q negative” in immunofluorescence microscopy.
The patient was consulted to internal medicine with blood results. No malignancy was found in the further examinations.
The patient was diagnosed with generalized prurigo nodularis together with clinical and histopathological findings. The patient was evaluated as having atopic dermatitis related PN because of the pruritus, xerosis, atopy history, and high IgE values. The peak pruritus numerical rating scale was 9 before treatment.
Oral iron replacement therapy was given to the patient by the internal medicine department, but clinical findings did not improve. Cyclosporine and other immunosuppressive treatment options could not be planned for the patient due to concomitant hypertension and other systemic diseases. Due to severe clinical findings and the presence of comorbidities, dupilumab treatment was planned for the patient. Dupilumab therapy was administered at the standard dose: An initial induction dose of 600 mg followed by 300 mg every 14 d.
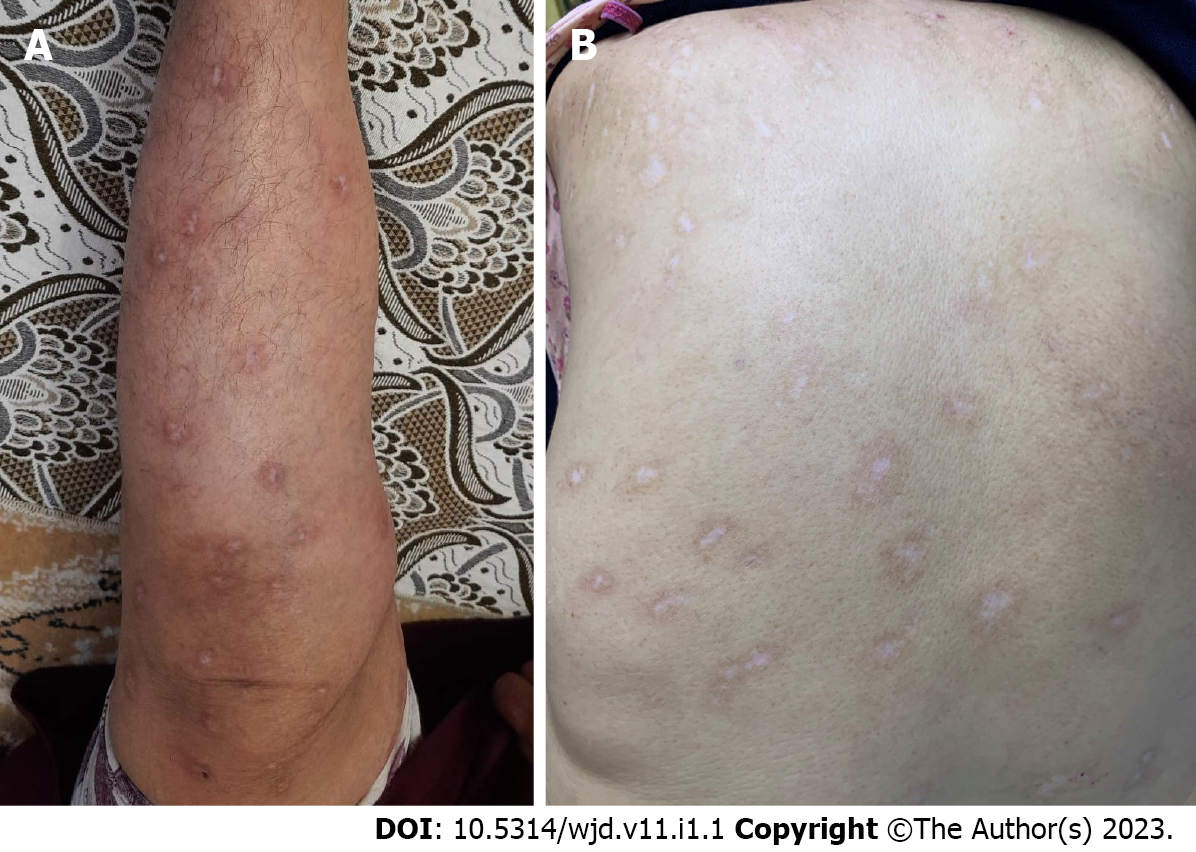
In the follow-up 4 mo later, it was observed that all nodular lesions healed with postinflammatory hypopigmentation (Figure 2) in the patient whose itching complaint completely resolved in a short time. The IgE value decreased to 1500 IU/mL after 4 mo of dupilumab treatment. The peak pruritus numerical rating scale was 0 after 4 mo of dupilumab treatment.
The mechanisms underlying the development of PN are still not fully known. The pathogenesis of PN involves T cells and their cytokines, particularly IL-31. IL-31 is mainly produced by activated Th2 cells, cluster of differentiation 45R0 cutaneous lymphocyte antigen T cells, and mast cells. IL-31 has a significant role in the induction of chronic cutaneous inflammation. It has been shown to have an important role in the etiology of atopic dermatitis and has been accepted as a major dermal pruritogen[7,8]. In comparison to healthy skin, messenger RNA for IL-31 is found more frequently in PN lesional skin[3]. IL-31 and its receptor have become potential therapeutic targets for a range of pruritic diseases, including PN[9].
