Published online Dec 18, 2015. doi: 10.5312/wjo.v6.i11.902
Peer-review started: May 12, 2015
First decision: July 27, 2015
Revised: August 4, 2015
Accepted: October 16, 2015
Article in press: October 19, 2015
Published online: December 18, 2015
Processing time: 221 Days and 2.1 Hours
Lesions of the rotator cuff (RC) are a common occurrence affecting millions of people across all parts of the globe. RC tears are also rampantly prevalent with an age-dependent increase in numbers. Other associated factors include a history of trauma, limb dominance, contralateral shoulder, smoking-status, hypercholesterolemia, posture and occupational dispositions. The challenge lies in early diagnosis since a high proportion of patients are asymptomatic. Pain and decreasing shoulder power and function should alert the heedful practitioner in recognizing promptly the onset or aggravation of existing RC tears. Partial-thickness tears (PTT) can be bursal-sided or articular-sided tears. Over the course of time, PTT enlarge and propagate into full-thickness tears (FTT) and develop distinct chronic pathological changes due to muscle retraction, fatty infiltration and muscle atrophy. These lead to a reduction in tendon elasticity and viability. Eventually, the glenohumeral joint experiences a series of degenerative alterations - cuff tear arthropathy. To avert this, a vigilant clinician must utilize and corroborate clinical skill and radiological findings to identify tear progression. Modern radio-diagnostic means of ultrasonography and magnetic resonance imaging provide excellent visualization of structural details and are crucial in determining further course of action for these patients. Physical therapy along with activity modifications, anti-inflammatory and analgesic medications form the pillars of nonoperative treatment. Elderly patients with minimal functional demands can be managed conservatively and reassessed at frequent intervals. Regular monitoring helps in isolating patients who require surgical interventions. Early surgery should be considered in younger, active and symptomatic, healthy patients. In addition to being cost-effective, this helps in providing a functional shoulder with a stable cuff. An easily reproducible technique of maximal strength and sturdiness should by chosen among the armamentarium of the shoulder surgeon. Grade 1 PTTs do well with debridement while more severe lesions mandate repair either by trans-tendon technique or repair following conversion into FTT. Early repair of repairable FTT can avoid appearance and progression of disability and weakness. The choice of surgery varies from surgeon-to-surgeon with arthroscopy taking the lead in the current scenario. The double-row repairs have an edge over the single-row technique in some patients especially those with massive tears. Stronger, cost-effective and improved functional scores can be obtained by the former. Both early and delayed postoperative rehabilitation programmes have led to comparable outcomes. Guarded results may be anticipated in patients in extremes of age, presence of comorbidities and severe tear patters. Overall, satisfactory results are obtained with timely diagnosis and execution of the appropriate treatment modality.
Core tip: Close attention to history and examination enables early diagnosis in the frequently asymptomatic rotator cuff tear. Ultrasonography and magnetic resonance imaging serve as excellent visualization tools. While conservative measures are successful in elderly patients with minimal lesions and demands, regular monitoring helps in isolating the surgical candidate. Early surgery should be considered in younger, healthier, active and symptomatic patients. Lower grades of tears do well with debridement alone while more severe lesions warrant a repair. Arthroscopic double-row repairs are superior in patients with massive tears. Satisfactory results are obtained with timely diagnosis and execution of the appropriate treatment modality.
- Citation: Sambandam SN, Khanna V, Gul A, Mounasamy V. Rotator cuff tears: An evidence based approach. World J Orthop 2015; 6(11): 902-918
- URL: https://www.wjgnet.com/2218-5836/full/v6/i11/902.htm
- DOI: https://dx.doi.org/10.5312/wjo.v6.i11.902
“Its been an ache and a joy both to look over this big shoulder of mine at all my yesterdays” - Ethel Waters. Tears of the rotator cuff (RC) have been inherited by man from his ancestors with an association leading to the great apes[1,2]. With the advent of newer techniques, good to excellent results can be expected in the appropriately selected and compliant patient[3]. The aim of this article is to provide a comprehensive review of evidence-based concepts and present understanding of the epidemiology, etiopathogenesis, natural history, clinical evaluation, imaging, management and healing of RC tears.
Abundant data from across the world has been published on the prevalence of RC tears. Yamaguchi et al[4], from Missouri, United States, evaluated 588 patients with unilateral (U/L) shoulder complaints. Their analysis revealed 199 (33.8%) U/L and 177 (30.1%) bilateral (B/L) RC tears with average ages of 58.7 and 67.8 years respectively. The authors found high correlation between advancing age and RC tears[4]. In 2009, a study from the same institution on ultrasonographic (USG) screening of both shoulders in 237 asymptomatic individuals revealed a 17.3% prevalence of RC tear in at least one shoulder. Age-wise prevalence observed was 20% in 60-69 years old and 40.7% in subjects 70 years of age or older[5]. In a larger cohort of 683 Japanese villagers with a mean age of 57.9 years, Yamamoto et al[6] observed RC tears in 36% symptomatic against 16.9% in asymptomatic subjects with an overall prevalence of 20.7%. In a recent systematic review of 30 studies, Teunis et al[7] analysed 6112 shoulders with 1452 cuff abnormalities. Overall prevalence of RC abnormalities ranged from 9.7% in patients younger than or 20 years and increased to 62% in patients aged 80 years and older (P < 0.001) regardless of symptoms, among the general population and in patients with a dislocated shoulder.
A German prospective study on 411 asymptomatic shoulders by Tempelhof et al[8] revealed 23% overall prevalence of RC tears with high occurrence in patients over the age 70 and 80 years of 31% and 51% respectively. Further, Fehringer et al[9] observed a 22% prevalence of full-thickness tears (FTT) of the RC in patients aged 65 and above. Other European studies have shown lower figures. In a study from Austria on 212 asymptomatic shoulders, Schibany et al[10] reported a 6% prevalence of FTT. Likewise, Moosmayer et al[11] in a Norwegian study on 420 asymptomatic volunteers aged 50-79, revealed FTT in 32 subjects (7.6%).
Cadaveric studies on the RC tears have also revealed varying results. Neer’s study on 500 cadavers more than 3 decades ago observed a less than 5% occurrence of FTT[12]. In 456 cadaver shoulders (mean age = 64.7) years, Lehman et al[13] reported an higher, 17% prevalence of FTT. Cadavers below and above 60 years of age had tear incidences of 6% and 30% respectively[13]. Still higher rates were observed by Reilly et al[14] in a review comparing cadaveric against radiological studies. Overall prevalence among 30 cadaveric studies was 30.3% while 11 ultrasound studies reported a prevalence of 38.9% in asymptomatic individuals which rose to 41.4% in symptomatic patients. Further, 14 magnetic resonance imaging (MRI) based studies reported 26.2% prevalence in the asymptomatic population vs 49.38% in symptomatic patients[14]. The disparity between human and cadaveric studies can be attributed to differences in subject population and unknown historical and clinical backgrounds in cadavers.
Despite varied regional distribution, RC tears are prevalent in up to 39% of asymptomatic individuals. An age-related increase in incidence leaves one with the thought of whether the tears are a part of the normal aging process. Their overall prevalence in symptomatic individuals is up to 64%. Presence of pain and decreasing shoulder strength and scores, which increases with age, heralds the onset or increase in size of existing RC tears. The opposite shoulder must be evaluated in U/L complaints to rule out B/L RC tears.
Advancing age has been consistently held accountable as one of the major risk factor for the development of cuff tears in various studies. Gumina et al[15] on 586 patients with a history of arthroscopic tear repair reported a mean age of 59 years. Patients older than 60 were twice as likely to develop tear which were larger and more massive[15]. The prevalence in human and cadaveric studies in patients 60 years and older ranges from 20% to 30% and touches 62% in individuals aged 80 and more[4,5,7-9,11,13]. With a 10-year increase in age, the odds of an RC tear increase 2.69 fold (P = 0.005)[8].
A study by Abate et al[16] on menopausal women revealed increased prevalence of asymptomatic FTT in the postmenopausal period. Both sexes have otherwise been quoted as being equally predisposed to the development of RC tears[17,18].
While some evidence suggests greater risk of the dominant hand for developing RC tears, others find this predilection not significant[6,18]. Dominant shoulders in veteran tennis players were more frequently torn suggesting an association of high energy activity with RC tears[19].
In patients operated for ipsilateral partial-thickness tears (PTT) or FTT, opposite shoulders are at increased risks of developing the same[20]. Ro et al[21] reported a higher prevalence of RC tears in contralateral (C/L) asymptomatic shoulders in U/L symptomatic RC tears, medium-sized or large operated RC tears and in patients with symptomatic RC tears of the non-dominant arm. B/L tears are common in U/L symptomatic tears with 35.5% prevalence FTT on the opposite side as observed by Yamaguchi et al[4]. The likelihood of a bilateral tear after 60 years of age is as high as 50%[4].
A strong dose and time-dependent association has been established between smoking and the development of RC tears. Baumgarten et al[22], among 586 consecutive patients with unilateral shoulder pain, found 375 patients with RC tears. A smoking history was elicited in 61.9% patients with a mean 23.4 years of smoking 1.25 packs per day and 30.1 mean pack-years. In a systematic review by Bishop et al[23], increased rates and sizes of RC degeneration and symptomatic RC tears were seen in smokers which could consequently increase the number of surgical procedures in these patients. In a study on 408 patients, Carbone et al[24] found higher frequencies of smokers with at least a type II tear (34.8%) differing significantly from the type I patients (23.2%) and concluded that smoking negatively affects vascularity of tendons.
Increased risk in relatives of individuals with RC disease has been identified. In a study by Tashjian et al[25], patients diagnosed before 40 years showed significant relatedness for individuals with RC disease in close and distant relationships (up to 3rd cousins) (P = 0.001).
In a recent cross-sectional study by Yamamoto et al[26], RC tears were observed in 65.8% patients with kyphotic-lordotic postures, 54.3% with flat-back postures and 48.9% with sway-back postures while only 2.9% patients with ideal alignment had RC tears. The authors found poor posture as an independent predictor of symptomatic and asymptomatic RC tears[26]. In an Italian study, Gumina et al[27] compared the radiologically calculated subacromial space (SAS) width in 47 patients with thoracic hyperkyphosis with normal controls. They found reduced acromio-humeral space in hyperkyphotic patients, females and patients older than 60 years. The authors attributed this decrease to less posterior tilting and dyskinesis of the scapula[27].
They are history of trauma, hypercholesterolemia and occupational demands of heavy labour[6].
