Published online Oct 18, 2013. doi: 10.5312/wjo.v4.i4.229
Revised: August 5, 2013
Accepted: August 28, 2013
Published online: October 18, 2013
Processing time: 124 Days and 7.3 Hours
Tendon ruptures remain a significant musculoskeletal injury. Despite advances in surgical techniques and procedures, traditional repair techniques maintain a high incidence of rerupture or tendon elongation. Mechanical loading and biochemical signaling both control tissue healing. This has led some researchers to consider using a technique based on tension regulation at the suture line for obtaining good healing. However, it is unknown how they interact and to what extent mechanics control biochemistry. This review will open the way for understanding the interplay between mechanical loading and the process of tendon healing.
Core tip: Ruptured tendons heal poorly compared to skin, muscles and bones. Immobilization during repair has been shown to be detrimental for the healing process. Mechanical loading of the tendon callus gives rise to intracellular signaling, increases gene expression and protein synthesis. However, early loading reported clinical complications. A surgical technique based on control of the mechanical environment at the suture line provided satisfactory results. Therefore, understanding the interplay between loading and the healing process seems necessary. This review focuses on the biological processes that regulate tendon repair and timing of mechanical loading during the healing process. How do tendon cells sense mechanical forces?
- Citation: Massoud EIE. Healing of subcutaneous tendons: Influence of the mechanical environment at the suture line on the healing process. World J Orthop 2013; 4(4): 229-240
- URL: https://www.wjgnet.com/2218-5836/full/v4/i4/229.htm
- DOI: https://dx.doi.org/10.5312/wjo.v4.i4.229
This article is limited to the subcutaneous tendons, i.e., tendons that do not glide through synovial sheaths responsible for their nutrition. It describes the function, structure and mechanobiology of tendons, reviews the phases of tendon healing and reviews the influence of the mechanical environment at the site of repair on the healing process.
Healthy tendons are brilliant white in color and have a fibroelastic texture. Tendons demonstrate marked variation in form; generally, extensor tendons are more flattened than flexor tendons which tend to be round or oval[1,2]. The dry mass of human tendons is approximately 30% of the total tendon mass, with water accounting for the remaining 70%[1,3]. Lemoine et al [3] reported that men had a significantly greater amount of tendon dry mass than women. Many authors have explained this dissimilarity on the fact that estrogen directly alters collagen kinetics and inherently higher estrogen levels in women may therefore chronically depress collagen production as tendon cells have estrogen receptors[4,5]. Thus, blunted collagen production via estrogen could explain the lower amount of dry mass in female tendons as collagen comprises 90% of dry mass[3,4].
Tenoblasts and tenocytes constitute about 90% to 95% of the cellular elements of tendons. They lie between the collagen fibers along the long axis of the tendon[6]. The remaining 5% to 10% of the cellular elements consists of chondrocytes at the insertion sites, synovial cells and vascular cells, including capillary endothelial cells and smooth muscle cells of arterioles[1]. Tenocytes are mature tendon cells. They are active in energy generation and synthesize collagen and all components of the extracellular matrix network[7].
Extracellular matrix (ECM) of tendons largely consist of collagens and proteoglycans and are dominated by type I collagen as it accounts for 65% to 80% of the dry mass of tendons. However, other collagens (e.g., type II, III, V, VI, IX, XI) are also present[2,8]. Collagen molecules consist of polypeptide chains (tropocollagen). Soluble tropocollagen molecules form cross-links to create insoluble collagen molecules. Three such chains combine together to form a densely packed, helical tropocollagen molecule (triple-helix polypeptide chain). In turn, five tropocollagens constitute a microfibril and microfibrils aggregate together to form fibrils. Fibrils are then grouped into fibers, fibers into fiber bundles (primary bundles) and fiber bundles into fascicles (secondary bundles), tertiary bundles and the tendon itself. A collagen fiber is the smallest tendon unit that can be tested mechanically and is visible under light microscopy[1,2]. Some of the larger collections of fascicles are visible in gross dissections[2].
The principal role of the collagen fibers is to resist tension, although they still allow for a certain degree of compliance (i.e., reversible longitudinal deformation). Such apparently conflicting demands are probably resolved because the collagen is arranged in hierarchical levels of increasing complexity, beginning with tropocollagen[1,2]. At various levels of tendon organization, including the whole tendon, fascicles and fibrils, a helical architecture (often with superimposed “crimp”, i.e., a zigzag undulation of collagen fibrils) occurs in certain tendons. This helical organization of tendon components makes them comparable to man-made ropes and the presence of crimp contributes to their inherent flexibility[9-11]. Roukis et al[10] have suggested that the twisting that characterizes the tendon of tibialis posterior reduces the need for longitudinal slippage between fascicles during triplanar movements of the foot. The angle of torsion of the inner fibrils in a helical tendon fascicle may be less oblique than that of the outer fibrils and this may give the tendon regionally distinct compliance[10]. The fibers of the Achilles tendon twist through its descent, thus elastic recoil within the tendon are possible[12]. This point will be detailed under subheading elastic recoil of the tendon.
The ground substance of the extracellular matrix network surrounding the collagen and the tenocytes is composed of proteoglycans, glycosaminoglycans, glycoproteins and several other small molecules[1]. Proteoglycans make up less than 1% of the dry weight of most tensile tendons[8]. Proteoglycans are strongly hydrophilic, enabling rapid diffusion of water-soluble molecules and the migration of cells[1]. Additionally, the proteoglycans have the function of providing a viscous environment, allowing the collagen fibrils, fibers or fascicles to slide relative to each other, as well as to stretch and dissipate the force of sudden loads[8,13].
