Published online Dec 18, 2015. doi: 10.5312/wjo.v6.i11.961
Peer-review started: April 11, 2015
First decision: June 24, 2015
Revised: September 8, 2015
Accepted: October 1, 2015
Article in press: October 8, 2015
Published online: December 18, 2015
Processing time: 251 Days and 19.9 Hours
AIM: To explore the effect of platelet-rich plasma on protein expression patterns of transforming growth factor-beta1 (TGF-β1) in cartilage following autologous osteochondral transplantation (AOT) in a rabbit knee cartilage defect model.
METHODS: Twelve New Zealand white rabbits received bilateral AOT. In each rabbit, one knee was randomized to receive an autologous platelet rich plasma (PRP) injection and the contralateral knee received saline injection. Rabbits were euthanized at 3, 6 and 12 wk post-operatively. Articular cartilage sections were stained with TGF-β1 antibody. Histological regions of interest (ROI) (left, right and center of the autologous grafts interfaces) were evaluated using MetaMorph. Percentage of chondrocytes positive for TGF-β1 was then assessed.
RESULTS: Percentage of chondrocytes positive for TGF-β1 was higher in PRP treated knees for selected ROIs (left; P = 0.03, center; P = 0.05) compared to control and was also higher in the PRP group at each post-operative time point (P = 6.6 × 10-4, 3.1 × 10-4 and 7.3 × 10-3 for 3, 6 and 12 wk, respectively). TGF-β1 expression was higher in chondrocytes of PRP-treated knees (36% ± 29% vs 15% ± 18%) (P = 1.8 × 10-6) overall for each post-operative time point and ROI.
CONCLUSION: Articular cartilage of rabbits treated with AOT and PRP exhibit increased TGF-β1 expression compared to those treated with AOT and saline. Our findings suggest that adjunctive PRP may increase TGF-β1 expression, which may play a role in the chondrogenic effect of PRP in vivo.
Core tip: Despite the prevalence of platelet rich plasma (PRP) in both practice and literature, there is a dearth of data exploring the specific factors crucial to its role as an adjunct to cartilage repair surgeries. Our results suggest that the increased expression pattern of transforming growth factor-beta1 in PRP-treated rabbit femoral condyles, compared to saline treated controls, is associated with enhanced cartilage repair at the graft-host interface following autologous osteochondral transplantation. Our results serve as an initial step in building a body of evidence behind the specific growth factors crucial to cartilage repair and promise to help us understand how formulations of PRP are effective in musculoskeletal healing.
- Citation: Boakye LA, Ross KA, Pinski JM, Smyth NA, Haleem AM, Hannon CP, Fortier LA, Kennedy JG. Platelet-rich plasma increases transforming growth factor-beta1 expression at graft-host interface following autologous osteochondral transplantation in a rabbit model. World J Orthop 2015; 6(11): 961-969
- URL: https://www.wjgnet.com/2218-5836/full/v6/i11/961.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i11.961
The poor regenerative response of articular cartilage to mechanical injury is largely attributed to its avascularity and hypocellularity[1]. Thus, trauma to the articular surface of joints, particularly to the knee and ankle, may lead to osteochondral lesions (OCL). Few articular cartilage lesions heal without surgical treatment and most OCLs require intervention in hopes of preventing the eventual onset of post-traumatic osteoarthritis[2]. Generally, smaller defects are repaired with arthroscopic bone marrow stimulation (microfracture and drilling)[3]. Replacement procedures, including autologous osteochondral transplantation (AOT) are reserved for larger size lesions. In the ankle, AOT has excellent short to medium term clinical outcomes[3,4]. However, recent concern over poor graft host interface incorporation leading to subchondral bone voids and graft failure, has prompted investigators to identify biological adjuncts that may improve the cartilage graft-host interface incorporation[5-8].
Platelet rich plasma (PRP) may act as a biologic stimulant to influence cartilage repair at the graft-host interface. Although the exact combination of growth factors essential to the regenerative properties of PRP is unknown, transforming growth factor-beta1 (TGF-β1) has been suggested to stimulate mesenchymal stem cells (MSC), chondrocyte proliferation and inhibit catabolic activity[9-11]. Several in vivo and in vitro studies have attempted to investigate the milieu of growth factors that play a role in articular cartilage deposition, repair, and survival[9,12]. PRP contains concentrated platelets as well as growth factors that are three to five fold greater in concentration than whole blood[13,14]. Smyth et al[15] found that PRP improved osteochondral graft integration at the cartilage interface and decreased graft degeneration in an in vivo AOT rabbit model. Other reports have demonstrated that PRP promotes type II collagen deposition and chondrocyte proliferation in vitro substantiating the chondrogenic potential of PRP[14,16].
