Revised: July 13, 2012
Accepted: July 26, 2012
Published online: August 2, 2012
Pigmentary mosaicism is proposed to encompass all pigment anomalies caused by chromosomal mosaicism. The concept includes, not only pigment anomalies following the lines of Blaschko, but also pigmentary disorders with phylloid, checkerboard and patchy pigmentation without midline separation. The representative disorders are hypomelanosis of Ito (pigmentary mosaicism of hypopigmented or Ito type), linear and whorled nevoid hypermelanosis (pigmentary mosaicism of hyperpigmented type), pigmentary mosaicism of hypopigmented and hyperpigmented type, and phylloid hypo- and hypermelanosis. Pigmentary mosaicism is nowadays recognized as a pigmentary disorder caused by somatic chromosomal abnormalities disrupting or accelerating the function of pigmentary genes. Affected individuals with pigmentary mosaicism commonly have multiple congenital abnormalities, developmental delays and/or mental retardation. However, the complication is not a syndrome because functional loss or acquisition due to various chromosomal abnormalities induces pigment abnormalities and specific complications. Cytogenetic abnormalities, including polyploidy, aneuploidy, deletions, insertions and translocations, are associated with almost any chromosome and tissue-limited mosaicism for chromosome abnormalities. Cytogenetic findings in cases with the phylloid pattern demonstrate the obvious causal relationship between phylloid hypomelanosis and mosaic trisomy 13. The pattern of cutaneous mosaicism depends on the trajectory of migration and proliferation during embryogenesis. The chromosomal regions of hot breakpoints in pigmentary mosaicism may contain pigmentation-associated genes. The accumulation of relationships between cases and chromosomal analyses may provide the opportunity to identify and understand the pigmentation-associated genes because more than 800 phenotypic alleles are known in the mice models of pigmentary anomalies and not all color loci have been identified. Here, we summarize the clinical features of pigmentary mosaicism and specific forms of phylloid hypo- and hypermelanosis.
- Citation: Oiso N, Kawada A. Pigmentary mosaicism and specific forms of phylloid hypo- and hypermelanosis. World J Dermatol 2012; 1(2): 6-9
- URL: https://www.wjgnet.com/2218-6190/full/v1/i2/6.htm
- DOI: https://dx.doi.org/10.5314/wjd.v1.i2.6
Hypomelanosis of Ito is characterized by hypopigmented whorls and streaks following the lines of Blaschko[1]. Accurate recognition of skin color is frequently difficult because a hypopigmented lesion may be present in the hyperpigmentation[2]. Linear and whorled nevoid hypermelanosis is typified by hyperpigmented whorls and streaks following Blaschko lines[3]. In some cases of linear and whorled nevoid hypermelanosis, the concomitant presence of hypopigmented and hyperpigmented lines has been observed[4]. Therefore, the term ‘pigmentary mosaicism’ is now used for a group of abnormal skin-colored whorls and streaks following the Blaschko’s lines[1,2]. Hypomelanosis of Ito is redefined as pigmentary mosaicism of the Ito type or hypopigmented type, linear and whorled nevoid hypermelanosis as pigmentary mosaicism of the hyperpigmented type, and cases of hypopigmented and hyperpigmented whorls and streaks following Blaschko lines as pigmentary mosaicism of the hypo- and hyperpigmented type[2].
As pigmentary mosaicism is proposed to encompass all pigment anomalies caused by chromosomal mosaicism[1], the concept includes, not only pigment anomalies following the lines of Blaschko, but also pigmentary disorders with phylloid, checkerboard and patchy pigmentation without midline separation[1,5,6]. Phylloid hypomelanosis is characterized by hypochromic lesions with various elements, including round or oval patches and oblong macules mimicking the leaves of a begonia[7,8], while phylloid hypermelanosis is characterized by hyperchromic lesions[9,10].
In this review, we summarize the clinical features of pigmentary mosaicism and specific forms of phylloid hypo- and hypermelanosis.
Pigmentary mosaicism is now recognized as a pigmentary disorder caused by somatic chromosomal abnormalities disrupting or accelerating the function of pigmentary genes[1]. The complication is not a syndrome, even although individuals showing pigmentary mosaicism frequently have multiple congenital abnormalities, developmental delays and mental retardation. Functional loss or acquisition due to various chromosomal abnormalities induces pigment abnormalities and specific complications.