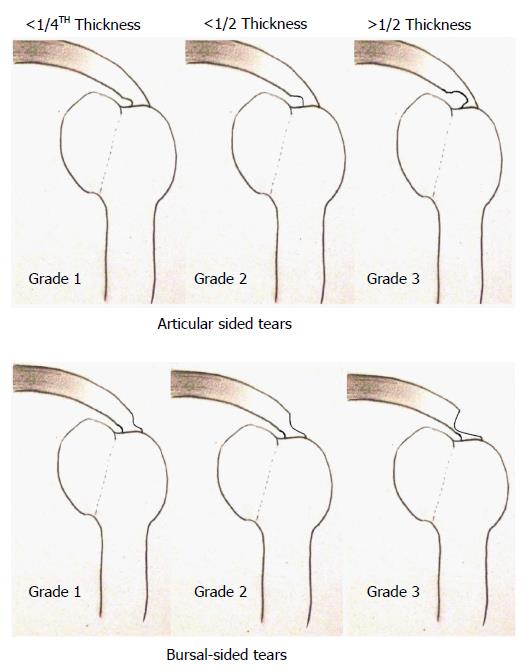
The classical description by Ellman classifies RC tears on the basis of location into articular-sided tears (AST) and bursal-sided tears (BST) which are further staged according to their depths (Figure 1)[28]. These two varieties have differing properties and vasculature.
The precarious vascularity of the articular side has been demonstrated by Lohr and Uhthoff[29] who found the predominance of a zone of hypovascularity on the on the articular side. While ASTs result more commonly from intrinsic factors alone, intrinsic and extrinsic factors and greater wear and impingement more often result in BST. Ozaki et al[30] in a cadaveric study on 200 shoulders confirmed the association of BST with attritional lesions on the coracoacromial ligament (CAL) and anterior third of the undersurface of the acromion while ASTs had a normal acromion. Although an acromial lesion was always associated with an RC tear, the reverse was not true[30].
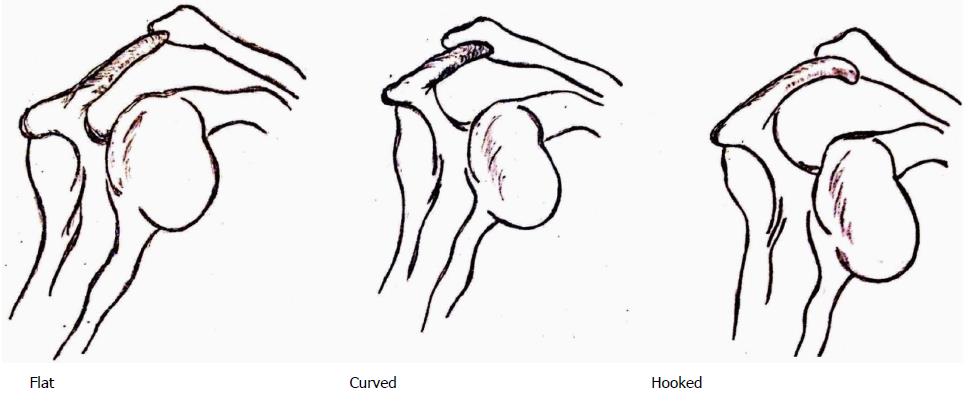
Tears of the RC have been theorized by Neer et al[31] to occur as a sequel of shoulder impingement due to repetitive translation of the cuff tendons under the acromion. Anatomical factors implicated in impingement and consequent tears of the RC include, among others, abnormalities in the coracoacromial arch. The acromion has been classified as flat, curved and hooked by Bigliani et al[32] (Figure 2).
Extrinsic sources arise from developmental aberrations in the form of os acromiale, hooked and keeled acromion, enthesophytes at acromial insertion of CAL and acromion-uncovered portion of humeral head (HH) leading to SSP impingement between the two[33]. Lower successful rates of nonoperative methods have been demonstrated in curved (73%) and hooked (58.3%) vs flat types (89%) by Wang et al[34]. Neer[35] in 1972 on 50 shoulders managed by anterior acromioplasty for impingement over 5 years demonstrated good results in 39. He concluded that acromioplasty provides good pain relief in mechanical impingement, exposure for SSP repair and prevents further impingement and wear. Moor et al[36], on the other hand, found no association between the acromion morphology and slope and RC tears. They avowed higher acromial indices, smaller lateral acromial angles and larger critical shoulder angles in degenerative RC tears. Anterior and lateral acromial bony spurs have been associated with FTT in symptomatic patients[37]. Other structures implicated in RC tears include spurs in CAL, altered coracoid morphology, increased pillar angulation, reduced inter-pillar distance or pillar length resulting in subcoracoid impingement. The subdeltoid, subcoracoid and subacromial bursae (SAB) are recognized causes of adhesions, bursitis and impingement[33]. The SAB may however, prove beneficial in tendon healing[38].
Besides extrinsic factors, the degeneration-microtrauma theory links advancing age and chronic microtrauma with PTT. Involvement of deep fibres leads to retraction, increased tension on intact fibres and conversion to FTT. Inflammatory changes and oxidative stresses causing tenocyte apoptosis along tissue remodelling are responsible for these tears[39]. Hypovascularity predisposes to development of RC tears in an age-dependent manner as shown by Rudzki et al[40] in 2008. A critical hypovascular zone exists 10-15 mm proximal to the RC insertion on the HH. In a study on 18 human specimens, the presence this zone close to SSP insertion was confirmed by selective vascular injection with a silicon-rubber compound. Uniformly sparse vascular distribution at the articular side against a well-vascularized bursal side could explain development of AST[29]. A few, however, have refuted this theory[41,42]. Laser Doppler flowmetry analysis by Levy et al[41] reported areas of increased flux at the RC tear edges suggesting areas of hypervascularity.
Walch et al[42] in young, athletic individuals described impingement occurring between the deep side of SSP and posterosuperior edge of glenoid. Arthroscopic visualization confirmed AST involving posterior and anterior regions of the SSP and infraspinatus (ISP) respectively in the abducted and externally rotated shoulder.
In a recent study, Choo et al[43] found patients with bursitis and tendinopathy expressing pro-myogenic genes while those with FTT expressed genes linked with fatty atrophy and fibrosis. Massive tears had down-regulation of most genes except inhibition of myogenesis which explains the difficulty in treating them. The authors also suggested earlier surgical intervention for a more favourable response.
To conclude, RC tears result from multiple etiological factors ranging from tendon injury due to narrowed SAS and anatomical aberrations, degeneration from within the tendon and predilections resulting from non-physiological positioning in extremes of abduction and external rotation (ER). A relation of the tear process with regulation of gene expression may have a negative effect in the outcome of repairs.
Tears have been described as the ultimate consequence of impingement occurring in the final stage III of RC disease[13].
Studies in the past have revealed a substantial symptomatic conversion of asymptomatic FTT. Moosmayer et al[44], in a three-year follow-up of 50 patients found 18 symptomatic at the end of 3 years with significantly larger mean tear sizes (10.6 mm vs 3.3 mm, P = 0.02), faster muscular atrophy (MA), fatty degeneration (FD) and development of a pathological long head of biceps (LHB). Yamaguchi et al[45], while evaluating asymptomatic C/L tears in patients with U/L symptoms over 5 years, found greater symptoms and tear progression in 23 (51%) of 45 previously asymptomatic patients over a mean 2.8 years. In 2010, Mall et al[46] compared 44 newly symptomatic subjects over a two-year period, with 55 subjects who remained asymptomatic. The development of pain and increase in size of FTT by more than 5 mm occurred in 18% of the FTT. A 40% conversion of PTT to FTT was associated with significant reductions in the American Shoulder and Elbow Surgeons (ASES) scores. Newly symptomatic patients had significantly larger tears at initial evaluation. The development of symptoms in asymptomatic tears should, therefore, alert the surgeon towards suspecting tear enlargement[46].
The natural history of symptomatic RC tears often puzzles the reader with doubts regarding worsening and improvement of symptoms, risk of tear progression, indications and benefits of nonoperative and surgical intervention and the presence of FD and MA. Improved pain relief and arm function was seen in 19 of 33 symptomatic FTT at 3.8 years of follow-up by Hawkins et al[47] in 1995. Goldberg et al[48] in a 2.5-year follow-up of 46 nonoperatively managed symptomatic FTT, found functional improvement in 59% patients. Fucentese et al[49] in 24 nonoperatively managed FTT found no increase in tear size and FD over a mean 3.5 years. Tear progression, reported in 25% patients wasn’t related to tear reparability. A sample containing small (mean 1.6 cm), isolated tears in patients aged around 54 years could adversely affect generalization of these results[49]. MRI monitored progression in 33 nonoperatively treated FTT by Maman et al[50] in 2009 demonstrated increased sizes in 52% patients. Older patients (> 60 years) with longer symptom duration (> 18 mo) and initial fatty infiltration were likely to progress (FI). Safran et al[51] in 2011, prospectively evaluated 61 nonoperatively managed FTT with a minimum size of 5 mm in patients younger than 60 years of age. At follow-up (mean 29 mo), 30 (49%) of the 61 tears were found to be larger with significant association with pain (P = 0.002).
Progression rates of symptomatic PTT to FTT have ranged from 10% to 50%[46,50]. The presence of pain has shown to be significantly indicative of an increase in tear size and should be, therefore, closely watched.
Tissue quality plays an important role in RC healing[52]. Cuff pathology has effects on both muscles and tendons. Muscle retraction from FTT can alter angulations between the muscle fibres and allow adhesions and eventual FI. The twofold effects of FI produces MA and higher failure rates of surgical procedures[53,54].
The term fatty infiltration signifies infiltration of adipose cells[55,56]. Melis et al[57] in 1688 shoulders found statistical correlation (P < 0.0005) between FI, type of tendon lesion and patient age for SSP, ISP and subscapularis (SS). Severe FI was observed in extensive lesions, longer duration following rupture and increased patient age. A study involving 251 FTT revealed eighty-seven (34.7%) tears with FI having significantly greater dimensions and shorter distances posterior from the biceps than those without FI (P < 0.0001). The latter was the most important predictor for SSP FI. Tear width and length were found to be the most important predictors for ISP FI[58]. ISP FI has shown poorer results following repairs attributed to traction injuries of suprascapular nerve, disturbance to the anterior-posterior glenohumeral (GH) force couple and under-diagnosis[59].
As a part of the RC pathology, muscle atrophy has been documented by various authors. Swan et al[60] observed increases in muscle mass by 30%, fibre length by 7%, and physiological cross-sectional area by 27% in normal growing rats. The sarcomere length, however, were nearly constant. A comparative 53% SSP and 45% ISP rat RC mass reduction 30 d after tear was reported by Gumucio et al[61]. Barton et al[62] demonstrated rapid yet reversible loss of muscle mass, increase in fast muscle fibres, and fibrotic content of the muscle bed and tendon adhesions following RC tears in rats. Adhesion-induced reversal of changes may however, occur on return of load to the muscle. Ditsios et al[63] observed 30% and 35% reduction in SSP and ISP forces respectively in massive tears vs normal rat shoulders. FI and MA were more evident near the tendon on the dorsal aspect. Recently, Mendias et al[64] in 13 human shoulders elicited reductions in muscle fibre force production correlating with ASES scores and tear sizes. Disordered sarcomeres with lipid-laden macrophages in the extracellular matrix were seen surrounding SSP fibres. Reports exist on compensatory hypertrophy with MA. Kikukawa et al[65] in 2014, in a retrospective review of 279 subjects, confirmed hypertrophic changes in teres minors of patients with RC tear involving ISP. Mechanical unloading and denervation have been shown to result in FI and MA. These two independent predictors (part of the same pathological process) along with increased connective tissue content and fibrosis eventually result in decreased elasticity, viability and healing of the RC[59]. These unique, specific changes differ from denervation-induced changes after suprascapular nerve entrapment with respect to muscle border, perineural fat and overall FI distribution[66].