The potential chondrogenic effects of PRP are attributed to cytokines and growth factors released from the alpha granules of circulating platelets, which suppress inflammation, promote angiogenesis and support collagen synthesis[14]. These growth factors include TGF-β1, vascular endothelial growth factor, basic fibroblast growth factor, and a host of other factors[14]. The role of TGF-β1 remains to be defined, however it is known that it assists in recruiting cells that mediate injury repair and has the capacity to promote chondrogenesis in vivo[15]. Thus, it is of interest to understand whether TGF-β1 is crucial to the reparative properties of PRP. The aim of the current study was to explore the effect of PRP on expression patterns of TGF-β1 in a previously published rabbit femoral condyle OCL AOT study. We hypothesized that TGF-β1 would have an increased expression pattern in the PRP treated rabbit femoral condyles compared to saline treated controls.
The Institutional Animal Care and Use Committee at our institution approved the pre-cursor study (HSS Project # 09-11-03B) by Smyth et al[15]. The current study utilized unaltered archived samples obtained from the same cohort as the prior study. All appropriate measures were taken to minimize pain or discomfort of the rabbits during the interventions. The rabbits were cared for at the HSS veterinary facilities.
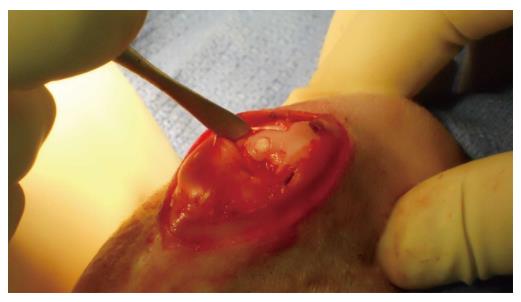
Twelve New Zealand white rabbits were treated with bilateral AOT as previously described by the current authors (Figure 1)[15]. After giving each rabbit adequate anesthesia, 27 mL of blood was aspirated from the great aural artery, combined with 4 mL of anticoagulant citrate dextrose solution A. One milliliter was set aside for cytological analysis and the remainder of the blood was used to prepare the platelet concentrated solution. Surgical procedures and PRP preparation (Magellan Autologous Platelet Separator; Arteriocyte Inc., Cleveland, Ohio) were performed as previously described[15]. Knees were randomly treated with an injection of 0.5 mL of either PRP or saline. Each rabbit received treatment in one knee, and saline in the contralateral knee to serve as its own control following wound closure. Osteochondral grafts were also soaked in PRP or saline for 10 min prior to implantation.
Rabbits were a mean weight of 4 kg (range, 3.7 to 4.2 kg) and a mean age of 23 wk. Rabbits were allowed unrestricted cage activity in individual cages for 7 d postoperatively, with rabbits euthanized (n = 4) at 3, 6 and 12 wk following surgery. Both knees were then removed en bloc and prepared for histological processing. A sample of synovium was also retrieved from each knee. Surgical protocol for harvesting of samples is as reported by Smyth et al[15]. Following the procedures, the rabbits were each given a fentanyl skin patch (12 mcg/h) and were granted free range within a cage. Rabbits were then euthanized with intravenous pentobarbital (100-150 mg/kg) at 3, 6, and 12 wk after the initial surgery.
Following euthanasia and removal of knees, specimens were fixed in 10% formalin for 7 d. Decalcification protocol included placing specimens in sodium citrate-formic acid solution for a further seven days. Specimens were then embedded in paraffin and cut into 8-μm sections. TGF-β1 antibody (R and D Systems Inc., Minneapolis, Minnesota) was used to stain articular cartilage and synovial sections.