The epitenon, a fine loose connective-tissue sheath containing the vascular, lymphatic and nerve supply to the tendon, surrounds the tendon as a whole and forms the gross structure of the tendon[14,15]. The epitenon extends deeply as the endotenon, which is a thin reticular network of connective tissue investing each tendon fiber. The endotenon protects tendon vasculature and allows fascicles to slide over one another in particularly malleable parts of the tendon[2,14]. Due to the epitenon being directly continuous with the endotenon, the points of continuity help to bind it firmly to the surface of the tendon[14]. The epitenon is further enclosed by paratenon, a loose areolar connective tissue separated from the epitenon by a thin layer of fluid to allow tendon movement with reduced friction[2,14,15]. The paratenon may be quite vascular and is a source of the blood supply to the tendon itself[8].
The myotendinous junction (MTJ) is the interface between muscle and tendon; it is tailored for transmitting the mechanical force generated by a muscle contraction to the extracellular matrix of the muscle and onto the tendon[16-18]. The characteristic morphology of the MTJ is the folding of the sarcolemma into finger-like projections at the interface between muscle and tendon at sites of myocyte termination[16,17]. The projections increase the area of muscle-tendon contact to more than 10 fold over the cross-section of the muscle fiber. Thus, the local stress (force per unit area) is reduced. Additionally, the longitudinal arrangement of the projections ensures that the stresses experienced by the MTJ are shear stresses[19,20]. Mechanical loading of the MTJ activates cell-signaling pathways that instruct the cells located at the interface to secrete and deposit proteins to form a specialized extracellular matrix at the MTJ. However, lack of the expression of these proteins has been shown to lead to structural damage of the interface during contraction[18].
The osteotendinous junctions (OTJ) are sites of stress concentration at the region where tendons attach to bone[21]. These regions are characterized by the presence of a unique transitional tissue called “entheses” at the interface, which can effectively transfer the stress from tendon to bone and vice versa through its gradual change in structure, composition and mechanical behavior[21,22]. There are two types of entheses, based on how the collagen fibers attach to bone[14,21,22]. Direct insertions (also called the fibrocartilaginous entheses), such as the insertion of the Achilles tendon and patellar tendon, are composed of four zones in order of gradual transition: tendon, uncalcified fibrocartilage, calcified fibrocartilage and bone[21,22]. The continuous change in tissue composition from tendon to bone is presumed to aid in the efficient transfer of load between the two materials[22]. Indirect insertions (also called fibrous entheses), such the insertion of the deltoid tendon into the humerus, have no fibrocartilage interface. The tendon passes obliquely along the bone surface and inserts at an acute angle either directly to the bone or indirectly to it via the periosteum[21,22]. The main factors affecting the type of insertion seem to be strain, site, length and angle of insertion. When a tendon runs parallel to the bone, the insertion is more likely to be indirect, while when the tendon enters the bone perpendicularly, the insertion is direct[22].
The majority of tendons attach not only to bone, but also to adjacent fascia. This is a basic strategy for dissipating stress concentration at entheses and thus reducing the risk of failure or local wear and tear. One of the classic examples of subcutaneous tendons that have both bony and fibrous attachments is the quadriceps tendon. This not only attaches to the superior pole of the patella, but also sends a sheet of fibers anterior to the patella that become continuous with the patellar tendon[23].
Tendons are still vascularized and the presence of vessels is important for the normal functioning of tendon cells and the ability of tendons to repair[2]. Tendons receive their blood supply from three sources: the peritendinous tissues (the extrinsic source) that have a richer blood supply than the tendons themselves[24]. In the tendon itself, the vessels run longitudinally, parallel to the fascicles and within the endotenon and anastomoses between parallel vessels are common[25]. Intrinsic sources include vessels that enter tendons at their myotendinous junctions and at entheses[2]. Nevertheless, the direct role of the blood vessels in tendon nutrition has been called into question. Edwards has reported that tendons may be cut and transplanted with impunity[25]. Recently, many investigators have pointed out that diffusion from surrounding tissues may play a significant role in metabolic exchange in intact tendons[26-28].
Tendon innervation originates from cutaneous, muscular and peritendinous nerve trunks[1]. The majority of nerve fibers are located within the paratenon and not the tendon itself[29]. Paratenon nerves form rich plexuses that send a few branches penetrating the epitenon. These branches are described to cross the myotendinous junction and to continue into the endotenon septa[30]. Deep in the tendon tissue proper, where innervation is reported to be relatively scarce, the nerves follow the blood vessels running along the axis of the tendon[2,30]. Four types of nerve endings have been identified: free nerve endings, Ruffini corpuscles, Pacinian corpuscles and Golgi tendon organs[30]. Vessel-associated fibers are autonomic nerves that immunolabel for neuropeptide Y and noradrenaline (vasoconstrictive factors) and for vasoactive intestinal peptide (VIP), a vasodilator factor. It has been suggested that the nerve fibers regulate blood flow within the tendon. Furthermore, free nerve fibers containing substance P and calcitonin gene-related peptide (CGRP) might be involved in collecting sensory information (including pain) and relaying this to the central nervous system[29]. Zaffagnini et al[31] have reported the presence of Ruffini and Pacinian corpuscles within the pes anserinus tendons, particularly at their tibial attachment sites. Benjamin et al[32] confirm that Pacinian corpuscles can be found on the surface of subcutaneous entheses.