Tear-induced changes in GH kinematics have been described by Keener et al[67]. HH migration in 98 asymptomatic and 62 symptomatic RC tears showed significant correlation between PHM and RC tear size (≥ 175 mm2), tear extension into ISP and presence of pain. The net result, cuff-tear arthropathy occurs more commonly than realized[68]. Neer et al[69] ascribed this phenomenon to inactivity, disuse, synovial fluid leakage and HH migration. Next, cartilage atrophy, subchondral bone osteoporosis and impingement leads to acromial and acromioclavicular (AC) joint erosion. The collapsed HH finally erodes into the scapula from glenoid to as far as the coracoid resulting in a condition extremely difficult to manage[69].
Muscle retraction leads to tear-size, site, duration and age-dependent FI and reduction in muscle mass and force. Incongruent GH surfaces result in a challenging cuff-deprived joint. Surgical decision-making solely based on natural history seems inadequate and inappropriate due to conflicting results with nonoperative treatment. Further studies are required to establish guidelines assisting surgeons with the decision making process. Early surgical intervention in young, active adults with RC tears can avert advanced changes and GH arthropathy, both of which have poor outcomes.
Progressive shoulder pain typically occurs around the anterolateral shoulder margin, lateral surface of the arm down to the elbow[70]. Night pain can occur in 83% patients while 41% may experience muscle weakness[71]. Local examination reveals disuse-related SSP and ISP MA. Among various tests, the empty can test is most sensitive (68.4%), drop arm and lift-off tests most specific (100%) and Neer test most accurate (75%) for RC tears overall[71].
For the SSP and ISP, the Jobe sign and the full can test have comparable accuracies[72]. High sensitivities (83% and 97%) and low specificities (23% and 5%) are reported with Hawkins sign and the painful arc test. Higher specificities (91% and 86%) have been observed with the external rotation lag sign (ERLS) and the drop-arm test (DAT) in diagnosing FTT. The sensitivity of lag tests reduces after subacromial lidocaine injection, while specificities of the Jobe, ERLS and DAT have been seen to improve[73]. A positive lift-off test is highly specific for diagnosing FTT and severe FI of SS[74]. No test in isolation however, is adequate for diagnosing an RC tear and a combination of tests improves the diagnostic yield[75].
Once clinically suspected, the RC tear requires radiological establishment of diagnosis. Ultrasonography (USG) is invaluable providing excellent tendon visualization. In a meta-analysis of 6066 shoulders, USG showed good sensitivity (84%) and specificity (89%) for the assessment of PTT and FTT (sensitivity 0.96; specificity 0.93)[76]. Orthopaedician-performed USG displayed better accuracy in large and massive tears against small tears (96.5% vs 91.6%)[77]. In a study by Iannotti et al[78] on 99 shoulders with RC disease, comparable sensitivities and specificities of MRI and USG were reported. Errors with USG were due to inability to distinguish between PTT and FTT around 1 cm in size[78]. FI can also be reported by USG with remarkable precision. Wall et al[79] have reported, as percentage-agreement with MRI, 92.5% and 87.5% accuracy in detection of FI with USG in SSP, ISP and SS respectively.
Read et al[80] compared preoperative and postoperative USG findings in 42 consecutively operated patients obtaining excellent sensitivity and specificity for FTT (100% and 97%). The results were more dismal for PTT with sensitivity of 46%. A Cochrane Database review has similarly reported poor sensitivity of USG in diagnosis of PTT[81]. The 3D technique has demonstrated lower inter and intra-observer reliability than 2D USG for RC tears especially in the interpretation of small hypoechoic lesions close to the footprint as PTT[82]. Ok et al[83], in a prospective analysis of orthopaedician-performed USG in 51 shoulders, reported significantly poor correlation of USG-reported and arthroscopically-determined tear sizes (P < 0.05). In contrast, Roy et al[84] in a meta-analysis have demonstrated similar accuracy of USG by the radiologist/sonographer or orthopaedician.
A steep learning curve, operator and technique-dependence with inaccuracies in measuring tear size and PTT are few drawbacks of USG. Nonetheless it remains a reliable, fast, accurate, cost and time-saving tool in experienced hands providing excellent depiction of the RC tendon fibres. It also possesses easy availability, portability and speed, lacks motion artefacts and allows instant comparison with C/L side, dynamic evaluation of tendons and quantitative and qualitative assessment of FI[85,86].
The MRI can provide vital information and outstanding details not only on the RC tear size, extent, location retraction, FI and MA, but also on LHB, acromial morphology, AC joint and SAS[87]. A prospective follow-up of 48 patients revealed 100% PPV of MRI in detecting surgical tears[88]. Lower MRI accuracy has been reported in severe GH arthritis. Sershon et al[89] in 100 patients reported a 100% sensitivity, 68% specificity and 6% PPV. The MRI thus has an edge over USG in detecting smaller tears and possibly better evaluation of PTT.
Literature is flooded with studies comparing the two techniques. Teefey et al[90] in 124 consecutive painful shoulders reported similar accuracies of USG and MRI (87%). Likewise, Lenza et al[81] evaluating 20 studies with 1147 shoulders failed to illustrate any significant difference. Roy et al[84], in their meta-analysis, observed sensitivity and specificity of over 90% for FTT with USG and MRI. While specificity for PTT with both modalities was over 90%, lower sensitivities (67%-83%) were seen for PTT. Dinnes et al[91] in a systematic review found USG more cost-effective and accurate at picking up PTT than MRI. Over a 4-year period, Rutten et al[92] evaluated 5216 patients with shoulder symptoms and reported comparable accuracies of 95% and 100% for USG and MRI in diagnosing FTT and 89% and 67% in diagnosing PTT respectively. A smaller study on 21 patients demonstrated similar sensitivity, specificity, PPV, NPV and accuracy of USG and MRI[93].
Other, less frequently utilized and indication-specific modalities of imaging include plain radiographs with arthrography, computed tomography scans and MRI arthrography[80].
The decision regarding which to perform can be made on parameters like importance of obtaining data on lesions of the glenoid labrum, joint capsule or surrounding structures, the presence of an implanted device, patient tolerance and cost[78]. The ultimate decision needs to be based on these facts as well as availability, affordability and high-quality training of personnel performing the USG.
Management options RC tears includes nonsurgical measures, partial repair and/or debridement, open or arthroscopic repair, reconstruction and arthroplasty[94]. While the benefits of nonoperative measures include: avoiding surgery and its potential complications, less obvious risks include persisting and recurring symptoms, lack of healing, tear extension, fatty infiltration, muscle atrophy, tendon retraction and arthritis[5]. In a follow-up of 19 massive, nonoperatively managed RC tears, Zingg et al[95] observed satisfactory shoulder function for at least four years despite significant progression of degenerative structural joint changes. This risk of a reparable tear progressing to an irreparable tear needs to be averted by prompt recognition and early surgical intervention. Various modalities include analgesics, anti-inflammatory medications, physical therapy, activity modification and subacromial injections of local anaesthetics and/or steroids[96].
PTT are managed nonoperatively by tailored rehabilitation, ROM optimization and RC and scapular rotator strengthening. Anterior capsular tightness is addressed by ER stretching with the shoulder adducted while posterior capsular contractures require stretching of the abducted and internally rotated arm. With subsequent improvements, a strengthening programme is initiated of the shoulder girdle, core abdominal and thoracic muscles[97]. In a retrospective study on conservatively managed 26 PTT, Maman et al[50] found 2 increased and one decreased tears sizes. Contrasting reports however, exist. Hamada et al[98] in 1997 utilized in-situ hybridization to localize cells containing α1 type-I procollagen mRNA in 13 PTT to determine RC healing. Maintained numbers of labelled cells at the tear and margins of concomitant intratendinous extensions led the authors to conclude that PTT and these extensions can continue to rupture after initial injury. Further, Yamanaka et al[99] in 40 AST observed tear reduction and disappearance in 4 cases each while tear enlargement and progression to FTT was seen in 21 and 11 patients respectively at 2 years.
Existing evidence on nonsurgical management of FTT is also conflicting. In a series by Fucentese et al[49], 24 symptomatic, isolated FTT of SSP were offered operative treatment only to be declined by the patients. Re-examination and MRI (at median 42 mo after diagnosis) reported high patient satisfaction and no increase in the average tear size. While 9 tears each remained constant and decreased, 2 were no longer detected at follow-up. In another 20 conservatively managed FTT, Baydar et al[100] observed statistically significant improvements in ROM, pain and function scores at 6 mo follow-up. In a study by the Moon shoulder group on 452 patients with atraumatic FTT, significantly improved patient reported outcomes were observed at 6 and 12 wk after diagnosis and patients opted for surgical treatment in only 25% of instances[101]. In a long-term follow-up of 43 RC tears over 13 years, Kijima et al[102] observed nil or only slight pain in 90% of the patients while 70% patients had no disturbance in the ADLs. Younger patients had more significant symptoms.
Poor results with conservative treatment for FTT have been show by Maman et al[50]. A 52% progression in 33 FTT over a mean 20 mo demonstrated SSP atrophy in 24% patients. Safran et al[51], in 61 nonoperatively treated symptomatic RC tears at mean 29 mo reported increased sizes in about 50% patients.
The effects of physical therapy were prospectively analysed in a multi-center study on 389 patients with symptomatic RC tears. Higher scores were seen in females (P = 0.001), higher education (P < 0.001), active abduction (P = 0.021) and strength in forward elevation (P = 0.002) and abduction (P = 0.007). Lower scores were seen in males (P = 0.001), supra and ISP atrophy (P = 0.04, 0.003) and scapulothoracic dyskinesis (P < 0.001). The authors concluded that factors associated with pain and loss of function including scapulothoracic dyskinesis, active abduction, and strength in forward elevation and abduction should be addressed nonoperatively with therapy[103]. The Appropriate Use Criteria (AUC) developed by the American Academy of Orthopaedic Surgeons recommend nonoperative treatment is always appropriate in patients have a response to conservative care[104].
Nonoperative management should be individualised considering patient expectations, symptoms and response to previous nonoperative treatment. Our experience suggests earlier surgery in patients below 60 years or healthy symptomatic 60-70 year olds having increased demand for activity. Patients older than 70 years with low functional demands can be managed nonoperatively.
Surgical repair of the RC is a cost-effective solution for all populations and reduces the societal burden of the disease[105]. Surgical techniques have evolved from open to arthroscopic procedures[106]. Arthroscopic repairs have witnessed a dramatic 600% increase in the United States over the last decade[107]. The ideal repair of the RC tear must have the potential to withstand physiological loads while allowing simultaneous healing to occur[39]. The repair configuration opted for must be most reproducible and provide the best possible outcome and healing[94]. Predictable success, pain relief and patient satisfaction has been met with after repair of RC tears[108]. In a recent study on 103 shoulder surgeons, Robinson et al[109] observed overall higher overall popularity of arthroscopic repairs while open and mini-open procedures were preferred by the long-timers.