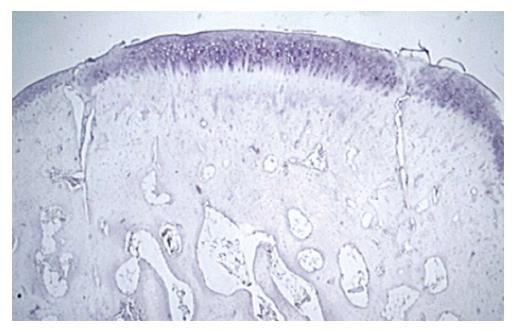
All slides were first dewaxed and rehydrated via 3-5 min treatments with xylenes, 5 min in 100% EtOH, 5 min in 95% EtOH, 5 min in 70% EtOH and 5 min in distilled water. Next, slides were blocked with H2O2 (3% in 1 × phosphate buffered saline (PBS)-10 mL H2O2 and 90 mL PBS), placed three times in PBS for 5 min each, retrieved without treatment, rinsed in deionized water twice for 5 min each, treated with protein block for 20 min, and exposed to antibody (1:20 in 1 × PBS-5 μL/slide), covered in parafilm, and left to react overnight in humidity chambers at room temperature. Samples were then rinsed three times in 1 × PBS. Anti-mouse immunoglobulin G was added and allowed to react for 1 h. After 45 min an Avidin Biotin Complex was prepared and added to the washed (3 × in PBS) samples for an hour. Samples were washed and then treated with 225 μL DAB and 3 mL PBS + 4 μL H2O2 until the cells darkened at about 7 min. Samples were then rinsed and counterstained in Harris Hematoxylin for 7 min. A mouse array containing various tissue samples served as a positive control and a negative control was not used.
Samples were visualized via confocal microscopy (Carl Zeiss, Inc., New York) and images were taken of predetermined regions of interest (ROI) in each graft sites in each knee at 400 × magnification. ROI included the interface between graft and unaltered cartilage on either side of the defect, the center of the autologous graft, and unaltered tissue remote from the repair sites. All samples were first analyzed qualitatively for area and density of apparent staining. A total of 144 samples were reviewed and the results of 120 histological sections were included in the study. The excluded images were not included due to poor or inadequate visualization of both femoral condyles and all ROIs. Two reviewers, who were blinded to the treatment groups, independently reviewed the histological sections and assessed percentage of chondrocytes staining positive for TGF-β1. Samples were evaluated quantitatively using computer software MetaMorph (Molecular Devices, Inc., Sunnyvale, California) to count the total number of chondrocytes and total number of chondrocytes expressing TGF-β1[17]. Analysis focused on the percentage of cells stained positive for TGF-β1 as the overall cellularity of each sample varied. Synovial sections were analyzed qualitatively. The presence of any synoviocytes staining positively with TGF-β1-antibody, or lack of any stained synoviocytes within the synovial specimen, was recorded. The International Cartilage Repair Society (ICRS) histological scoring system was originally developed in 2001 as a universal and standardized scoring system to aid in the assessment of cartilage repair[18]. An analysis of ICRS Score and overall percentage of chondrocytes positive for TGF-β1 with TGF-β1 for each specimen was performed using the Spearman Rho Coefficient to determine if higher ICRS scores correlated with a higher percentage of chondrocytes positive for TGF-β1.
Statistical review of the study methods was performed by a biomedical statistician. A Student’s t-test was used to determine whether there was a significant difference in TGF-β1 staining between samples. The percentage of chondrocytes stained positive for TGF-β1 in the PRP and saline groups in all ROI and all time intervals were compared. A value of P≤ 0.05 was considered significant. Comparison of data distribution between observers was calculated via a two-sample independent t-test. The intraclass correlation coefficient (ICC) was calculated to measure interobserver reliability. Statistical analysis was performed using commercially available software (Epi Info7™, Centers for Disease Control and Prevention, Atlanta, Georgia).