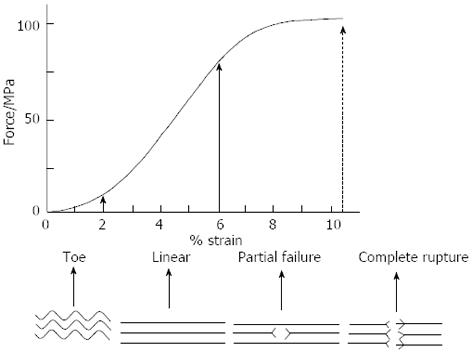
Tendons transmit force from muscle to bone and act as a buffer by absorbing external forces to limit muscle damage[1]. We will discuss the response of the tendon to mechanical stimuli at fibrillar and cellular levels. At rest, a tendon has a wavy configuration, a result of crimping of the collagen fibrils. The stress-strain curve of tendons usually exhibits three distinct regions[33], which can be correlated to deformations at different structural levels (Figure 1). In the first region that is usually called the toe region, a very small stress is sufficient to strain (elongate) the tendon up to 2% of its length and the straightening of the macroscopic crimp in the collagen fibrils[34]. In the second region of the curve, at higher strains, the stiffness of the tendon increases[13,35]. If the strain placed on the tendon remains at less than 4%, the tendon behaves as a mechanical spring and returns to its original length and crimps when unloaded[35]. The most probable processes are thought to be the ability of the fascicles to slide independently against each other. This allows them to transmit tension despite the changing angles of a joint as it moves and allows tendons to change shape as their muscles contract[2,36]. Sliding within fascicles occurs between fibrils and this may account for up to 50% of the longitudinal deformation (i.e., strain) of a tendon[37]. Sliding of fibrils or fascicles relative to each other occurs within the proteoglycan-rich matrix surrounding them[13]. The presence of the endotenon between fascicles and/or fiber bundles facilitates the sliding movement[2,14,38]. Lubricin, a molecule often associated with joint lubrication, is also present between the fascicles of certain tendons[39].
At strain levels between 4% and 8%, the tendon becomes progressively easier to extend but its length still returns to its original value. However, the wave pattern does not reappear[34]. On the other hand, recent work has suggested that strain values of 6% and even up to 8% may be physiological. Within the physiological range, particularly towards the higher range, microscopic degeneration within the tendon may start to occur, especially with repeated and/or prolonged stressing[40]. Beyond 8% to 10% strain, macroscopic failure occurs from intrafibril damage by molecular slippage[40-42]. The probable process has previously been investigated using synchrotron radiation diffraction. Initially, collagen fibril elongation occurs as a result of molecular elongation. When the stress increases the stretching of the collagen triple helices and the cross-links between the helices, a considerable gliding of neighboring molecules occur[43,44].
There is now considerable evidence to suggest that tendons and tendon cells can respond to altered mechanical load. In man, collagen synthesis in the patellar tendon increases by nearly 100% as a result of just a single bout of acute exercise and the effect is still evident 3 d later[45]. At a cellular level, there seems to be no difference in the response of tenocytes to mechanical load between cells that have been extracted from different tendons, e.g., those associated with antagonistic muscles[46]. However, in a given tendon, different stress patterns provoke different cellular reactions depending on the amount and duration of the tensional stress applied. Cell proliferation, for example, is stimulated by short periods of repetitive tension but inhibited by more extended periods[47]. The response seems to depend on gap junctional communication between neighboring cells, for when gap junctions are blocked, the cells no longer increase collagen synthesis in response to stretching forces applied in vitro. The modulation of ECM synthesis involves two types of gap junctions: those characterized by the presence of connexin 32 and those containing connexin 43. The former junctions stimulate and the latter inhibit collagen synthesis[48]. In addition to its effects on collagen synthesis, the repetitive stretching of tenocytes in vitro up regulates proinflammatory cytokine production and the gene expression of mediators such as Cox-2, prostaglindin E2 and matrix metalloproteinase (MMP)-1[49,50]. Smaller levels of repetitive tensile stress reduce the production of proinflammatory agents. Thus, repetitive small magnitude stretching seems to be anti-inflammatory, whereas large magnitude stretching is pro-inflammatory. If the findings also prove to be applicable in vivo, then it follows that moderate exercise may be beneficial for reducing tendon inflammation[50]. It is interesting to note that tenocytes themselves may produce IL-1β, especially if they are located next to a site where the tendon is injured. Expression is highest 1 day after injury but can persist for several days[51]. The significance of IL-1β production in an injured tendon is that it can induce the expression of a wide range of pro-inflammatory agents such as Cox2, MMP1, MMP3, MMP13, ADAMTS-4 and IL-6. It also triggers the further expression of IL-1β mRNA[52] and this is presumably a mechanism for rapidly raising its local concentration. It should be noted, however, that in addition to such actions, IL-1β reduces the elastic modulus of tenocytes by disrupting actin filaments[53]. The authors suggest that this acts as a protective mechanism against mechanical overuse of tendon cells during healing. How do tendon cells sense mechanical forces? This question will be answered in detail in this review.
Suppression of proteoglycan and collagen synthesis in cultured tenocytes can be induced by glucocorticoids[54,55]. These are among the substances commonly used by clinicians to suppress inflammation in patients with tendon injuries. Glucocorticoids can also suppress tenocyte proliferation and progenitor cell recruitment[56]. If such effects also occur in vivo, then this may explain why the integrity of the tendon as a whole may be affected by corticosteroid treatment. In contrast to corticosteroids, nitric oxide generally benefits tendon healing and enhances collagen synthesis[57]. Nitric oxide synthetases are normally expressed at low levels and are up regulated by mechanical stimuli[58,59]. The absence of nitric oxide from tendons during wound healing is associated with prolonged inflammation[60].
Tendons can recoil elastically when a stretching force is removed. The elastic recoil property seems to be structurally related to crimp and/or knots within fibrils in regions where fibrils are twisted or bent. When a tendon is physiologically stretched in vivo, the crimp numbers within it may decrease by nearly 50%[61].
The ability of tendons to stretch and recoil enables them to save energy in running by allowing the limb to have shorter muscle fascicles or slower muscle fibers that can generate force more economically[12]. The fibers of the Achilles tendon spiral through 90 degrees during its descent, such that the fibers that lie medially in the proximal portion become posterior distally. In this way, elongation and elastic recoil within the tendon are possible and stored energy can be released during the appropriate phase of locomotion. In addition, this stored energy allows the generation of higher shortening velocities and greater instantaneous muscle power than could be achieved by contraction of the triceps surae alone[12,15]. The stiffness of tendons varies with sex, age and physical activity. The Achilles tendon of women can recoil elastically more than that of men[62]. Stiffness is greater in young men than in young boys; however, it decreases with training in adults[63,64]. The greater compliance of tendons in young boys may reduce the risk of sport injuries[63]. The elasticity of a fatigued tendon tends to be greater, as evidenced by its ability to lengthen further with the same load[65].