We describe examples of chromosomal rearrangements that are involved in aberrant skin colors. The relationship between chromosomal rearrangements and aberrant skin color has been observed on the region of 15q11-q13[11-13]. The P gene is located on the region of 15q11-q13 and mutations in the P gene result in oculocutaneous albinism type 2 (OCA2)[14]. An increase in the number of the P gene is associated with hyperpigmentation[12]. We previously reported generalized skin hypopigmentation in a case of Angelman syndrome with OCA2 caused by a hemizygous P gene amino acid substitution[13]. Akahoshi et al[11] reported two interesting cases; a patient with generalized skin hyperpigmentation with chromosomal duplication of 15q11.2-q14 and another with mottled and linear patterns of hyperpigmentation with 47,XY,+idic(15)(pter→q14::q14→pter)/46,XY[12].
A wide range of cytogenetic abnormalities, including polyploidy, aneuploidy, deletions, insertions and translocations, are involved in almost any chromosome and tissue-limited mosaicism for chromosome abnormalities[1,15-17].These are induced by different expressions of the various pigmentation-associated genes. The pattern of cutaneous mosaicism depends on the trajectory of migration and proliferation during embryogenesis[6].
Pigmentation-associated genes may be located on the regions of hot breakpoints in pigmentary mosaicism[2,18]. This concept suggests that accumulating more cases and chromosomal analyses may provide the opportunity to identify and understand the pigmentation-associated genes[19], since over 800 phenotypic alleles are known in the mice models of pigmentary anomalies and not all color loci have been identified[20].
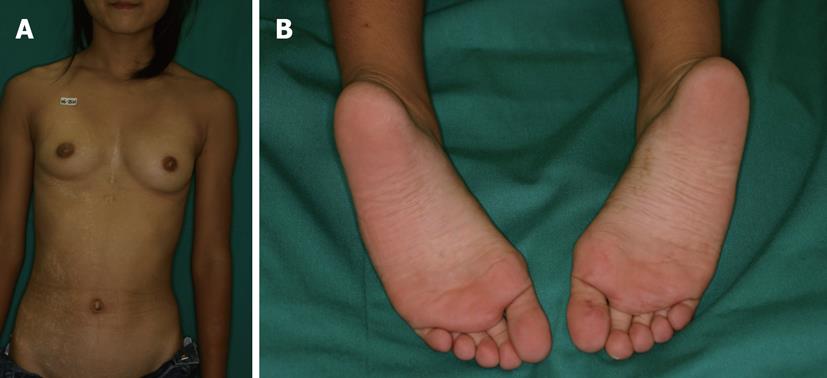
We show a typical case of pigmentary mosaicism[19]. A 12 year old Japanese girl with a history of congenital atrial septal defect showed asymptomatic hypopigmented whorls and streaks encircled by slight hyperpigmentation along the Blaschko lines on the right trunk and extremities and hyperpigmented streaks on the right sole and palm[19] (Figure 1). Karyotyping of peripheral blood lymphocytes showed balanced X; autosome translocations 46, X, t(X; 9) (p11.21; q34.1)[19]. These were representative wholes and streaks intermingled with hypopigmentation and hyperpigmentation. Hyperpigmented streaks were notable in the pale, skin-colored palmoplantar region and hypopigmented whorls and streaks were visible on the trunk and extremities.
Happle[7] reviewed the cytogenetic findings in six cases with the phylloid pattern and demonstrated the obvious causal relationship between phylloid hypomelanosis and mosaic trisomy 13[21-25]. Additionally, cases of phylloid hypomelanosis associated with mosaic trisomy 13[26,27], mosaic partial trisomy[28] and tetrasomy 13[17] have been described. Reporting cases of phylloid hypomelanosis caused by mosaic partial trisomy, Gonzalez-Ensenat et al[28] indicated that the disorder is most likely related to the 13q region. It is proposed that mosaic overexpression of the candidate pigmentary genes, such as endothelin receptor type B (EDNRB) which is related to melanoblast migration, may cause impaired melanoblast migration and melanocyte formation, resulting in phylloid hypomelanosis[17]. Phylloid hypomelanosis and pigmentary mosaicism of the Ito type (hypomelanosis of Ito) is clearly different because the distribution of Blaschko’s lines and the phylloid pattern is dissimilar, as shown by Molho-Pessach et al[6]. González-Enseñat et al[28] insist that a hypopigmented phylloid pattern indicates the presence of a specific chromosomal abnormality in the form of mosaic trisomy 13.