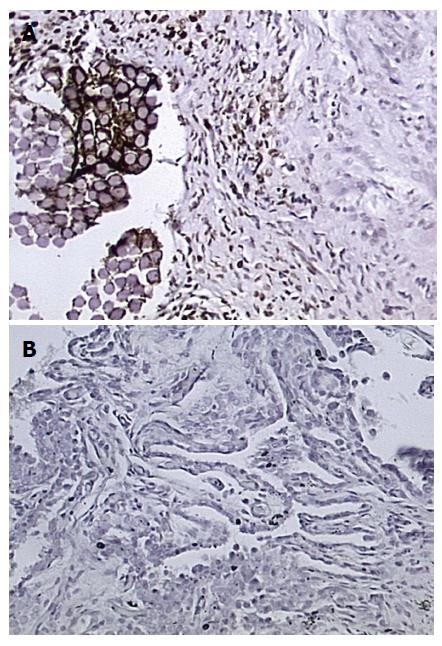
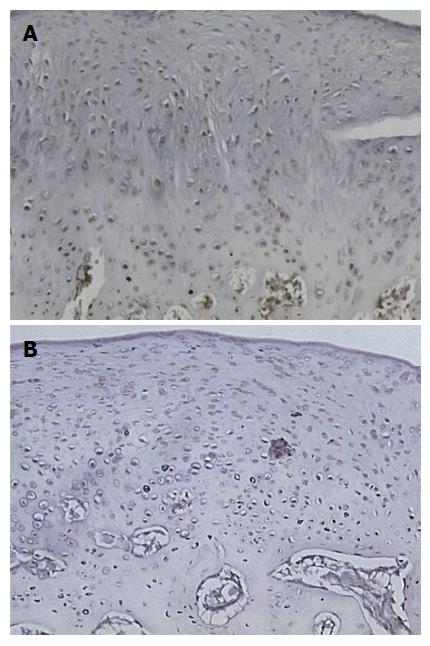
Qualitative microscopic analysis showed differences in the expression pattern of TGF-β1, with stronger (a higher percent concentration of chondrocytes) staining in the superficial cartilage of PRP treated joints. PRP treated knees showed strong TGF-β1 staining at all time periods (3, 6 and 12 wk). The mean modified ICRS histological score was significantly higher for all PRP-treated samples with a value of 18.2 ± 2.7 vs 13.5 ± 3.3 for saline treated controls (P = 0.002)[17]. A statistically insignificant correlation of percentage of TGF-β1 stained chondrocytes to ICRS scoring existed for the samples (r = 0.06, P = 0.77). Two-sample independent t-test indicated that there was no difference in distribution of the data between observers (P = 0.79). There was good reliability between observers (ICC, 0.79).
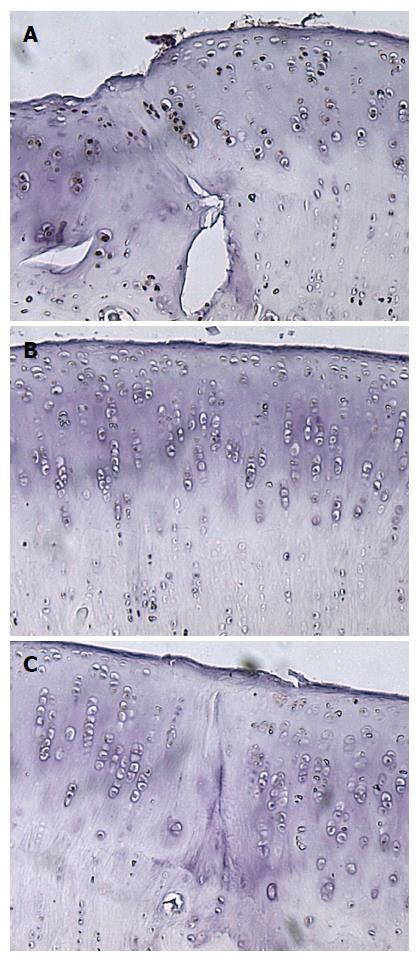
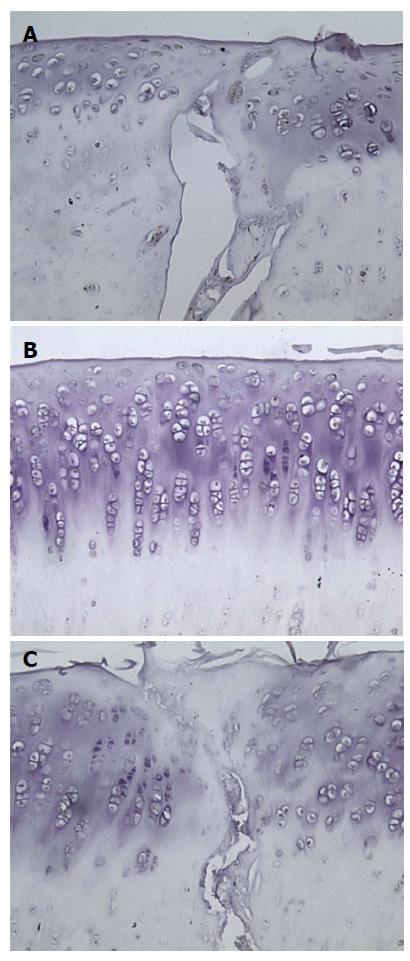
The results of staining quantification are detailed in Tables 1 and 2. Total mean percentage of chondrocytes positive for TGF-β1, when combining data from all ROIs and time points, indicated that TGF-β1 expression was higher in the PRP group compared to saline treated group, with values of 36% ± 29% vs 15% ± 18% (P = 1.8 × 10-6) (Figure 2, Figure 3, Figure 4, Figure 5). Mean percentage of chondrocytes positive for TGF-β1 was also higher in PRP treated knees at each individual ROI when combining data from all post-operative time points. Findings for each ROI are detailed in Table 1. Staining was significantly higher left of the graft interface and in the center of the graft (P = 0.03, P = 0.05), but the difference at the right interface of graft and host tissue was not significant (P = 0.72). The percentage of chondrocytes positive for TGF-β1 was higher in the PRP treated group at each euthanasia time point (P = 6.6 × 10-4; 3.1 × 10-4 and 7.3 × 10-3 for 3, 6 and 12 wk, respectively). Findings based on time from surgery to euthanasia are detailed in Table 2. Data from all ROIs combined were utilized for calculations by post-operative time point.