Studies in goats have shown that it is the muscle rather than the tendon that provides the extra length within the muscle-tendon unit necessary for limb lengthening by distraction osteotomy. While the muscle may elongate by almost 10% of its initial length, the tendon only does so by 3%-4%[66]. Elastic recoil of tendon stumps will elongate the spontaneously healed tendon. This emphasizes the necessity for tension relief at the site of repair in the early phase of tendon healing.
Tendon rupture occurs spontaneously or following direct trauma such as severance of a tendon by sharp objects or being caught between bones and traumatizing agent. A spontaneous rupture may be defined as a rupture that occurs during movements and activities that should not, and usually do not, damage the involved musculotendinous units[67]. Although many investigators reported that spontaneous rupture of a tendon is preceded by degenerative changes, there is little agreement with regard to its etiology. Degenerative changes of the tendon have been linked to genetic abnormalities of the collagen tissues[68], chronic diseases or metabolic disorders[69] and neurological conditions[70]. Fluoroquinolones and locally or systemically administered corticosteroids have been implicated in the etiology of tendon rupture[71-74]. In the literature, there are four basic types of tendon degeneration: hypoxic, mucoid degeneration, lipomatosis and calcification of the tendon. Extensive tendo lipomatosis by itself may lead to rupture of the tendon without degenerative changes in the collagen tissues[75]. For reasons that are not clear, most reported cases of tendo lipomatosis have been in the quadriceps or patellar tendon. The described histopathological changes predispose the tendon to rupture through decrease of its tensile strength[67]. Strength of the tendons and resistance to tensile forces are related to the angles of tendon crimps in providing a resistance to sudden elongation and to the diameter of the collagen fibers[61,76]. Järvinen et al[77] investigated the crimp angle and the diameter of the collagen fibers in spontaneously ruptured tendons and compared them to healthy tendons. They concluded that the collagen fibers in ruptured tendons are substantially thinner than in normal tendons. The crimp angle of the collagen fibers is also significantly decreased in ruptured tendons[77].
After tendon rupture, the body restores tendon continuity through a cascade of events can be divided into three overlapping phases: tissue inflammation, cell proliferation and remodeling phases[78-80].
This phase starts immediately post injury and persists for about 24 h[78]. In this phase, injured blood vessels that are in the tendon envelope cause the formation of a hematoma, which activates the release of various chemotactic factors such as vasodilators and proinflammatory molecules[78,79]. The chemotactic factors attract inflammatory cells (e.g., neutrophils, monocytes and macrophages) that migrate to the wound site and clean the site of necrotic materials by phagocytosis. Tendon fibroblasts recruited to the site begin to synthesize various components of the ECM[81]. Moreover, during this phase, the angiogenic factors initiate the formation of a vascular network[82]. These processes include an increase in DNA and ECM, which establishes continuity and partial stability at the site of injury[78].
In this phase that lasts a few weeks, tendon fibroblasts synthesize collagen and other ECM components and deposit them at the wound site[78]. These components are initially arranged randomly within the ECM, which at this time is composed largely of type III collagen[83]. An extensive blood vessel network is present and the wound has a scar-like appearance[84]. During this phase, the repair tissue is highly cellular and contains relatively large amounts of water and an abundance of ECM components[78,79].
This phase that begins by nearly the 6th week after injury is characterized by decreased cellularity, reduced matrix synthesis, decrease in type III collagen, and an increase in type I collagen synthesis[78,79]. Type I collagen fibers are organized longitudinally along the tendon axis and are responsible for the mechanical strength of the regenerate tissue[85]. During the later remodeling phase, covalent bonding between collagen fibers consequently increases tendon stiffness and tensile strength. In addition, both the metabolism of tenocytes and tendon vascularity decline[78,79].
It is known that the fibroblasts during healing generate and exert force on the ECM. This force is referred to as fibroblast contraction, which is essential for wound closure[86]. However, excessive cell contraction may lead to tissue scarring. On the other hand, inhibiting fibroblast contraction results in impaired wound healing[87]. Therefore, an optimal level of fibroblast contraction is desirable to facilitate wound closure while minimizing scar tissue formation.
Cell contraction involves the actin cytoskeleton[88]. The interaction between actin and myosin generates cell contraction[89] and the contractile forces transmit through actin filaments to integrins and the ECM[90].
In the literature, most studies concerned with cell contraction focus on skin fibroblasts[78]. Using a cell force monitor, contractile forces of tendon and skin fibroblasts were measured over time. It was found that tendon and skin fibroblasts exhibited different patterns of contraction where tendon fibroblasts produced a lower maximum contraction force than skin fibroblasts[91]. In healing tissues, myofibroblasts are thought to play a major role in tissue contraction. These cells have phenotypic characteristics of both fibroblasts and smooth muscle cells, including the formation of stress fibers parallel with the long axis of the cell[92] Mechanical loading also influences myofibroblast differentiation. Increased tension on granulation tissue in rats increases the formation of stress fibers and the expression levels of a-SMA and ED-A fibronectin[93], which are two protein markers of myofibroblasts[78].
As described above, tendon cells respond to mechanical forces by altering gene expression, protein synthesis and cell phenotype. These early adaptive responses may proceed, initiate long-term tendon structure modifications, and thus lead to changes in the tendon’s mechanical properties. After that, we need to understand how tendon cells sense mechanical forces and convert them into cascades of cellular and molecular events that eventually lead to changes in tendon structure. This question comprises of the definition of mechanotransduction and necessitates a review of mechanotransduction mechanisms. Cellular components implicated in the transduction of mechanical forces are extracellular matrix, cytoskeleton, integrins, G proteins, receptor tyrosine kinases (RTKs), mitogen-activated protein kinases (MAPKs) and stretching-activated ion channels.