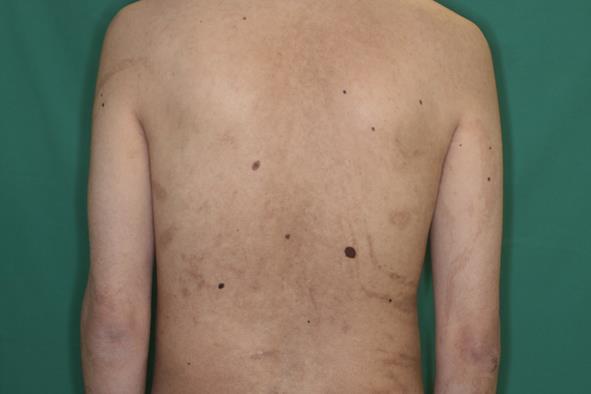
In 2009, Hwang et al[29] reported a case of phylloid hypermelanosis but did not perform a cytogenetic analysis. In 2010, we described a 29-year-old Japanese male with mental retardation and phylloid hypermelanosis that were associated with three aberrant chromosome 13 cell lines (Figure 2)[10]. The karyotyping of thirty peripheral blood lymphocytes showed 46,XY,r(13)(p11.2q34) in twenty-one cells, 45,XY,-13 in seven cells, and 46,XY,dic r(13)(p11.2q34) in two cells[10]. Happle[9] also described two additional cases of phylloid hypermelanosis. The questions of whether or not a consistent cytogenetic aberration of chromosome 13 exists and what determines the different clinical shapes or patterns are unanswered. More case reports of phylloid hypermelanosis, which is extremely rare, are needed to answer these questions.
The proposed concept of pigmentary mosaicism and phylloid hypo- and hyperpigmentation elucidates the obvious relationship with genetic mosaicism. Additional cases with clinical, cytogenetic and molecular research will allow us to identify the pigment-associated genes.
| 1. | Taibjee SM, Bennett DC, Moss C. Abnormal pigmentation in hypomelanosis of Ito and pigmentary mosaicism: the role of pigmentary genes. Br J Dermatol. 2004;151:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Kuster W, Happle R. “Hypomelanosis of Ito” and mosaicism. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne JP, editors. The Pigmentary System. 2nd ed. Massachusetts: Blackwell Publishing Ltd 2006; 636-645. |
| 3. | Kalter DC, Griffiths WA, Atherton DJ. Linear and whorled nevoid hypermelanosis. J Am Acad Dermatol. 1988;19:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 62] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Nehal KS, PeBenito R, Orlow SJ. Analysis of 54 cases of hypopigmentation and hyperpigmentation along the lines of Blaschko. Arch Dermatol. 1996;132:1167-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Happle R. Mosaicism in human skin. Understanding the patterns and mechanisms. Arch Dermatol. 1993;129:1460-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 254] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 6. | Molho-Pessach V, Schaffer JV. Blaschko lines and other patterns of cutaneous mosaicism. Clin Dermatol. 2011;29:205-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Happle R. Phylloid hypomelanosis is closely related to mosaic trisomy 13. Eur J Dermatol. 2000;10:511-512. [PubMed] |
| 8. | Happle R. Pigmentary patterns associated with human mosaicism: a proposed classification. Eur J Dermatol. 1993;3:170-174. |
| 9. | Happle R. Phylloid hypermelanosis: an unusual form of pigmentary mosaicism. Dermatology. 2010;220:183-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Oiso N, Tsuruta D, Imanishi H, Sayasa H, Narita T, Kobayashi H, Ikegami H, Kawada A. Phylloid hypermelanosis and melanocytic nevi with aggregated and disfigured melanosomes: causal relationship between phylloid pigment distribution and chromosome 13 abnormalities. Dermatology. 2010;220:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Akahoshi K, Spritz RA, Fukai K, Mitsui N, Matsushima K, Ohashi H. Mosaic supernumerary inv dup(15) chromosome with four copies of the P gene in a boy with pigmentary dysplasia. Am J Med Genet A. 2004;126A:290-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Akahoshi K, Fukai K, Kato A, Kimiya S, Kubota T, Spritz RA. Duplication of 15q11.2-q14, including the P gene, in a woman with generalized skin hyperpigmentation. Am J Med Genet. 2001;104:299-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Saitoh S, Oiso N, Wada T, Narazaki O, Fukai K. Oculocutaneous albinism type 2 with a P gene missense mutation in a patient with Angelman syndrome. J Med Genet. 