| Region of interest | Platelet rich plasma | Saline | P-value |
| Left | 45 ± 36 | 15 ± 17 | 0.03 |
| Center | 30 ± 31 | 7 ± 12 | 0.05 |
| Right | 36 ± 24 | 11 ± 23 | 0.72 |
| Unaltered | 24 ± 25 | 11 ± 12 | 0.19 |
| All ROIs | 32 ± 31 | 15 ± 19 | 2.60 × 10-3 |
| Time | Platelet rich plasma | Saline | P-value |
| 3 wk | 42 ± 19 | 12 ± 7 | 6.6 × 10-4 |
| 6 wk | 30 ± 11 | 7 ± 8 | 3.1 × 10-4 |
| 12 wk | 44 ± 19 | 21 ± 8 | 7.3 × 10-3 |
Percentage of chondrocytes positive for TGF-β1 in unaltered cartilage remote from the graft site was higher in the PRP group (24% ± 25%) than the saline group (11% ± 12%) but this difference was not statistically significant (P = 0.19) (Table 1).
Synovial samples that stained positively for TGF-β1 were associated with a lower mean ICRS score (14.6 ± 29.3) than those that did not stain positively (18.3 ± 4.9, P = 0.008). The mean percentage of chondrocytes stained positive for TGF-β1 in the samples with a positive synovial TGF-β1 stain was (29.3% ± 22.5%) compared to (44.9% ± 24.6%) for synovial samples without positive TGF-β1 staining- thus a difference was not statistically different (P = 0.80). Synovial tissue specimens demonstrated hypertrophy in the PRP treated group when compared to the saline treated group microscopically. This was more pronounced at the 6-wk time point than either of the other two experimental time points. An analysis of the synovial samples staining positively for TGF-β1 showed only 6 of 12 stained positively for TGF-β1 in the PRP treated group and four of the 12 stained positively in the saline treated group. This was not a statistically significant difference.
The current study is the first to investigate the effect of PRP on TGF-β1 expression in the setting of cartilage repair in an in vivo model. It follows a previous study indicating PRP was chondrogenic using the same animals. The current results indicate that PRP increases expression of TGF-β1 in the same model[15]. Increased expression of TGF-β1 appears to be present at 3, 6 and 12 wk after surgery. Platelet-rich plasma also appears to increase the presence of TGF-β1 in synovium, primarily 6 wk after surgery, although this was only qualitatively assessed, as images were not amenable to quantitative analysis of number of synoviocytes stained.
Increased TGF-β1 expression was noted in both PRP and saline treated knees. This was expected as the saline treated knees underwent AOT surgery. TGF-β1 expression was more prevalent in AOT repair sites treated with PRP compared to the saline control groups. An increase in TGF-β1 expression in histological sections exhibiting better graft integration could potentially indicate that TGF-β1 participates in this chondrogenic process in a rabbit model. The samples treated with PRP showed higher TGF-β1 expression and when compared to results published previously by Smyth et al[15] had higher ICRS macroscopic scores (Figure 6). In the previously published investigation, the authors showed, using the same rabbit femoral AOT model, that PRP facilitated chondrogenic integration at the graft-host interface using immunohistochemistry and safranin-o staining evaluation and quantitative data showed that PRP treated AOT produced a better infill at the graft site[15]. As this data is considered in light of the current data, we believe the effect of PRP is in large part as a result of TGF-β1 expression.