ECM is composed of cell-produced proteins and polysaccharides[94,95]. It defines tissue shape, structure and acts as the substrate for cell adhesion, growth and differentiation[96]. Mechanical loading increases ECM protein production by promoting the release of growth factors which mediate collagen secretion[97,98].
ECM transmits mechanical loads, stores and dissipates loading-induced elastic energy. Moreover, mechanical deformations in the ECM can transmit to the actin cytoskeleton and cause the remodeling of the actin cytoskeleton[99,100], which is known to control cell shape, affect cell motility and mediate various cellular functions, including DNA and protein syntheses[101].
The cytoskeleton is composed of microfilaments, microtubules and intermediate filaments[101,102]. The cytoskeleton responds to extracellular forces, participates in transmembrane signaling and provides a network for translocating signaling molecules. Mechanical forces applied to the cell surface transmit directly to the cytoskeleton and cause changes in its structure[103]. Consequently, these changes due to applied mechanical forces can initiate transduction cascades within the cell through the activation of integrins and the stimulation of G protein receptors, RTKs and MAPKs[78].
Integrins are transmembrane proteins composed of a and b subunits[104]. It mediates mechanotransduction between the extracellular matrix and the cell through ‘‘outside in’’ and ‘‘inside out’’ fashions[104,105]. Mechanical forces stimulate the conformational activation of integrins in cells and increase cell binding to the extracellular matrix[106].
G proteins are membrane proteins that are involved in mechanotransduction. Mechanical forces may simultaneously activate G proteins and integrins[107].
RTKs and MAPKs are a class of cell membrane proteins that are phosphorylated when subjected to cyclic stretching or shear stress. They can travel into the nucleus and alter gene expression[107,108]. It has been shown that cyclic stretching activates MAPKs in patellar tendon fibroblasts[109].
The activation of these channels permit calcium (Ca2+) and other ions (e.g., sodium and potassium) to influx, followed by membrane depolarization[110].
Mechanical stretching induced Ca2+ signal transmission involves the actin microfilament system because an actin polymerization inhibitor was found to abolish Ca2+ responses induced by mechanical stimulation[111]. This means that calcium is an important mediator in cellular mechanotransduction.
It has been agreed that injured tendons require mechanical loading for optimal healing but early loading is not without risks of adverse effects. Loading might create excessive damage to the repair tissue leading to failure of the healing process or mostly plastic deformation (elongation) of the callus with subsequent tendon lengthening [112]. Lengthening during tendon healing is one potential clinical adversity. It is therefore important to understand the interplay between the loading and healing process.
The inflammatory phase of tendon repair seems to prepare the way for the formation of a fibrous callus. If early inflammation is inhibited, the fibrous callus can lose a third of its strength due to inferior material properties (lower stress at failure). During the inflammatory phase, there is little mechanical strength and mostly plastic deformation[112]. About the effect of loading during the inflammatory phase, Eliasson et al[113] noticed suppression of genes related to inflammation and extracellular matrix components. Moreover, they observed the loaded tendons by the third day also had a lower expression of collagens III and I than unloaded ones, emphasizing, again, the value of mechanical protection in the early phase[113]. Once some elasticity has been obtained in the early fibrous callus, however, deformed tissues will resume their pre-load shape and cyclic loading will lead to biological signals[114]. It is remarkable that the investigators have suggested the period between the 4th and 6th postoperative week for the institution of mechanical loading at the tendinous interface as well as at the tendon-bone junction[114-117]. Eliasson et al[113] reported that later during healing, loading was related to a higher expression of extracellular matrix-related and tendon-specific genes, perhaps suggesting that the tissue was to some extent undergoing transformation from scar to tendon regeneration.
Because of the great distraction forces that arise from the muscles, long-lasting devices have been used for reinforcement of the repaired tendon[118-120]. However, prolonged reinforcement adversely influences the healing progress. Current knowledge indicates the appropriate time during the healing process for loading to start, but a suitable method for initiation of the mechanical loading during the healing process needs to be found. In our practice, we use an absorbable reinforcement device for tension regulation at the suture line. The utility of absorbable devices for reinforcement has been mentioned in several studies[121-123]. In two published studies[124,125], we used a reinforcement device made of Vicryl (polyglactin 910) suture, which has initial tensile strength equal or superior to nonabsorbable sutures[126,127]. Thereby, it serves initially as “a suture line tension-relieving suture”. By the fourth postoperative week, the suture loses about 75% of its tensile strength[128]; fortunately, this coincides with the remodeling phase.
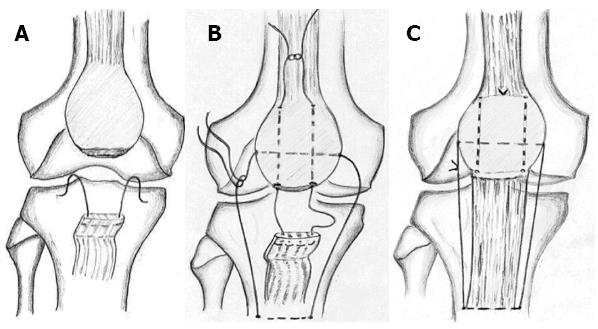
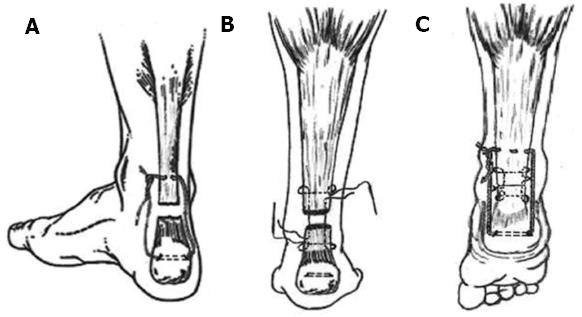
We used the technique for the repair of a fresh rupture of the patellar and Achilles tendon in two groups of patients (Figures 2 and 3). Our philosophy is based on the protection of the tendon callus in the early healing phase. The spontaneous loss of tensile strength of the suture let the callus be exposed to the muscle tone, which continually changes. Continuous change of the muscle tone, theoretically, equals the cyclic mechanical loading in its effect. The group treated for patellar tendon rupture resumed their pre-injury activities at an average of 6.1 mo; knee motion reported no extension lag or flexion deficit and radiologically no patella alta, patella baja or degenerative changes in the patellofemoral joints were noted[124]. The group treated for Achilles tendon rupture returned to pre-injury daily activities by the fourth month and reported no tendon lengthening or reruptures[125]. Moreover, it is doubtless that we can attribute the noticed preservation of thigh and calf girths to our technique. The lack of tension on the immobilized musculotendinous unit is a major factor in the development of atrophy in the muscles[129,130]. This is due to the muscle spindle relaxing and afferent impulses to type-I fibers ceasing. The human soleus muscle contains a high portion of type-I muscle fibers as they are responsible for postural tone and are continually activated while the person is standing[131]. Our technique is designed to protect the suture line and places the muscle fibers under tension as long as the Vicryl suture preserves its tensile strength (Figures 2 and 3). By the end of the fourth week, patients were allowed active joint motion and weight bearing as tolerated.