Several biomechanical factors to consider while repairing RC tears include the suture material properties which should be sufficiently strong, stiff, requiring a simple and reliable operative technique and configuration. Bioabsorbable anchors over their metallic counterparts offer no lasting foreign object, graduated loss of strength, minimum imaging artefacts and suture-abrading eyelets. Potential drawbacks include unpredictable loss of strength, eyelet rupture and foreign body reaction. Anchor insertion at standard depths (manufacturer-dependent), 0°-45° angulation with double loaded suture in the anterior and middle regions of the greater tuberosity with screw-type metal anchors for osteoporotic bones impart a good fixation[39].
PTT can be operated after a fair trial with conservative therapy. Repairs in non-overhead and overhead athletes are considered for tears more than 30%-50% and 75% respectively. Higher positional forces in throwers threaten repair integrity and preservation of motion. Options available include arthroscopic cuff debridement and open/arthroscopic repair with or without subacromial decompression[97]. Excellent postoperative outcomes have been reported, in data from 16 studies, ranging from 28.7% to 93%[110]. Thirty-nine PTT managed by acromioplasty, debridement and suturing, at a mean follow-up of 55 mo revealed satisfactory to excellent results in 33 (85%) patients. Four of six unsatisfactory results had unsuccessful previous surgery[111]. Evidence to suggest good to excellent results in more than 80% patients treated by debridement exists in literature[112,113]. Cordasco et al[114] in 162 patients with either normal, grade 1 (frayed) or grade 2 (< 50%) PTT in an average 4.5 years postoperatively reported significantly higher failure rates in grade 2B BST while the outcomes of in grades 1 and 2 and normal tears were not significantly different. The authors believed grade 2B BST may have been better served with primary repair.
Satisfactory results with arthroscopic BST repairs have been obtained. Koh et al[115] retrospectively evaluated 38 patients with a mean age of 50.8 years undergoing full-layer repair for more than 50% PTT with preservation of articular fibres. At a mean 26.9 mo of follow-up of 33 shoulders, 29 (87.9%) had intact repaired tendons with significant improvements in the patient scores. Investigators have also evaluated various techniques of AST repairs. In a prospective study by Franceschi et al[116], 2 arthroscopic modalities were compared. While 32 AST were repaired with TTT, 28 were converted to FTT and repaired. The authors reported significant improvements in clinical and functional outcomes and healing. Both methods were safe, comparable and effective. The addition of a biceps tendon tenotomy and augmentation to the TTT for AST has shown significant improvements in pain, function and ROM with no re-tears in preliminary results on 39 consecutive patients[117]. The direct visualization of the retracted AST layer to its insertion on the GT followed by DR fixation has been also shown to provide a stable shoulder with good ROM[118]. A reduction in GH contact pressures has been identified with TTT for AST, in a study on 9 cadavers leading to diminished GH and SA impingement. Laxity however, can unwantedly result[119]. Reports also exist of FTT medial side tearing with TTT of AST in 21.2 mo follow-up of 7 out of 8 patients despite improved clinical outcomes[120].
Excellent healing after arthroscopic conversion of PTT to FTT has been reported by Kamath et al[121] in repairs of 42 PTT (after conversion to FTT). Thirty-seven (88%) shoulders had intact repairs 11 mo postoperatively which were significantly younger (average age 51.8, P = 0.02) than those with persistent defects (62.6). Overall patient satisfaction rate was 93%. No significant differences have been reported between AST and BST following conversion to FTT. A recent paper by Kim et al[122] comparing 20 AST with 23 BST conversions to FTT and repairs reported comparable improvements in both groups at mean 35.53 mo follow-up with higher re-tear rates in BST (9.5% vs 0%).
Outcomes of repaired PTT and FTT have had comparisons. Peters et al[123] compared 105 arthroscopically repaired small-medium FTT with 64 PTT demonstrating excellent results in both groups. Postoperative stiffness and re-tear rates were similar. Chung et al[124] compared repairs of 34 high-grade PTT with 21 small FTT and observed healing failure in 12 PTT related to severity of tendinosis (7.64 times higher in high-grade tendinosis). Despite good to excellent results in a majority of PTT repairs, patients operated previously with poor tendon quality and advanced age can disappoint.
The AUC recommendations suggest repair of full-thickness RC tears: maybe appropriate for reparable tears (even in patients responding conservatively), is appropriate for healthy symptomatic patients failing conservative treatment, maybe appropriate in chronic massive tears while arthroplasty maybe appropriate for healthy pseudoparalytic irreparable tears[104]. Early surgical intervention is needed in the setting of weakness and substantial functional disability[108]. Factors contributing to optimal repair include intact tendon-footprint motion and contact area, tendon and bone quality. Hypoxia, decreased vascularity, fibrocartilaginous changes and extrinsic compression along with MA and FI reduce the healing response[125].
Comparable long-term results have been demonstrated for open and all-arthroscopic modalities. Lindley et al[126] in a systematic review of 10 studies comparing postoperative outcomes in mini-open repair and all-arthroscopic repair techniques found no significant differences in patient demographics, RC pathology, rehabilitation protocols, ASES scores and recurrent defects. Short-term pain reduction was seen in the all-arthroscopic repairs. A recent meta-analysis on 12 studies with 770 patients failed to identify any significant differences in functional outcomes, pain scores, re-tear rates or presence of adhesive capsulitis between the 2 groups[127]. Parallel results have been demonstrated by other authors[128,129]. A randomized study on 125 patients receiving either mini-open or arthroscopic procedures for RC tears revealed similar results in both groups. Functional outcomes and re-tears were higher in FTT managed arthroscopically[130]. A lateral approach for mini-open RC repair permits improved visualization and function as described by Cho et al[131].
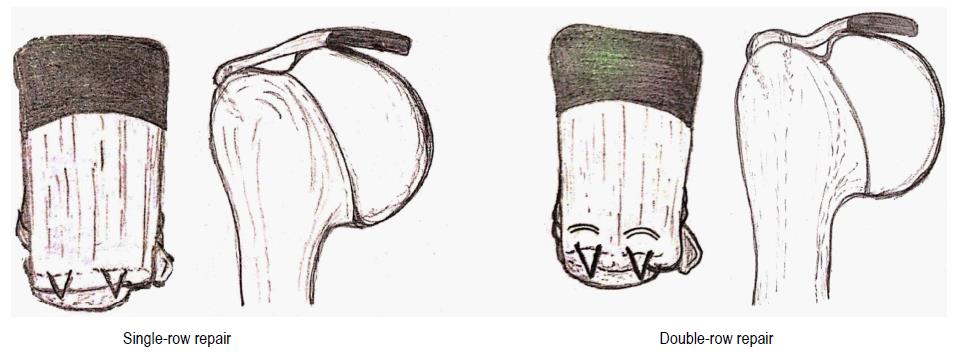
Popular among arthroscopic techniques are the single-row repair (SRR) and the double-row repairs (DRR) (Figure 3).
Varying degrees of clinical outcomes, healing and repair integrity and cost-effectiveness have been reported of DRR over SRR. A randomized controlled trial by Carbonel et al[132] on 160 patients revealed significantly greater improvements with DRR. Higher ASES scores, IR and ER along with lower re-tears following DRR have been reported in 2 recent meta-analyses[133,134]. A systematic review by Duquin et al[135] demonstrated significantly lower re-tear rates with DRR when compared to SRR for tears more than 10 mm in size. Another recent meta-analysis by Millett et al[136] comparing SRR with DRR revealed significantly higher re-tear rates in SRR especially in PT re-tears.
The repair of large, massive tears with DRR have shown better, though not statistically significant, functional outcome than with SRR in a systematic review by Saridakis and Jones[137]. This improvement in larger tears has been confirmed in a meta-analysis by Chen et al[138]. Other investigators have found equally improved outcomes after SRR and DRR[139-141]. Superiority of DRR in terms of healing rates has more clearly documented. Significantly higher healing has been observed in DRR surgeries[134,142]. This has also been confirmed in biomechanical studies. A systematic review by Wall et al[143] found DRR stronger than SRR with lower rates of failure and gap formation. A cadaveric study by Ahmad et al[144] demonstrated lesser extravasation of fluid from DRR suggesting an enhanced healing potential. Pauly et al[145] have also, in a systematic review demonstrated biomechanical and radiographic superiority with DRR in terms of structural integrity and reduced re-tear rates.
Few studies have criticized the DRR and proclaimed superiority for the simpler time-saving SRR method. Aydin et al[146], documented no significant differences in clinical outcomes and found DRR to be demanding, expensive and time consuming. Longer operating times with DRR have been supported by other studies[134,147]. The cost-effectiveness of DRR has been questioned by Genuario et al[148]. However, costs variations and probability of re-tear with SRR can have profound effects and prove DRR to be more cost effective in the first place. Higher re-tears with DRR have been reported. Kim et al[149] in a comparative analysis in 78 larger than medium-sized RC tears observed higher re-tear, lower UCLA and ASES scores in remnant tendons lengths less than 10 mm. Park et al[150] observed improved ultimate failure loads, better footprint restoration and stronger repairs with transosseous-equivalent (TOE) RC repair technique when compared to DRR. Gap formation was similar for both techniques.
Double-loaded suture anchors and margin-convergence sutures have been shown by Miškulin et al[151] to provide excellent results with significant improvements in functional parameters. The use of triple-loaded anchors in SRR has been shown to yield comparable and have shown to increase footprint coverage in cadaveric shoulders and potentially reduce costs[152]. The results of knotless self-reinforcing DRR have shown high patient satisfaction and serve as an alternative option in managing RC tears[153].
Based on the above discussion of the existing evidence, the suture technique should be chosen based on tissue properties, tear pattern and dimension and surgeon experience and comfort. PTT (BST or AST), do well with surgery. In FTT, mini-open and arthroscopic repairs provide comparable outcomes with better short-term pain control and more re-tears in arthroscopic methods. While the best biomechanical characteristics are possessed by the TOE repair, DRR may prove beneficial in tears more than 10 mm by providing stronger repairs closely replicating the native cuff at the cost of increased expenditure and failure at the MTJ which is harder to repair. Smaller (< 10 mm) tears can be repaired successfully by SRR the biomechanical superiority of which can be increased by increasing the suture limbs[104].
Physical rehabilitation is a vital component of postoperative patient care involving strict adherence and compliance. Poor patient cooperation, observed most frequently between 6-12 wk postoperatively, can lead to re-tears and failure[154]. Despite several reviews of rehabilitative programmes, high-level evidence is presently lacking[155]. Primary goals include restoring function while maintaining repair integrity. Gradual rehabilitation has been advocated in 4 phases beginning with 4 to 6 wk of immobilization, followed by protected passive ROM, followed by a gradual progression to active ROM and appropriate resistance exercise program[156]. Millett et al[157] have advocated protecting the repair initially and gradually progressing from early passive ROM through return to preoperative levels of activity.