A number of other studies have investigated the role of TGF-β1 in articular cartilage synthesis with comparable results. Fortier et al[9] compiled the findings of several prevalent studies investigating the role of various growth factors in vitro and in vivo and found that TGF-β1 has effects on chondrocytes, cartilage, synovium and MSC. TGF-β1 stimulates MSCs and chondrocytes to facilitate chondrogenic proliferation and inhibits the catabolic activity of interleukin-1 (IL-1) and matrix metalloproteinases, which prompt degradation of cartilage extracellular matrix (ECM)[10]. TGF-β1 has also been shown to stimulate the synthesis of the ECM and downregulate type I collagen deposition, which is associated with fibrosis and potential scar formation[9]. However, TGF-β1 has also been associated with chemotaxis of inflammatory cytokines and synovial proliferation and fibrosis[9]. These potential drawbacks have served to create some ambiguity regarding TGF-β1’s interactions with ECM components.
More specifically, Qiao et al[19] studied in vivo growth factor expression in a rabbit model by exposing chondrocytes from articular cartilage of 7-week-old New Zealand white rabbits samples to recombinant TGF-β1 activated kinase1 (TAK1), TGF-β1 activated kinase1 antagonist (TAK1a), or over-expression of TAK1 via transfection with adenovirus. Results demonstrated enhanced synthesis of type II collagen protein by TGF-β1 and bone morphogenic protein-2. Möller et al[20] found that chondrocytes retrovirally transfected with TGF-β1 demonstrated a 96% increase in proteoglycan synthesis and a 304% increase in collagen synthesis (predominantly type II) compared to untreated samples, thereby providing significant stimulation of ECM synthesis and hyaline cartilage deposition. Galéra et al[11] found that TGF-β1 successfully increases collagen synthesis via enhanced gene expression based on mRNA encoding of type II procollagen of rabbit articular cartilage in monolayer culture. TGF-β1 may also contribute to mediating catabolic and inflammatory processes within the joint[9]. Smith et al[21] found that although IL-1 reduced proteoglycan synthesis by 50% on its own, treatment with TGF-β1 was able to restore proteoglycan synthesis to control levels.
In contrast to the aforementioned studies, PRP was the source of TGF-β1 in our study, rather than direct exposure of TGF-β1. The molecular effects of PRP have been shown to have a temporal sequence, with a maximal TGF-β1 expression at about 5 d, which is associated with the anabolic components of chondrocyte proliferation and maturation[22].
The effect of PRP is mediated therefore largely by its anabolic and anti-inflammatory effects[23]. A recent systematic review of the basic science literature found that 17 of 21 studies reported that PRP had a positive effect on cartilage repair[24]. An in vitro study by Park et al[22] assessed the effect of treating isolated primary chondrocytes with 0.1%-20% PRP. Results of reverse transcription-polymerase chain reaction and cytochemical staining indicated increased TGF-β1 expression and proteoglycan expression, along with a number of other growth factors[22]. Other animal studies have shown direct enhancement of articular cartilage repair using PRP[25-27].
This study had several limitations. Firstly, the use of PRP limited our ability to understand the effect of TGF-β1 in isolation from other growth factors and cytokines introduced by PRP. However, the purpose of our study was to investigate the effect of PRP on TGF-β1 expression patterns, rather than the effect of TGF-β1 on cartilage repair. Secondly, a lack of overall staining and ability to quantify percentage of synoviocytes or synovial fibroblasts stained positive for TGF-β1 antibody limited our ability to draw conclusions regarding the effect of PRP on synovial TGF-β1 expression. Lastly, the surgeon was not blinded to the treatment group at the time of operation, leading to possible bias during graft implantation. However, histological observers were blinded during data collection.
Several studies have reported that PRP may be an effective peri- or post-operative adjunct for cartilage injury, but there is a paucity of data elucidating the exact milieu of factors and cytokines essential to forming hyaline-like repair cartilage. The results of our study demonstrate that PRP increases TGF-β1 expression in AOT and may play a role in enhancing cartilage repair. Further evaluation of TGF-β1’s restorative capacity and temporal sequence of activity would help to optimize PRP’s use as an adjunctive therapy. Future research should focus on determining the specific role of growth factors and inflammatory cytokines to determine the most effective PRP formulation for musculoskeletal healing.