In addition to alteration of the morphological character, lack of tension in immobilized muscle also alters its physiological properties that appear as a liability to rerupture. In a study of transected sheep Achilles tendons that had spontaneously healed, the rupture force was only 56.7% of normal at twelve months[132]. One possible reason for this is the absence of mechanical loading during the period of immobilization[1]. Immobilization reduces the water and proteoglycan content of tendons and increases the number of reducible collagen cross links[133,134]. Collagen fascicles from stress-shielded rabbit patellar tendons displayed lower tensile strength and strain at failure than control samples[135]. Additionally, as detailed above, lack of tension in the musculotendinous unit leads to structural damage of MTJ[18].
Perception of tendon biology and the biological processes that regulate tendon repair have progressed to a great extent; however, many challenges need to be addressed to bring about a successful treatment strategy. The simplicity of a surgical technique that is based on control of the mechanical loading at the suture line may reduce the requirement for demanding tissue engineering, particularly in simple circumstances. Moreover, this technique may open the way for earlier plaster removal and institution of more vigorous rehabilitation programs; thereby, the morbidity period can be reduced.
| 1. | Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 531] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 2. | Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 279] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 3. | Lemoine JK, Lee JD, Trappe TA. Impact of sex and chronic resistance training on human patellar tendon dry mass, collagen content, and collagen cross-linking. Am J Physiol Regul Integr Comp Physiol. 2009;296:R119-R124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Hansen M, Koskinen SO, Petersen SG, Doessing S, Frystyk J, Flyvbjerg A, Westh E, Magnusson SP, Kjaer M, Langberg H. Ethinyl oestradiol administration in women suppresses synthesis of collagen in tendon in response to exercise. J Physiol. 2008;586:3005-3016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Miller BF, Hansen M, Olesen JL, Schwarz P, Babraj JA, Smith K, Rennie MJ, Kjaer M. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol. 2007;102:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Kirkendall DT, Garrett WE. Function and biomechanics of tendons. Scand J Med Sci Sports. 1997;7:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Jozsa L, Balint JB, Reffy A, Demel Z. Histochemical and ultra structural study of adult human tendon. Acta Histochem. 1979;65:250-257. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Vogel KG. Tendon structure and response to changing mechanical load. J Musculoskelet Neuronal Interact. 2003;3:323-335; discussion 333-334;. [PubMed] |
| 9. | Bozec L, van der Heijden G, Horton M. Collagen fibrils: nanoscale ropes. Biophys J. 2007;92:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Roukis TS, Hurless JS, Page JC. Functional significance of torsion of the tendon of tibialis posterior. J Am Podiatr Med Assoc. 1996;86:156-163. [PubMed] |
| 11. | Stolinski C. Disposition of collagen fibrils in human tendons. J Anat. 1995;186:577-583. [PubMed] |
| 12. | Alexander RM, Bennet-Clark HC. Storage of elastic strain energy in muscle and other tissues. Nature. 1977;265:114-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 436] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Puxkandl R, Zizak I, Paris O, Keckes J, Tesch W, Bernstorff S, Purslow P, Fratzl P. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos Trans R Soc Lond B Biol Sci. 2002;357:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 310] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments--an adaptation to compressive load. J Anat. 1998;193:481-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 498] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Maffulli N. Rupture of the Achilles tendon. J Bone Joint Surg Am. 1999;81:1019-1036. [PubMed] |
| 16. | Tidball JG, Lin C. Structural changes at the myogenic cell surface during the formation of myotendinous junctions. Cell Tissue Res. 1989;257:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Trotter JA. Functional morphology of force transmission in skeletal muscle. A brief review. Acta Anat (Basel). 1993;146:205-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Larkin LM, Calve S, Kostrominova TY, Arruda EM. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 2006;12:3149-3158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Tidball JG. Myotendinous junction: morphological changes and mechanical failure associated with muscle cell atrophy. Exp Mol Pathol. 1984;40:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Trotter JA, Hsi K, Samora A, Wofsy C. A morphometric analysis of the muscle-tendon junction. Anat Rec. 1985;213:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208:471-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 494] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 22. | Lui P, Zhang P, Chan K, Qin L. Biology and augmentation of tendon-bone insertion repair. J Orthop Surg Res. 2010;5:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Toumi H, Higashiyama I, Suzuki D, Kumai T, Bydder G, McGonagle D, Emery P, Fairclough J, Benjamin M. Regional variations in human patellar trabecular architecture and the structure of the proximal patellar tendon enthesis. J Anat. 2006;208:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Langberg H, Bülow J, Kjaer M. Blood flow in the peritendinous space of the human Achilles tendon during exercise. Acta Physiol Scand. 1998;163:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Edwards DA. The blood supply and lymphatic drainage of tendons. J Anat. 1946;80:147-152. [PubMed] |
| 26. | Hooper G, Davies R, Tothill P. Blood flow and clearance in tendons. Studies with dogs. J Bone Joint Surg Br. 1984;66:441-443. [PubMed] |
| 27. | Matthews P. The fate of isolated segments of flexor tendons within the digital sheath--a study in synovial nutrition. Br J Plast Surg. 1976;29:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Manske PR, Lesker PA. Nutrient pathways of flexor tendons in primates. J Hand Surg Am. 1982;7:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 61] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Ackermann PW, Li J, Finn A, Ahmed M, Kreicbergs A. Autonomic innervation of tendons, ligaments and joint capsules. A morphologic and quantitative study in the rat. J Orthop Res. 2001;19:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Jozsa LG, Kannus P. Human Tendons: Anatomy, Physiology, and Pathology. Champaign (IL): Human Kinetics 1997; 3-96. |