Aggressive and early rehabilitation with continuous passive motion has been associated with improved early ROM, pain relief and outcomes[158]. In a prospective randomized controlled trial of 88 patients who underwent SRR, postoperative immobilization for 4 and 8 wk were compared. No significant differences were observed in terms of re-tears, ROM or clinical scores. Increased stiffness was however, higher in the 8 wk group (P = 0.038)[159]. Keener et al[160], in a study on 124 patients comparing early with delayed motion at 6 wk after DRR reported no differences in function, ROM and strength between the 2 groups. Active elevation and ER were better in early mobilization group at 3 mo. Gallagher et al[161] observed improved function in the first 3-6 mo with early ROM. Increased re-tears in medium to large tears, though statistically insignificant, occurred with early ROM. Identical outcomes were seen at 1 year. Lee et al[162] compared early and limited early passive rehabilitation in 64 patients after arthroscopic RC repair. Significant and faster improvements were seen in the early rehabilitation group in forward flexion, ER and IR at 90° of abduction and abduction 3 mo postoperatively. No significant differences were seen at 1-year follow-up. Postoperative MRI scans showed higher re-tears in the early vs limited early rehabilitated group (23.3% against 8.8%). The authors advocated gentler rehabilitation after arthroscopic RC repair for improved for tendon healing.
Less aggressive delayed immobilization has been associated with stiffness. Parsons et al[163] evaluating 43 arthroscopically repaired FTT in full-time sling immobilization without formal therapy for 6 wk found 10 patients (23%) developing initial stiffness. No difference in mean forward elevation, ER or IR and functional scores with the remaining patients at 1 year postoperatively. Lower re-tears were seen among the stiff patients. Newer modalities including combined aquatic and land-based postoperative therapy have shown encouraging results with significant improvements in both ROM and outcome scores (P < 0.001)[164].
An individualized rehabilitation approach is warranted in order to achieve a strong and mobile with maximum function and preserved repair integrity.
Healing, as described by Mall et al[52], is formation of a continuous layer of tissue from the RC muscle belly to its insertion on the greater tuberosity. The evidence of spontaneous RC healing, without surgical repair, has been shown to be inadequate, inferior and limited in animal models.
Pathological changes described earlier along with delay in repair adversely influence postoperative outcomes as shown in animal studies[54,165,166]. Studies have however, reported excellent outcomes despite significant rates of recurrence, re-tearing and poor cuff healing. Deniz et al[167] reported excellent results in 66 out of 87 arthroscopically repaired shoulders despite re-ruptures in 26 patients. Neither the MA nor FI, over 30 mo, demonstrated any MRI documented improvements. While the MA pattern in re-tears was identical in the intact and the re-torn tendons, FI was significantly greater in the latter. Though complete healing demonstrates a greater improvement on follow-up, patient age, sizes of initial and re-tears are other factors correlating with final results[168-171]. Postoperative USG is an asset in evaluating repair integrity with 85% concordance with MRI readings and can be employed by sufficiently trained and experienced surgeons[172].
In recent literature, patient related factors affecting RC healing have been identified as demographic variables, comorbidity-status and tear-related factors. Demographic variables include: advancing patient age, longer duration of symptoms and longer follow-up[52,173-175]. Comorbidities including: Diabetes, hypercholesterolemia, smoking-status and nonsteroidal anti-inflammatory drug (NSAID) use have been shown to affect and delay bone-tendon healing[52,173,174,176]. Smoking has been associated with worsened histopathology and degenerative changes and increased apoptosis[175]. A reduced bone mineral density in a cohort study by Chung et al[173] was identified as an independent factor affecting postoperative RC healing. Tear-related factors with poor healing have included: Larger tears of longer duration with multi-tendon involvement. Small to medium-sized tears show higher healing rates (87%) as against large to massive-sized tears (62%)[177]. Tashjian et al[175] followed-up 49 shoulders at mean 16 mo postoperatively after DR-repairs identified lower healing rates (36%) in multi-tendon tears against single-tendon tears (67%). Retraction of the muscle-tendon units, tendon shortening, FI and MA along with presence of matrix metalloproteinases -1, 9 and tumor necrosis factor-alpha have also yielded poor results[52,128,173-175,177]. Medialization of the muscle-tendon junction (MTJ) is associated with significantly poorer healing rates (55%) when compared to MTJs lateral to the face of the glenoid (93%)[177]. The anterior sub-region of tendon has been associated with significantly higher gap formation[178]. Large tears with additional biceps or AC procedures have a negative impact on cuff integrity[179].
Various surgeon-associated factors influencing healing have included the choice and timing of technique, greater tuberosity preparation, acromioplasty, structural augmentation, platelet-rich plasma (PRP) and rehabilitation protocol[52]. Open vs arthroscopic methods have been systematically reviewed with no significant differences in the rates on healing[126,180]. While DRR has been shown to be stronger with improved healing rates as compared to single-row techniques, the functional outcomes have been equivalent in all except large and massive tears where DRR has provided a functional advantage[173]. Knotted vs knotless techniques have shown comparable functional outcomes and repair integrity. Mall et al[181], however, have reported greater hysteresis, reduced gap formation and higher ultimate load in medially knotted shoulders. Repair of chronically torn tears can result in injury at the time of repair. Davis et al[182] observed injury to almost 70% rat muscle fibres at the time of repair. Concomitant acromioplasty and the use of PRP have failed to demonstrate improvements in structural healing[52]. While early and delayed mobilizations continue to be debated, slower rehabilitation programmes can be safely adopted in light of improved healing and equivocal outcomes[52,173].
Compromised RC healing ability has questionable association with outcomes. Tear and patient age, comorbidities, NSAIDS, smoking-status, osteoporosis and tendon shortening and retraction adversely affect outcomes. Surgical and rehabilitation techniques have varying degrees of impact on the final result.
With increasing likelihood of occurrence RC tears with advancing age and longevity, the future poses a unique challenge to the orthopaedic surgeon. The morbidity of activity restriction added to severe pain makes matters worse for the debilitated patient. In such circumstances, it becomes essential for us to give due regard and promptly recognise cuff tears and timely intervene by providing the most appropriate treatment. Nonoperative treatment should be offered and continued in those with a good initial response and improvement of symptoms. Surgical intervention however, must not be postponed endlessly, especially in the younger and active population with worsening symptoms and progressing tears. With inherent advantages and promising outcomes, arthroscopic repair seems a propitious approach. The addition of a rehabilitation plan to the above provides the requisite nourishment and environment essential for the repaired and recovering tendons. An evidence-based and systematic modus operandi can help the surgeon in comprehensively managing RC tears with high degrees of success in the present scenario. Future directions point towards application of principles of tissue engineering to achieve enhanced repairs and functional outcomes.
The authors would like to acknowledge the efforts of Dr. Surya Khanna for her contribution in preparing the figures.
| 1. | Craik JD, Mallina R, Ramasamy V, Little NJ. Human evolution and tears of the rotator cuff. Int Orthop. 2014;38:547-552. [PubMed] |
| 2. | Tashjian RZ, Saltzman EG, Granger EK, Hung M. Incidence of familial tendon dysfunction in patients with full-thickness rotator cuff tears. Open Access J Sports Med. 2014;5:137-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Park SE, Panchal K, Jeong JJ, Kim YY, Kim JH, Lee JY, Ji JH. Intratendinous rotator cuff tears: prevalence and clinical and radiological outcomes of arthroscopically confirmed intratendinous tears at midterm follow-up. Am J Sports Med. 2015;43:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006;88:1699-1704. [PubMed] |
| 5. | Kim HM, Teefey SA, Zelig A, Galatz LM, Keener JD, Yamaguchi K. Shoulder strength in asymptomatic individuals with intact compared with torn rotator cuffs. J Bone Joint Surg Am. 2009;91:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 6. | Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, Kobayashi T. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 962] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 7. | Teunis T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J Shoulder Elbow Surg. 2014;23:1913-1921. [PubMed] |
| 8. | Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8:296-299. [PubMed] |
| 9. | Fehringer EV, Sun J, VanOeveren LS, Keller BK, Matsen FA. Full-thickness rotator cuff tear prevalence and correlation with function and co-morbidities in patients sixty-five years and older. J Shoulder Elbow Surg. 2008;17:881-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Schibany N, Zehetgruber H, Kainberger F, Wurnig C, Ba-Ssalamah A, Herneth AM, Lang T, Gruber D, Breitenseher MJ. Rotator cuff tears in asymptomatic individuals: a clinical and ultrasonographic screening study. Eur J Radiol. 2004;51:263-268. [PubMed] |
| 11. | Moosmayer S, Smith HJ, Tariq R, Larmo A. Prevalence and characteristics of asymptomatic tears of the rotator cuff: an ultrasonographic and clinical study. J Bone Joint Surg Br. 2009;91:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Neer CS. Impingement lesions. Clin Orthop Relat Res. 1983;70-77. [PubMed] |
| 13. | Lehman C, Cuomo F, Kummer FJ, Zuckerman JD. The incidence of full thickness rotator cuff tears in a large cadaveric population. Bull Hosp Jt Dis. 1995;54:30-31. [PubMed] |
| 14. | Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88:116-121. [PubMed] |
| 15. | Gumina S, Carbone S, Campagna V, Candela V, Sacchetti FM, Giannicola G. The impact of aging on rotator cuff tear size. Musculoskelet Surg. 2013;97 Suppl 1:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 16. | Abate M, Schiavone C, Di Carlo L, Salini V. Prevalence of and risk factors for asymptomatic rotator cuff tears in postmenopausal women. Menopause. 2014;21:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 17. | Pauly S, Stahnke K, Klatte-Schulz F, Wildemann B, Scheibel M, Greiner S. Do patient age and sex influence tendon cell biology and clinical/radiographic outcomes after rotator cuff repair? Am J Sports Med. 2015;43:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Milgrom C, Schaffler M, Gilbert S, van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br. 1995;77:296-298. [PubMed] |
| 19. | Brasseur JL, Lucidarme O, Tardieu M, Tordeur M, Montalvan B, Parier J, Le Goux P, Gires A, Grenier P. Ultrasonographic rotator-cuff changes in veteran tennis players: the effect of hand dominance and comparison with clinical findings. Eur Radiol. 2004;14:857-864. [PubMed] |