The results of the current study indicate that repair cartilage treated with PRP exhibits increased TGF-β1 expression compared to saline treated controls in a rabbit model. This finding, along with enhanced repair in the PRP group previously reported in the same model, indicates that TGF-β1 may play a role in the chondrogenic effect of PRP in vivo.
The authors would like to gratefully acknowledge and thank Dr. Orla O’Shea for her assistance in histological sectioning and staining and Dr. Steve Doty for his assistance with imaging.
Trauma to the articular surface of joints may lead to osteochondral lesions (OCL) due to poor regenerative potential. Most OCLs require intervention in hopes of preventing subsequent post-traumatic osteoarthritis. There are options for repair and/or replacement of articular cartilage. Replacement procedures, including autologous osteochondral transplantation (AOT) are reserved for larger size lesions. Although AOT has shown excellent short to medium term clinical outcomes, concern regarding poor graft host interface incorporation has prompted investigators to identify biological adjuncts that may improve the cartilage graft-host interface incorporation. Platelet rich plasma (PRP) may act as a biologic stimulant to influence cartilage repair at the graft-host interface. The potential chondrogenic effects of PRP are attributed to cytokines and growth factors released from the alpha granules of circulating platelets, which prevent inflammation, promote angiogenesis and support collagen synthesis. Although the exact combination of growth factors essential to the regenerative properties of PRP is unknown, transforming growth factor-beta1 (TGF-β1) has been suggested to stimulate mesenchymal stem cells, chondrocyte proliferation and inhibit catabolic activity.
The use of biologic adjuncts in articular cartilage repair or replacement surgeries is a burgeoning field as there remains a dearth of information of what factors are most influential in the effectiveness of the most commonly used adjuncts. In an effort to align clinical practices with evidence-based research, much of the recent research on articular cartilage has focused on the pathophysiology of adjuncts.
The study was driven by an interest in addressing the paucity of data elucidating the exact milieu of factors and cytokines essential to forming hyaline-like repair cartilage. The aim of the current study was to explore the effect of PRP on expression patterns of TGF-β1 in a previously published rabbit femoral condyle OCL AOT study. The results of the study demonstrate that PRP increases TGF-β1 expression in AOT and may play a role in enhancing cartilage repair.
Further evaluation of TGF-β1’s restorative capacity and temporal sequence of activity would help to optimize PRP’s use as an adjunctive therapy. Future research should focus on determining the specific role of growth factors and inflammatory cytokines to determine the most effective PRP formulation for musculoskeletal healing.
PRP: An autologous blood product rich in cytokines and growth factors increasingly studied as an adjunct to cartilage repair and replacement procedures. Above baseline quantities of platelets or at least 1.1 × 106 platelets/μL as well as growth factors that are 3 to 5-fold greater in concentration than whole blood; AOT: Replacement of the articular cartilage defect with a tubular unit of viable hyaline cartilage and bone from a donor site in the ipsilateral knee joint.
The manuscript is well written and has scientifically relevant data.