| 31. | Zaffagnini S, Golanò P, Farinas O, Depasquale V, Strocchi R, Cortecchia S, Marcacci M, Visani A. Vascularity and neuroreceptors of the pes anserinus: anatomic study. Clin Anat. 2003;16:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Benjamin M, Redman S, Milz S, Büttner A, Amin A, Moriggl B, Brenner E, Emery P, McGonagle D, Bydder G. Adipose tissue at entheses: the rheumatological implications of its distribution. A potential site of pain and stress dissipation? Ann Rheum Dis. 2004;63:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Vincent J. Structural biomaterials. Princeton: Princeton University Press 1990; 56-62. |
| 34. | Diamant J, Keller A, Baer E, Litt M, Arridge RG. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci. 1972;180:293-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 295] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Misof K, Rapp G, Fratzl P. A new molecular model for collagen elasticity based on synchrotron X-ray scattering evidence. Biophys J. 1997;72:1376-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 96] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Fallon J, Blevins FT, Vogel K, Trotter J. Functional morphology of the supraspinatus tendon. J Orthop Res. 2002;20:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Screen HR, Lee DA, Bader DL, Shelton JC. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc Inst Mech Eng H. 2004;218:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 368] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Sun Y, Berger EJ, Zhao C, An KN, Amadio PC, Jay G. Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res. 2006;47:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (Oxford). 2006;45:508-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 273] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 41. | O’Brien M. Functional anatomy and physiology of tendons. Clin Sports Med. 1992;11:505-520. [PubMed] |
| 42. | Kastelic J, Baer E. Deformation in tendon collagen. Symp Soc Exp Biol. 1980;34:397-435. [PubMed] |
| 43. | Folkhard W, Mosler E, Gercken W, Knörzer E, Nemetschek-Gansler H, NemetschekTh . Quantitative analysis of the molecular sliding mechanism in native tendon collagen: time resolved dynamic studies using synchrotron radiation. Int J Biol Macromol. 1987;9:169-175. [RCA] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S. Fibrillar structure and mechanical properties of collagen. J Struct Biol. 1998;122:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 423] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 45. | Miller BF, Olesen JL, Hansen M, Døssing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 448] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 46. | Evans CE, Trail IA. An in vitro comparison of human flexor and extensor tendon cells. J Hand Surg Br. 2001;26:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Barkhausen T, van Griensven M, Zeichen J, Bosch U. Modulation of cell functions of human tendon fibroblasts by different repetitive cyclic mechanical stress patterns. Exp Toxicol Pathol. 2003;55:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Waggett AD, Benjamin M, Ralphs JR. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur J Cell Biol. 2006;85:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Wang JH, Li Z, Yang G, Khan M. Repetitively stretched tendon fibroblasts produce inflammatory mediators. Clin Orthop Relat Res. 2004;243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 51. | Koshima H, Kondo S, Mishima S, Choi HR, Shimpo H, Sakai T, Ishiguro N. Expression of interleukin-1beta, cyclooxygenase-2, and prostaglandin E2 in a rotator cuff tear in rabbits. J Orthop Res. 2007;25:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, Almekinders L, Bynum D, Yang X, Banes AJ. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res. 2003;21:256-264. [RCA] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 292] [Article Influence: 12.7] [Reference Citation Analysis (4)] |
| 53. | Qi J, Fox AM, Alexopoulos LG, Chi L, Bynum D, Guilak F, Banes AJ. IL-1beta decreases the elastic modulus of human tenocytes. J Appl Physiol. 2006;101:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Wong MW, Tang YN, Fu SC, Lee KM, Chan KM. Triamcinolone suppresses human tenocyte cellular activity and collagen synthesis. Clin Orthop Relat Res. 2004;277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Wong MW, Tang YY, Lee SK, Fu BS. Glucocorticoids suppress proteoglycan production by human tenocytes. Acta Orthop. 2005;76:927-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Scutt N, Rolf CG, Scutt A. Glucocorticoids inhibit tenocyte proliferation and Tendon progenitor cell recruitment. J Orthop Res. 2006;24:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 57. | Xia W, Szomor Z, Wang Y, Murrell GA. Nitric oxide enhances collagen synthesis in cultured human tendon cells. J Orthop Res. 2006;24:159-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Flick J, Devkota A, Tsuzaki M, Almekinders L, Weinhold P. Cyclic loading alters biomechanical properties and secretion of PGE2 and NO from tendon explants. Clin Biomech (Bristol, Avon). 2006;21:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Szomor ZL, Appleyard RC, Murrell GA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res. 2006;24:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Darmani H, Crossan JC, Curtis A. Single dose of inducible nitric oxide synthase inhibitor induces prolonged inflammatory cell accumulation and fibrosis around injured tendon and synovium. Mediators Inflamm. 2004;13:157-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Franchi M, Quaranta M, De Pasquale V, Macciocca M, Orsini E, Trirè A, Ottani V, Ruggeri A. Tendon crimps and peritendinous tissues responding to tensional forces. Eur J Histochem. 2007;51 Suppl 1:9-14. [PubMed] |
| 62. | Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol. 2003;88:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Kubo K, Kanehisa H, Kawakami Y, Fukanaga T. Growth changes in the elastic properties of human tendon structures. Int J Sports Med. 2001;22:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Kubo K, Kanehisa H, Ito M, Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. J Appl Physiol. 2001;91:26-32. [PubMed] |
| 65. | Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Effects of repeated muscle contractions on the tendon structures in humans. Eur J Appl Physiol. 2001;84:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Elsalanty M, Makarov M, Cherkashin A, Birch J, Samchukov M. Changes in pennate muscle architecture after gradual tibial lengthening in goats. Anat Rec (Hoboken). 2007;290:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507-1525. [PubMed] |