| 20. | Liem D, Buschmann VE, Schmidt C, Gosheger G, Vogler T, Schulte TL, Balke M. The prevalence of rotator cuff tears: is the contralateral shoulder at risk? Am J Sports Med. 2014;42:826-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Ro KH, Park JH, Lee SH, Song DI, Jeong HJ, Jeong WK. Status of the contralateral rotator cuff in patients undergoing rotator cuff repair. Am J Sports Med. 2015;43:1091-1098. [PubMed] |
| 22. | Baumgarten KM, Gerlach D, Galatz LM, Teefey SA, Middleton WD, Ditsios K, Yamaguchi K. Cigarette smoking increases the risk for rotator cuff tears. Clin Orthop Relat Res. 2010;468:1534-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Bishop JY, Santiago-Torres JE, Rimmke N, Flanigan DC. Smoking Predisposes to Rotator Cuff Pathology and Shoulder Dysfunction: A Systematic Review. Arthroscopy. 2015;31:1598-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 24. | Carbone S, Gumina S, Arceri V, Campagna V, Fagnani C, Postacchini F. The impact of preoperative smoking habit on rotator cuff tear: cigarette smoking influences rotator cuff tear sizes. J Shoulder Elbow Surg. 2012;21:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Tashjian RZ, Farnham JM, Albright FS, Teerlink CC, Cannon-Albright LA. Evidence for an inherited predisposition contributing to the risk for rotator cuff disease. J Bone Joint Surg Am. 2009;91:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Yamamoto A, Takagishi K, Kobayashi T, Shitara H, Ichinose T, Takasawa E, Shimoyama D, Osawa T. The impact of faulty posture on rotator cuff tears with and without symptoms. J Shoulder Elbow Surg. 2015;24:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Gumina S, Di Giorgio G, Postacchini F, Postacchini R. Subacromial space in adult patients with thoracic hyperkyphosis and in healthy volunteers. Chir Organi Mov. 2008;91:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Ellman H. Arthroscopic subacromial decompression: analysis of one- to three-year results. Arthroscopy. 1987;3:173-181. [PubMed] |
| 29. | Lohr JF, Uhthoff HK. The microvascular pattern of the supraspinatus tendon. Clin Orthop Relat Res. 1990;35-38. [PubMed] |
| 30. | Ozaki J, Fujimoto S, Nakagawa Y, Masuhara K, Tamai S. Tears of the rotator cuff of the shoulder associated with pathological changes in the acromion. A study in cadavera. J Bone Joint Surg Am. 1988;70:1224-1230. [PubMed] |
| 31. | Neer CS II, Poppen NK. Supraspinatus outlet. Orthop Trans. 1987;11:234. |
| 32. | Bigliani LU, Ticker JB, Flatow EL, Soslowsky LJ, Mow VC. The relationship of acromial architecture to rotator cuff disease. Clin Sports Med. 1991;10:823-838. [PubMed] |
| 33. | DeFranco MJ, Cole BJ. Current perspectives on rotator cuff anatomy. Arthroscopy. 2009;25:305-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Wang JC, Horner G, Brown ED, Shapiro MS. The relationship between acromial morphology and conservative treatment of patients with impingement syndrome. Orthopedics. 2000;23:557-559. [PubMed] |
| 35. | Neer CS. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54:41-50. [PubMed] |
| 36. | Moor BK, Wieser K, Slankamenac K, Gerber C, Bouaicha S. Relationship of individual scapular anatomy and degenerative rotator cuff tears. J Shoulder Elbow Surg. 2014;23:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 37. | Fujisawa Y, Mihata T, Murase T, Sugamoto K, Neo M. Three-dimensional analysis of acromial morphologic characteristics in patients with and without rotator cuff tears using a reconstructed computed tomography model. Am J Sports Med. 2014;42:2621-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Uhthoff HK, Sarkar K. Surgical repair of rotator cuff ruptures. The importance of the subacromial bursa. J Bone Joint Surg Br. 1991;73:399-401. [PubMed] |
| 39. | Yadav H, Nho S, Romeo A, MacGillivray JD. Rotator cuff tears: pathology and repair. Knee Surg Sports Traumatol Arthrosc. 2009;17:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Rudzki JR, Adler RS, Warren RF, Kadrmas WR, Verma N, Pearle AD, Lyman S, Fealy S. Contrast-enhanced ultrasound characterization of the vascularity of the rotator cuff tendon: age- and activity-related changes in the intact asymptomatic rotator cuff. J Shoulder Elbow Surg. 2008;17:96S-100S. [PubMed] |
| 41. | Levy O, Relwani J, Zaman T, Even T, Venkateswaran B, Copeland S. Measurement of blood flow in the rotator cuff using laser Doppler flowmetry. J Bone Joint Surg Br. 2008;90:893-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Walch G, Liotard JP, Boileau P, Noël E. [Postero-superior glenoid impingement. Another impingement of the shoulder]. J Radiol. 1993;74:47-50. [PubMed] |
| 43. | Choo A, McCarthy M, Pichika R, Sato EJ, Lieber RL, Schenk S, Lane JG, Ward SR. Muscle gene expression patterns in human rotator cuff pathology. J Bone Joint Surg Am. 2014;96:1558-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95:1249-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 45. | Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001;10:199-203. [PubMed] |
| 46. | Mall NA, Kim HM, Keener JD, Steger-May K, Teefey SA, Middleton WD, Stobbs G, Yamaguchi K. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 47. | Hawkins RH, Dunlop R. Nonoperative treatment of rotator cuff tears. Clin Orthop Relat Res. 1995;178-188. [PubMed] |
| 48. | Goldberg BA, Nowinski RJ, Matsen FA. Outcome of nonoperative management of full-thickness rotator cuff tears. Clin Orthop Relat Res. 2001;99-107. [PubMed] |
| 49. | Fucentese SF, von Roll AL, Pfirrmann CW, Gerber C, Jost B. Evolution of nonoperatively treated symptomatic isolated full-thickness supraspinatus tears. J Bone Joint Surg Am. 2012;94:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Maman E, Harris C, White L, Tomlinson G, Shashank M, Boynton E. Outcome of nonoperative treatment of symptomatic rotator cuff tears monitored by magnetic resonance imaging. J Bone Joint Surg Am. 2009;91:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 51. | Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39:710-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 52. | Mall NA, Tanaka MJ, Choi LS, Paletta GA. Factors affecting rotator cuff healing. J Bone Joint Surg Am. 2014;96:778-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 53. | Gerber C, Meyer DC, Frey E, von Rechenberg B, Hoppeler H, Frigg R, Jost B, Zumstein MA. Neer Award 2007: Reversion of structural muscle changes caused by chronic rotator cuff tears using continuous musculotendinous traction. An experimental study in sheep. J Shoulder Elbow Surg. 2009;18:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 741] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 55. | Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22:1004-1007. [PubMed] |
| 56. | Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A:1973-1982. [PubMed] |
| 57. | Melis B, Nemoz C, Walch G. Muscle fatty infiltration in rotator cuff tears: descriptive analysis of 1688 cases. Orthop Traumatol Surg Res. 2009;95:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 58. | Kim HM, Dahiya N, Teefey SA, Keener JD, Galatz LM, Yamaguchi K. Relationship of tear size and location to fatty degeneration of the rotator cuff. J Bone Joint Surg Am. 2010;92:829-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 59. | Kuzel BR, Grindel S, Papandrea R, Ziegler D. Fatty infiltration and rotator cuff atrophy. J Am Acad Orthop Surg. 2013;21:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 60. | Swan MA, Sato E, Galatz LM, Thomopoulos S, Ward SR. The effect of age on rat rotator cuff muscle architecture. J Shoulder Elbow Surg. 2014;23:1786-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Gumucio JP, Davis ME, Bradley JR, Stafford PL, Schiffman CJ, Lynch EB, Claflin DR, Bedi A, Mendias CL. Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res. 2012;30:1963-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 62. | Barton ER, Gimbel JA, Williams GR, Soslowsky LJ. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res. 2005;23:259-265. [PubMed] |
| 63. | Ditsios K, Boutsiadis A, Kapoukranidou D, Chatzisotiriou A, Kalpidis I, Albani M, Christodoulou A. Chronic massive rotator cuff tear in rats: in vivo evaluation of muscle force and three-dimensional histologic analysis. J Shoulder Elbow Surg. 2014;23:1822-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Mendias CL, Roche SM, Harning JA, Davis ME, Lynch EB, Sibilsky Enselman ER, Jacobson JA, Claflin DR, Calve S, Bedi A. Reduced muscle fiber force production and disrupted myofibril architecture in patients with chronic rotator cuff tears. J Shoulder Elbow Surg. 2015;24:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 65. | Kikukawa K, Ide J, Kikuchi K, Morita M, Mizuta H, Ogata H. Hypertrophic changes of the teres minor muscle in rotator cuff tears: quantitative evaluation by magnetic resonance imaging. J Shoulder Elbow Surg. 2014;23:1800-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Beeler S, Ek ET, Gerber C. A comparative analysis of fatty infiltration and muscle atrophy in patients with chronic rotator cuff tears and suprascapular neuropathy. J Shoulder Elbow Surg. 2013;22:1537-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 67. | Keener JD, Wei AS, Kim HM, Steger-May K, Yamaguchi K. Proximal humeral migration in shoulders with symptomatic and asymptomatic rotator cuff tears. J Bone Joint Surg Am. 2009;91:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 68. | Feeney MS, O’dowd J, Kay EW, Colville J. Glenohumeral articular cartilage changes in rotator cuff disease. J Shoulder Elbow Surg. 2003;12:20-23. [PubMed] |
| 69. | Neer CS, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232-1244. [PubMed] |
| 70. | Gumina S, Candela V, Passaretti D, Venditto T, Carbone S, Arceri V, Giannicola G. Intensity and distribution of shoulder pain in patients with different sized postero-superior rotator cuff tears. J Shoulder Elbow Surg. 2014;23:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | van Kampen DA, van den Berg T, van der Woude HJ, Castelein RM, Scholtes VA, Terwee CB, Willems WJ. The diagnostic value of the combination of patient characteristics, history, and clinical shoulder tests for the diagnosis of rotator cuff tear. J Orthop Surg Res. 2014;9:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | Beaudreuil J, Nizard R, Thomas T, Peyre M, Liotard JP, Boileau P, Marc T, Dromard C, Steyer E, Bardin T. Contribution of clinical tests to the diagnosis of rotator cuff disease: a systematic literature review. Joint Bone Spine. 2009;76:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 73. | Bak K, Sørensen AK, Jørgensen U, Nygaard M, Krarup AL, Thune C, Sloth C, Pedersen ST. The value of clinical tests in acute full-thickness tears of the supraspinatus tendon: does a subacromial lidocaine injection help in the clinical diagnosis? A prospective study. Arthroscopy. 2010;26:734-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Yoon JP, Chung SW, Kim SH, Oh JH. Diagnostic value of four clinical tests for the evaluation of subscapularis integrity. J Shoulder Elbow Surg. 2013;22:1186-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Somerville LE, Willits K, Johnson AM, Litchfield R, LeBel ME, Moro J, Bryant D. Clinical Assessment of Physical Examination Maneuvers for Rotator Cuff Lesions. Am J Sports Med. 2014;42:1911-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 76. | Smith TO, Back T, Toms AP, Hing CB. Diagnostic accuracy of ultrasound for rotator cuff tears in adults: a systematic review and meta-analysis. Clin Radiol. 2011;66:1036-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 77. | Al-Shawi A, Badge R, Bunker T. The detection of full thickness rotator cuff tears using ultrasound. J Bone Joint Surg Br. 2008;90:889-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Iannotti JP, Ciccone J, Buss DD, Visotsky JL, Mascha E, Cotman K, Rawool NM. Accuracy of office-based ultrasonography of the shoulder for the diagnosis of rotator cuff tears. J Bone Joint Surg Am. 2005;87:1305-1311. [PubMed] |