| 1. | Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460-466. [PubMed] |
| 2. | Jackson DW, Lalor PA, Aberman HM, Simon TM. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J Bone Joint Surg Am. 2001;83-A:53-64. [PubMed] |
| 3. | Kennedy JG, Murawski CD. The Treatment of Osteochondral Lesions of the Talus with Autologous Osteochondral Transplantation and Bone Marrow Aspirate Concentrate: Surgical Technique. Cartilage. 2011;2:327-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med. 2009;37 Suppl 1:105S-111S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Marcacci M, Kon E, Delcogliano M, Filardo G, Busacca M, Zaffagnini S. Arthroscopic autologous osteochondral grafting for cartilage defects of the knee: prospective study results at a minimum 7-year follow-up. Am J Sports Med. 2007;35:2014-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Siebert CH, Miltner O, Weber M, Sopka S, Koch S, Niedhart C. Healing of osteochondral grafts in an ovine model under the influence of bFGF. Arthroscopy. 2003;19:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Tibesku CO, Daniilidis K, Szuwart T, Jahn UR, Schlegel PM, Fuchs-Winkelmann S. Influence of hepatocyte growth factor on autologous osteochondral transplants in an animal model. Arch Orthop Trauma Surg. 2011;131:1145-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Siebert CH, Schneider U, Sopka S, Wahner T, Miltner O, Niedhart C. Ingrowth of osteochondral grafts under the influence of growth factors: 6-month results of an animal study. Arch Orthop Trauma Surg. 2006;126:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 441] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 10. | Maerz T, Herkowitz H, Baker K. Molecular and genetic advances in the regeneration of the intervertebral disc. Surg Neurol Int. 2013;4:S94-S105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Galéra P, Rédini F, Vivien D, Bonaventure J, Penfornis H, Loyau G, Pujol JP. Effect of transforming growth factor-beta 1 (TGF-beta 1) on matrix synthesis by monolayer cultures of rabbit articular chondrocytes during the dedifferentiation process. Exp Cell Res. 1992;200:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Civinini R, Nistri L, Martini C, Redl B, Ristori G, Innocenti M. Growth factors in the treatment of early osteoarthritis. Clin Cases Miner Bone Metab. 2013;10:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Miller Y, Bachowski G, Benjamin R, Eklund D, Hibbard A, Lightfoot T. Practice Guidelines for Blood Transfusion: A Compilation From Recent Peer-reviewed Literature. American Red Cross. 2007;2:56. |
| 14. | Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28:429-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 404] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 15. | Smyth NA, Haleem AM, Murawski CD, Do HT, Deland JT, Kennedy JG. The effect of platelet-rich plasma on autologous osteochondral transplantation: an in vivo rabbit model. J Bone Joint Surg Am. 2013;95:2185-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Milano G, Sanna Passino E, Deriu L, Careddu G, Manunta L, Manunta A, Saccomanno MF, Fabbriciani C. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Grimsey NL, Narayan PJ, Dragunow M, Glass M. A novel high-throughput assay for the quantitative assessment of receptor trafficking. Clin Exp Pharmacol Physiol. 2008;35:1377-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85-A Suppl 2:45-57. [PubMed] |
| 19. | Qiao B, Padilla SR, Benya PD. Transforming growth factor (TGF)-beta-activated kinase 1 mimics and mediates TGF-beta-induced stimulation of type II collagen synthesis in chondrocytes independent of Col2a1 transcription and Smad3 signaling. J Biol Chem. 2005;280:17562-17571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Möller HD, Fu FH, Niyibizi C, Studer RK, Georgescu HJ, Robbins PD, Evans CH. [TGF-beta-1 gene transfer in joint cartilage cells. Stimulating effect in extracellular matrix synthesis]. Orthopade. 2000;29:75-79. [PubMed] |
| 21. | Smith P, Shuler FD, Georgescu HI, Ghivizzani SC, Johnstone B, Niyibizi C, Robbins PD, Evans CH. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Park SI, Lee HR, Kim S, Ahn MW, Do SH. Time-sequential modulation in expression of growth factors from platelet-rich plasma (PRP) on the chondrocyte cultures. Mol Cell Biochem. 2012;361:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 23. | Smyth NA, Murawski CD, Haleem AM, Hannon CP, Savage-Elliott I, Kennedy JG. Establishing proof of concept: Platelet-rich plasma and bone marrow aspirate concentrate may improve cartilage repair following surgical treatment for osteochondral lesions of the talus. World J Orthop. 2012;3:101-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Smyth NA, Murawski CD, Fortier LA, Cole BJ, Kennedy JG. Platelet-rich plasma in the pathologic processes of cartilage: review of basic science evidence. Arthroscopy. 2013;29:1399-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 25. | Carneiro Mde O, Barbieri CH, Barbieri Neto J. Platelet-rich plasma gel promotes regeneration of articular cartilage in knees of sheeps. Acta Ortop Bras. 2013;21:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Altan E, Aydin K, Erkocak O, Senaran H, Ugras S. The effect of platelet-rich plasma on osteochondral defects treated with mosaicplasty. Int Orthop. 2014;38:1321-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Betsch M, Schneppendahl J, Thuns S, Herten M, Sager M, Jungbluth P, Hakimi M, Wild M. Bone marrow aspiration concentrate and platelet rich plasma for osteochondral repair in a porcine osteochondral defect model. PLoS One. 2013;8:e71602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Saniabadi AR S- Editor: Qiu S L- Editor: A E- Editor: Jiao XK