Lesional biopsies have shown that IL-4 and IL-13 have critical roles in the development of PN. Hence, there are similarities between PN and atopic dermatitis in terms of the involvement of Th2 activation and the signal transducers and activators of transcription pathway[10]. Therefore, it has been suggested that blocking IL-4/13 with dupilumab could help manage skin inflammation, which leads to itch[11].
Currently, there are no medications for PN that the United States Food and Drug Administration has approved, which leads to very variable practices in the prescription of off-label treatments. Treatment of PN is based on clinical experience rather than controlled trials. Studies are limited to case reports and case series. Thus, larger studies with a homogeneous design are required. However, corticosteroids, pimecrolimus, and calcipotriol can be used as topical treatments for PN. However, they have limited efficacy. Phototherapy is recommended as a second-line treatment agent. Phototherapy is an especially useful option for medically complex patients who have comorbidities and drug interactions with other medications. PN patients most commonly need treatment with systemic therapies because many patients are refractory to the therapies. Systemic neuromodulating drugs like gabapentin, pregabalin, aprepitant, naltrexone, butorphanol, duloxetine, paroxetine, fluvoxamine, and thalidomide, as well as systemic immunomodulating drugs like methotrexate, cyclosporine, mycophenolate mofetil, azathioprine, and dupilumab, can be used[1,3].
A literature review revealed that all 11 patients with recalcitrant prurigo nodularis treated with dupilumab had a good or perfect response to treatment and tolerated treatment well. Dupilumab seems to be a safe but costly alternative for the therapy of refractory PN patients[12]. In our patient, a complete response to dupilumab treatment was obtained within 4 mo, which is consistent with the patients previously reported in the literature.
It has been shown that patients with atopic dermatitis-related PN respond more slowly to treatment than patients with non-atopic dermatitis-related PN[6]. Although our patient had atopic dermatitis-related PN, she responded very well to the treatment in a short period of 4 mo, along with a significant decrease in very high IgE values. It was not possible to comment on whether the dramatic decrease in IgE values was due to dupilumab treatment or whether it was due to the discontinuation of omalizumab treatment.
As a result, complete remission was observed in our patient in a short time after dupilumab treatment. No side effects were observed in the follow-up of dupilumab treatment. No itching was observed in the patient's follow-up. Significant improvements in sleep and quality of life were observed. Based on our case, it can be predicted that dupilumab treatment is an effective and safe treatment for patients with refractory generalized PN.
PN is a disease that negatively affects the quality of life of patients due to severe itching. Patients often receive limited benefit from first-line treatments such as topical treatments and phototherapy and require systemic therapy. Dupilumab treatment stands out as an effective and safe systemic treatment agent among existing systemic treatments. There are few cases of PN treated with dupilumab in the literature, so more studies are needed to evaluate its efficacy. We present our case of generalized PN, in which we had a dramatic response with dupilumab.
| 1. | Lee MR, Shumack S. Prurigo nodularis: a review. Australas J Dermatol. 2005;46:211-18; quiz 219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: Epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: Pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 4. | Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, Ming JE, Ren H, Kao R, Simpson E, Ardeleanu M, Weinstein SP, Pirozzi G, Guttman-Yassky E, Suárez-Fariñas M, Hager MD, Stahl N, Yancopoulos GD, Radin AR. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1075] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 5. | Guttman-Yassky E, Bissonnette R, Ungar B, Suárez-Fariñas M, Ardeleanu M, Esaki H, Suprun M, Estrada Y, Xu H, Peng X, Silverberg JI, Menter A, Krueger JG, Zhang R, Chaudhry U, Swanson B, Graham NMH, Pirozzi G, Yancopoulos GD, D Hamilton JD. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143:155-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 517] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 6. | Husein-ElAhmed H, Steinhoff M. Dupilumab in prurigo nodularis: a systematic review of current evidence and analysis of predictive factors to response. J Dermatolog Treat. 2022;33:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19:347-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, Soumelis V, Feld M, Alenius H, Dillon SR, Carstens E, Homey B, Basbaum A, Steinhoff M. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 588] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 9. | Hashimoto T, Nattkemper LA, Kim HS, Kursewicz CD, Fowler E, Shah SM, Nanda S, Fayne RA, Paolini JF, Romanelli P, Yosipovitch G. Itch intensity in prurigo nodularis is closely related to dermal interleukin-31, oncostatin M, IL-31 receptor alpha and oncostatin M receptor beta. Exp Dermatol. 2021;30:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Fukushi S, Yamasaki K, Aiba S. Nuclear localization of activated STAT6 and STAT3 in epidermis of prurigo nodularis. Br J Dermatol. 2011;165:990-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Calugareanu A, Jachiet M, Lepelletier C, De Masson A, Rybojad M, Bagot M, Bouaziz JD. Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33:e303-e304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Holm JG, Agner T, Sand C, Thomsen SF. Dupilumab for prurigo nodularis: Case series and review of the literature. Dermatol Ther. 2020;33:e13222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rolla G, Italy; Velikova TV, Bulgaria S-Editor: Liu GL L-Editor: A P-Editor: Liu GL