| 79. | Wall LB, Teefey SA, Middleton WD, Dahiya N, Steger-May K, Kim HM, Wessell D, Yamaguchi K. Diagnostic performance and reliability of ultrasonography for fatty degeneration of the rotator cuff muscles. J Bone Joint Surg Am. 2012;94:e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 80. | Read JW, Perko M. Shoulder ultrasound: diagnostic accuracy for impingement syndrome, rotator cuff tear, and biceps tendon pathology. J Shoulder Elbow Surg. 1998;7:264-271. [PubMed] |
| 81. | Lenza M, Buchbinder R, Takwoingi Y, Johnston RV, Hanchard NC, Faloppa F. Magnetic resonance imaging, magnetic resonance arthrography and ultrasonography for assessing rotator cuff tears in people with shoulder pain for whom surgery is being considered. Cochrane Database Syst Rev. 2013;9:CD009020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 82. | Hayter CL, Miller TT, Nguyen JT, Adler RS. Comparative analysis of 2- versus 3-dimensional sonography of the supraspinatus tendon. J Ultrasound Med. 2012;31:449-453. [PubMed] |
| 83. | Ok JH, Kim YS, Kim JM, Yoo TW. Learning curve of office-based ultrasonography for rotator cuff tendons tears. Knee Surg Sports Traumatol Arthrosc. 2013;21:1593-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Roy JS, Braën C, Leblond J, Desmeules F, Dionne CE, MacDermid JC, Bureau NJ, Frémont P. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterisation of rotator cuff disorders: a systematic review and meta-analysis. Br J Sports Med. 2015;49:1316-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 214] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 85. | Rutten MJ, Maresch BJ, Jager GJ, Blickman JG, van Holsbeeck MT. Ultrasound of the rotator cuff with MRI and anatomic correlation. Eur J Radiol. 2007;62:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Watanabe T, Terabayashi N, Fukuoka D, Murakami H, Ito H, Matsuoka T, Seishima M. A pilot study to assess Fatty infiltration of the supraspinatus in patients with rotator cuff tears: comparison with magnetic resonance imaging. Ultrasound Med Biol. 2015;41:1779-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Tawfik AM, El-Morsy A, Badran MA. Rotator cuff disorders: How to write a surgically relevant magnetic resonance imaging report? World J Radiol. 2014;6:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 88. | Lambert A, Loffroy R, Guiu B, Mejean N, Lerais JM, Cercueil JP, Krausé D. [Rotator cuff tears: value of 3.0T MRI]. J Radiol. 2009;90:583-588. [PubMed] |
| 89. | Sershon RA, Mather RC, Sherman SL, McGill KC, Romeo AA, Verma NN. Low accuracy of interpretation of rotator cuff MRI in patients with osteoarthritis. Acta Orthop. 2013;84:479-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance imaging, and arthroscopic findings in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A:708-716. [PubMed] |
| 91. | Dinnes J, Loveman E, McIntyre L, Waugh N. The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review. Health Technol Assess. 2003;7:iii, 1-166. [PubMed] |
| 92. | Rutten MJ, Spaargaren GJ, van Loon T, de Waal Malefijt MC, Kiemeney LA, Jager GJ. Detection of rotator cuff tears: the value of MRI following ultrasound. Eur Radiol. 2010;20:450-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Swen WA, Jacobs JW, Algra PR, Manoliu RA, Rijkmans J, Willems WJ, Bijlsma JW. Sonography and magnetic resonance imaging equivalent for the assessment of full-thickness rotator cuff tears. Arthritis Rheum. 1999;42:2231-2238. [PubMed] |
| 94. | Murray J, Gross L. Optimizing the management of full-thickness rotator cuff tears. J Am Acad Orthop Surg. 2013;21:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | Zingg PO, Jost B, Sukthankar A, Buhler M, Pfirrmann CW, Gerber C. Clinical and structural outcomes of nonoperative management of massive rotator cuff tears. J Bone Joint Surg Am. 2007;89:1928-1934. [PubMed] |
| 96. | Clement ND, Nie YX, McBirnie JM. Management of degenerative rotator cuff tears: a review and treatment strategy. Sports Med Arthrosc Rehabil Ther Technol. 2012;4:48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 97. | Rudzki JR, Shaffer B. New approaches to diagnosis and arthroscopic management of partial-thickness cuff tears. Clin Sports Med. 2008;27:691-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 98. | Hamada K, Tomonaga A, Gotoh M, Yamakawa H, Fukuda H. Intrinsic healing capacity and tearing process of torn supraspinatus tendons: in situ hybridization study of alpha 1 (I) procollagen mRNA. J Orthop Res. 1997;15:24-32. [PubMed] |
| 99. | Yamanaka K, Matsumoto T. The joint side tear of the rotator cuff. A followup study by arthrography. Clin Orthop Relat Res. 1994;68-73. [PubMed] |
| 100. | Baydar M, Akalin E, El O, Gulbahar S, Bircan C, Akgul O, Manisali M, Torun Orhan B, Kizil R. The efficacy of conservative treatment in patients with full-thickness rotator cuff tears. Rheumatol Int. 2009;29:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Kuhn JE, Dunn WR, Sanders R, An Q, Baumgarten KM, Bishop JY, Brophy RH, Carey JL, Holloway BG, Jones GL. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg. 2013;22:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 251] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 102. | Kijima H, Minagawa H, Nishi T, Kikuchi K, Shimada Y. Long-term follow-up of cases of rotator cuff tear treated conservatively. J Shoulder Elbow Surg. 2012;21:491-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 103. | Harris JD, Pedroza A, Jones GL. Predictors of pain and function in patients with symptomatic, atraumatic full-thickness rotator cuff tears: a time-zero analysis of a prospective patient cohort enrolled in a structured physical therapy program. Am J Sports Med. 2012;40:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 104. | Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 105. | Mather RC, Koenig L, Acevedo D, Dall TM, Gallo P, Romeo A, Tongue J, Williams G. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95:1993-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 106. | Randelli P, Cucchi D, Ragone V, de Girolamo L, Cabitza P, Randelli M. History of rotator cuff surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23:344-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 107. | Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 589] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 108. | Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;455:52-63. [PubMed] |
| 109. | Robinson PM, Doll HA, Roy BR. Treating the torn rotator cuff: current practice in the UK. Ann R Coll Surg Engl. 2011;93:532-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Strauss EJ, Salata MJ, Kercher J, Barker JU, McGill K, Bach BR, Romeo AA, Verma NN. Multimedia article. The arthroscopic management of partial-thickness rotator cuff tears: a systematic review of the literature. Arthroscopy. 2011;27:568-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 111. | Wright SA, Cofield RH. Management of partial-thickness rotator cuff tears. J Shoulder Elbow Surg. 1996;5:458-466. [PubMed] |
| 112. | Snyder SJ, Pachelli AF, Del Pizzo W, Friedman MJ, Ferkel RD, Pattee G. Partial thickness rotator cuff tears: results of arthroscopic treatment. Arthroscopy. 1991;7:1-7. [PubMed] |
| 113. | Budoff JE, Nirschl RP, Guidi EJ. Débridement of partial-thickness tears of the rotator cuff without acromioplasty. Long-term follow-up and review of the literature. J Bone Joint Surg Am. 1998;80:733-748. [PubMed] |
| 114. | Cordasco FA, Backer M, Craig EV, Klein D, Warren RF. The partial-thickness rotator cuff tear: is acromioplasty without repair sufficient? Am J Sports Med. 2002;30:257-260. [PubMed] |
| 115. | Koh KH, Shon MS, Lim TK, Yoo JC. Clinical and magnetic resonance imaging results of arthroscopic full-layer repair of bursal-side partial-thickness rotator cuff tears. Am J Sports Med. 2011;39:1660-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 116. | Franceschi F, Papalia R, Del Buono A, Vasta S, Costa V, Maffulli N, Denaro V. Articular-sided rotator cuff tears: which is the best repair? A three-year prospective randomised controlled trial. Int Orthop. 2013;37:1487-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 117. | Ji JH, Shafi M, Jeong JJ, Lee YS, McFarland EG, Kim TK, Chung JY. Transtendon arthroscopic repair of high grade partial-thickness articular surface tears of the rotator cuff with biceps tendon augmentation: technical note and preliminary results. Arch Orthop Trauma Surg. 2012;132:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 118. | Dilisio MF, Miller LR, Higgins LD. Transtendon, double-row, transosseous-equivalent arthroscopic repair of partial-thickness, articular-surface rotator cuff tears. Arthrosc Tech. 2014;3:e559-e563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 119. | Mihata T, McGarry MH, Ishihara Y, Bui CN, Alavekios D, Neo M, Lee TQ. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 120. | Woods TC, Carroll MJ, Nelson AA, More KD, Berdusco R, Sohmer S, Boorman RS, Lo IK. Transtendon rotator-cuff repair of partial-thickness articular surface tears can lead to medial rotator-cuff failure. Open Access J Sports Med. 2014;5:151-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 121. | Kamath G, Galatz LM, Keener JD, Teefey S, Middleton W, Yamaguchi K. Tendon integrity and functional outcome after arthroscopic repair of high-grade partial-thickness supraspinatus tears. J Bone Joint Surg Am. 2009;91:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 122. | Kim KC, Shin HD, Cha SM, Park JY. Repair integrity and functional outcome after arthroscopic conversion to a full-thickness rotator cuff tear: articular- versus bursal-side partial tears. Am J Sports Med. 2014;42:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 123. | Peters KS, McCallum S, Briggs L, Murrell GA. A comparison of outcomes after arthroscopic repair of partial versus small or medium-sized full-thickness rotator cuff tears. J Bone Joint Surg Am. 2012;94:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 124. | Chung SW, Kim JY, Yoon JP, Lyu SH, Rhee SM, Oh SB. Arthroscopic repair of partial-thickness and small full-thickness rotator cuff tears: tendon quality as a prognostic factor for repair integrity. Am J Sports Med. 2015;43:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 125. | Lee TQ. Current biomechanical concepts for rotator cuff repair. Clin Orthop Surg. 2013;5:89-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 126. | Lindley K, Jones GL. Outcomes of arthroscopic versus open rotator cuff repair: a systematic review of the literature. Am J Orthop (Belle Mead NJ). 2010;39:592-600. [PubMed] |
| 127. | Shan L, Fu D, Chen K, Cai Z, Li G. All-arthroscopic versus mini-open repair of small to large sized rotator cuff tears: a meta-analysis of clinical outcomes. PLoS One. 2014;9:e94421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 128. | Nho SJ, Shindle MK, Sherman SL, Freedman KB, Lyman S, MacGillivray JD. Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J Bone Joint Surg Am. 2007;89 Suppl 3:127-136. [PubMed] |