2000;37:392-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Kato A, Fukai K, Oiso N, Hosomi N, Saitoh S, Wada T, Shimizu H, Ishii M. A novel P gene missense mutation in a Japanese patient with oculocutaneous albinism type II (OCA2). J Dermatol Sci. 2003;31:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Ritter CL, Steele MW, Wenger SL, Cohen BA. Chromosome mosaicism in hypomelanosis of Ito. Am J Med Genet. 1990;35:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Woods CG, Bankier A, Curry J, Sheffield LJ, Slaney SF, Smith K, Voullaire L, Wellesley D. Asymmetry and skin pigmentary anomalies in chromosome mosaicism. J Med Genet. 1994;31:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Dhar SU, Robbins-Furman P, Levy ML, Patel A, Scaglia F. Tetrasomy 13q mosaicism associated with phylloid hypomelanosis and precocious puberty. Am J Med Genet A. 2009;149A:993-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Gilgenkrantz S, Tridon P, Pinel-Briquel N, Beurey J, Weber M. Translocation (X; 9)(p11; q34) in a girl with incontinentia pigmenti (IP): implications for the regional assignment of the IP locus to Xp11. Ann Genet. 1985;28:90-92. [PubMed] |
| 19. | Oiso N, Amatsu A, Kawara S, Kawada A. Pigmentary mosaicism with hyperpigmented streaks on the palmoplantar lesion associated with balanced X; autosome translocations t(X; 9)(p11.21; q34.1). J Eur Acad Dermatol Venereol. 2009;23:359-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Bennett DC, Lamoreux ML. The color loci of mice--a genetic century. Pigment Cell Res. 2003;16:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 356] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Ohashi H, Tsukahara M, Murano I, Naritomi K, Nishioka K, Miyake S, Kajii T. Pigmentary dysplasias and chromosomal mosaicism: report of 9 cases. Am J Med Genet. 1992;43:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Horn D, Rommeck M, Sommer D, Körner H. Phylloid pigmentary pattern with mosaic trisomy 13. Pediatr Dermatol. 1997;14:278-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Pillay T, Winship WS, Ramdial PK. Pigmentary abnormalities in trisomy of chromosome 13. Clin Dysmorphol. 1998;7:191-194. [PubMed] |
| 24. | Ribeiro Noce T, de Pina-Neto JM, Happle R. Phylloid pattern of pigmentary disturbance in a case of complex mosaicism. Am J Med Genet. 2001;98:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Schepis C, Failla P, Siragusa M, Romano C. An additional case of macular phylloid mosaicism. Dermatology. 2001;202:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Fogu G, Maserati E, Cambosu F, Moro MA, Poddie F, Soro G, Bandiera P, Serra G, Tusacciu G, Sanna G. Patau syndrome with long survival in a case of unusual mosaic trisomy 13. Eur J Med Genet. 2008;51:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 27. | Chen CP. Prenatal diagnosis and genetic counseling for mosaic trisomy 13. Taiwan J Obstet Gynecol. 2010;49:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | González-Enseñat MA, Vicente A, Poo P, Catalá V, Mar Pérez-Iribarne M, Fuster C, Geán E, Happle R. Phylloid hypomelanosis and mosaic partial trisomy 13: two cases that provide further evidence of a distinct clinicogenetic entity. Arch Dermatol. 2009;145:576-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Hwang SW, Cho KJ, Kang JH, Seo JK, Lee D, Kim JW, Park SW, Sung HS. A case of hypermelanosis in a phylloid pattern. J Am Acad Dermatol. 2009;60:697-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Peer reviewer: Cheng Tan, MD, PhD, Associate Professor, Department of Dermatology, First Affiliated Hospital of Nanjing University of Traditional Chinese Medicine, No.155 Hanzhong Road, Nanjing 210029, Jiangsu Province, China
S- Editor Gou SX L- Editor Roemmele A E- Editor Zheng XM