| 129. | Morse K, Davis AD, Afra R, Kaye EK, Schepsis A, Voloshin I. Arthroscopic versus mini-open rotator cuff repair: a comprehensive review and meta-analysis. Am J Sports Med. 2008;36:1824-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 130. | Zhang Z, Gu B, Zhu W, Zhu L, Li Q. Arthroscopic versus mini-open rotator cuff repair: a prospective, randomized study with 24-month follow-up. Eur J Orthop Surg Traumatol. 2014;24:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 131. | Cho CH, Song KS, Min BW, Jung GH, Lee YK, Sin HK. Anterolateral approach for mini-open rotator cuff repair. Int Orthop. 2012;36:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 132. | Carbonel I, Martinez AA, Calvo A, Ripalda J, Herrera A. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears: a prospective randomized clinical study. Int Orthop. 2012;36:1877-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 133. | Xu C, Zhao J, Li D. Meta-analysis comparing single-row and double-row repair techniques in the arthroscopic treatment of rotator cuff tears. J Shoulder Elbow Surg. 2014;23:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 134. | Prasathaporn N, Kuptniratsaikul S, Kongrukgreatiyos K. Single-row repair versus double-row repair of full-thickness rotator cuff tears. Arthroscopy. 2011;27:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 135. | Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 136. | Millett PJ, Warth RJ, Dornan GJ, Lee JT, Spiegl UJ. Clinical and structural outcomes after arthroscopic single-row versus double-row rotator cuff repair: a systematic review and meta-analysis of level I randomized clinical trials. J Shoulder Elbow Surg. 2014;23:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 137. | Saridakis P, Jones G. Outcomes of single-row and double-row arthroscopic rotator cuff repair: a systematic review. J Bone Joint Surg Am. 2010;92:732-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 138. | Chen M, Xu W, Dong Q, Huang Q, Xie Z, Mao Y. Outcomes of single-row versus double-row arthroscopic rotator cuff repair: a systematic review and meta-analysis of current evidence. Arthroscopy. 2013;29:1437-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 139. | McCormick F, Gupta A, Bruce B, Harris J, Abrams G, Wilson H, Hussey K, Cole BJ. Single-row, double-row, and transosseous equivalent techniques for isolated supraspinatus tendon tears with minimal atrophy: A retrospective comparative outcome and radiographic analysis at minimum 2-year followup. Int J Shoulder Surg. 2014;8:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 140. | Grasso A, Milano G, Salvatore M, Falcone G, Deriu L, Fabbriciani C. Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy. 2009;25:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 141. | Lapner PL, Sabri E, Rakhra K, McRae S, Leiter J, Bell K, Macdonald P. A multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94:1249-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 142. | Gartsman GM, Drake G, Edwards TB, Elkousy HA, Hammerman SM, O’Connor DP, Press CM. Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. J Shoulder Elbow Surg. 2013;22:1480-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 143. | Wall LB, Keener JD, Brophy RH. Double-row vs single-row rotator cuff repair: a review of the biomechanical evidence. J Shoulder Elbow Surg. 2009;18:933-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 144. | Ahmad CS, Vorys GC, Covey A, Levine WN, Gardner TR, Bigliani LU. Rotator cuff repair fluid extravasation characteristics are influenced by repair technique. J Shoulder Elbow Surg. 2009;18:976-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 145. | Pauly S, Gerhardt C, Chen J, Scheibel M. Single versus double-row repair of the rotator cuff: does double-row repair with improved anatomical and biomechanical characteristics lead to better clinical outcome? Knee Surg Sports Traumatol Arthrosc. 2010;18:1718-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 146. | Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 147. | Tudisco C, Bisicchia S, Savarese E, Fiori R, Bartolucci DA, Masala S, Simonetti G. Single-row vs. double-row arthroscopic rotator cuff repair: clinical and 3 Tesla MR arthrography results. BMC Musculoskelet Disord. 2013;14:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 148. | Genuario JW, Donegan RP, Hamman D, Bell JE, Boublik M, Schlegel T, Tosteson AN. The cost-effectiveness of single-row compared with double-row arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94:1369-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 149. | Kim YK, Moon SH, Cho SH. Treatment outcomes of single- versus double-row repair for larger than medium-sized rotator cuff tears: the effect of preoperative remnant tendon length. Am J Sports Med. 2013;41:2270-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 150. | Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: Biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 306] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 151. | Miškulin M, Vrgoč G, Sporiš G, Dulic O, Gavrilovic G, Milanović Z. Single-row arthroscopic cuff repair with double-loaded anchors provides good shoulder function in long-term follow-up. Int Orthop. 2015;39:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 152. | Lorbach O, Kieb M, Raber F, Busch LC, Kohn D, Pape D. Comparable biomechanical results for a modified single-row rotator cuff reconstruction using triple-loaded suture anchors versus a suture-bridging double-row repair. Arthroscopy. 2012;28:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 153. | Vaishnav S, Millett PJ. Arthroscopic rotator cuff repair: scientific rationale, surgical technique, and early clinical and functional results of a knotless self-reinforcing double-row rotator cuff repair system. J Shoulder Elbow Surg. 2010;19:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 154. | Ahmad S, Haber M, Bokor DJ. The influence of intraoperative factors and postoperative rehabilitation compliance on the integrity of the rotator cuff after arthroscopic repair. J Shoulder Elbow Surg. 2015;24:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 155. | Baumgarten KM, Vidal AF, Wright RW. Rotator Cuff Repair Rehabilitation: A Level I and II Systematic Review. Sports Health. 2009;1:125-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 156. | Thigpen CA, Shaffer MA, Kissenberth MJ. Knowing the speed limit: weighing the benefits and risks of rehabilitation progression after arthroscopic rotator cuff repair. Clin Sports Med. 2015;34:233-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 157. | Millett PJ, Wilcox RB, O’Holleran JD, Warner JJ. Rehabilitation of the rotator cuff: an evaluation-based approach. J Am Acad Orthop Surg. 2006;14:599-609. [PubMed] |
| 158. | Ross D, Maerz T, Lynch J, Norris S, Baker K, Anderson K. Rehabilitation following arthroscopic rotator cuff repair: a review of current literature. J Am Acad Orthop Surg. 2014;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 159. | Koh KH, Lim TK, Shon MS, Park YE, Lee SW, Yoo JC. Effect of immobilization without passive exercise after rotator cuff repair: randomized clinical trial comparing four and eight weeks of immobilization. J Bone Joint Surg Am. 2014;96:e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 160. | Keener JD, Galatz LM, Stobbs-Cucchi G, Patton R, Yamaguchi K. Rehabilitation following arthroscopic rotator cuff repair: a prospective randomized trial of immobilization compared with early motion. J Bone Joint Surg Am. 2014;96:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 161. | Gallagher BP, Bishop ME, Tjoumakaris FP, Freedman KB. Early versus delayed rehabilitation following arthroscopic rotator cuff repair: A systematic review. Phys Sportsmed. 2015;43:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 162. | Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthroscopy. 2012;28:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 163. | Parsons BO, Gruson KI, Chen DD, Harrison AK, Gladstone J, Flatow EL. Does slower rehabilitation after arthroscopic rotator cuff repair lead to long-term stiffness? J Shoulder Elbow Surg. 2010;19:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 164. | Brady B, Redfern J, MacDougal G, Williams J. The addition of aquatic therapy to rehabilitation following surgical rotator cuff repair: a feasibility study. Physiother Res Int. 2008;13:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 165. | Galatz LM, Rothermich SY, Zaegel M, Silva MJ, Havlioglu N, Thomopoulos S. Delayed repair of tendon to bone injuries leads to decreased biomechanical properties and bone loss. J Orthop Res. 2005;23:1441-1447. [PubMed] |
| 166. | Papadopoulos P, Karataglis D, Boutsiadis A, Fotiadou A, Christoforidis J, Christodoulou A. Functional outcome and structural integrity following mini-open repair of large and massive rotator cuff tears: a 3-5 year follow-up study. J Shoulder Elbow Surg. 2011;20:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 167. | Deniz G, Kose O, Tugay A, Guler F, Turan A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: does it improve, halt or deteriorate? Arch Orthop Trauma Surg. 2014;134:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 168. | Harryman DT, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982-989. [PubMed] |
| 169. | DeFranco MJ, Bershadsky B, Ciccone J, Yum JK, Iannotti JP. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg. 2007;16:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 170. | Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1:96-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 171. | Abtahi AM, Granger EK, Tashjian RZ. Factors affecting healing after arthroscopic rotator cuff repair. World J Orthop. 2015;6:211-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 127] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (9)] |
| 172. | Codsi MJ, Rodeo SA, Scalise JJ, Moorehead TM, Ma CB. Assessment of rotator cuff repair integrity using ultrasound and magnetic resonance imaging in a multicenter study. J Shoulder Elbow Surg. 2014;23:1468-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 173. | Chung SW, Oh JH, Gong HS, Kim JY, Kim SH. Factors affecting rotator cuff healing after arthroscopic repair: osteoporosis as one of the independent risk factors. Am J Sports Med. 2011;39:2099-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 296] [Article Influence: 19.7] [Reference Citation Analysis (1)] |
| 174. | Meyer DC, Wieser K, Farshad M, Gerber C. Retraction of supraspinatus muscle and tendon as predictors of success of rotator cuff repair. Am J Sports Med. 2012;40:2242-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 175. | Tashjian RZ, Hollins AM, Kim HM, Teefey SA, Middleton WD, Steger-May K, Galatz LM, Yamaguchi K. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38:2435-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 176. | Lundgreen K, Lian OB, Scott A, Nassab P, Fearon A, Engebretsen L. Rotator cuff tear degeneration and cell apoptosis in smokers versus nonsmokers. Arthroscopy. 2014;30:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 177. | Tashjian RZ, Hung M, Burks RT, Greis PE. Influence of preoperative musculotendinous junction position on rotator cuff healing using single-row technique. Arthroscopy. 2013;29:1748-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 178. | Lorbach O, Pape D, Raber F, Busch LC, Kohn D, Kieb M. Influence of the initial rupture size and tendon subregion on three-dimensional biomechanical properties of single-row and double-row rotator cuff reconstructions. Knee Surg Sports Traumatol Arthrosc. 2012;20:2139-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 179. | Lambers Heerspink FO, Dorrestijn O, van Raay JJ, Diercks RL. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 180. | Kim KC, Shin HD, Lee WY. Repair integrity and functional outcomes after arthroscopic suture-bridge rotator cuff repair. J Bone Joint Surg Am. 2012;94:e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 181. | Mall NA, Lee AS, Chahal J, Van Thiel GS, Romeo AA, Verma NN, Cole BJ. Transosseous-equivalent rotator cuff repair: a systematic review on the biomechanical importance of tying the medial row. Arthroscopy. 2013;29:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 182. | Davis ME, Stafford PL, Jergenson MJ, Bedi A, Mendias CL. Muscle fibers are injured at the time of acute and chronic rotator cuff repair. Clin Orthop Relat Res. 2015;473:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Carbone S, Murata A S- Editor: Song XX L- Editor: A E- Editor: Jiao XK