| 68. | Dent CM, Graham GP. Osteogenesis imperfecta and Achilles tendon rupture. Injury. 1991;22:239-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Dodds WN, Burry HC. The relationship between Achilles tendon rupture and serum uric acid level. Injury. 1984;16:94-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Maffulli N, Irwin AS, Kenward MG, Smith F, Porter RW. Achilles tendon rupture and sciatica: a possible correlation. Br J Sports Med. 1998;32:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 71. | Hall MM, Finnoff JT, Smith J. Musculoskeletal complications of fluoroquinolones: guidelines and precautions for usage in the athletic population. PMR. 2011;3:132-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Newnham DM, Douglas JG, Legge JS, Friend JA. Achilles tendon rupture: an underrated complication of corticosteroid treatment. Thorax. 1991;46:853-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 73. | Hugate R, Pennypacker J, Saunders M, Juliano P. The effects of intratendinous and retrocalcaneal intrabursal injections of corticosteroid on the biomechanical properties of rabbit Achilles tendons. J Bone Joint Surg Am. 2004;86-A:794-801. [PubMed] |
| 74. | Kapetanos G. The effect of the local corticosteroids on the healing and biomechanical properties of the partially injured tendon. Clin Orthop Relat Res. 1982;170-179. [PubMed] |
| 75. | Clancy WG, Neidhart D, Brand RL. Achilles tendonitis in runners: a report of five cases. Am J Sports Med. 1976;4:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci. 1978;203:305-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 366] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 77. | Järvinen TA, Järvinen TL, Kannus P, Józsa L, Järvinen M. Collagen fibres of the spontaneously ruptured human tendons display decreased thickness and crimp angle. J Orthop Res. 2004;22:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Wang JH. Mechanobiology of tendon. J Biomech. 2006;39:1563-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 578] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 79. | James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 354] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 80. | Woo SL, Hildebrand K, Watanabe N, Fenwick JA, Papageorgiou CD, Wang JH. Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res. 1999;S312-S323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 161] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Lindsay WK, Birch JR. The fibroblast in flexor tendon healing. Plast Reconstr Surg. 1964;34:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Garner WL, McDonald JA, Koo M, Kuhn C, Weeks PM. Identification of the collagen-producing cells in healing flexor tendons. Plast Reconstr Surg. 1989;83:875-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Fenwick SA, Hazleman BL, Riley GP. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002;4:252-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 252] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 85. | Liu SH, Yang RS, al-Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing. A current review. Clin Orthop Relat Res. 1995;265-278. [PubMed] |
| 86. | Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 854] [Cited by in RCA: 841] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 87. | Nedelec B, Ghahary A, Scott PG, Tredget EE. Control of wound contraction. Basic and clinical features. Hand Clin. 2000;16:289-302. [PubMed] |
| 88. | Kolodney MS, Wysolmerski RB. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol. 1992;117:73-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 335] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 89. | Takayama Y, Mizumachi K. Effects of lactoferrin on collagen gel contractile activity and myosin light chain phosphorylation in human fibroblasts. FEBS Lett. 2001;508:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403-1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1294] [Cited by in RCA: 1296] [Article Influence: 43.2] [Reference Citation Analysis (10)] |
| 91. | Eastwood M, Porter R, Khan U, McGrouther G, Brown R. Quantitative analysis of collagen gel contractile forces generated by dermal fibroblasts and the relationship to cell morphology. J Cell Physiol. 1996;166:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 92. | Burridge K. Are stress fibres contractile? Nature. 1981;294:691-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 169] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 1062] [Article Influence: 42.5] [Reference Citation Analysis (12)] |
| 94. | Benjamin M, Ralphs JR. The cell and developmental biology of tendons and ligaments. Int Rev Cytol. 2000;196:85-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 95. | Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1053] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 96. | Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 2003;36:1529-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 370] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 97. | Yang G, Crawford RC, Wang JH. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 2004;37:1543-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 98. | Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur J Appl Physiol. 2001;86:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 99. | Wang JH. Substrate deformation determines actin cytoskeleton reorganization: A mathematical modeling and experimental study. J Theor Biol. 2000;202:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Wang JH, Goldschmidt-Clermont P, Wille J, Yin FC. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech. 2001;34:1563-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 228] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 101. | Janmey PA. Mechanical properties of cytoskeletal polymers. Curr Opin Cell Biol. 1991;3:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 113] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 102. | Ingber D. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 458] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 103. | Wang N, Ingber DE. Probing transmembrane mechanical coupling and cytomechanics using magnetic twisting cytometry. Biochem Cell Biol. 1995;73:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 148] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 104. | Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7430] [Cited by in RCA: 7414] [Article Influence: 218.1] [Reference Citation Analysis (0)] |
| 105. | Shyy JY, Chien S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr Opin Cell Biol. 1997;9:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 244] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 106. | Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci USA. 2001;98:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 264] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 107. | Lehoux S, Tedgui A. Cellular mechanics and gene expression in blood vessels. J Biomech. 2003;36:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 108. | Iwasaki H, Eguchi S, Ueno H, Marumo F, Hirata Y. Mechanical stretch stimulates growth of vascular smooth muscle cells via epidermal growth factor receptor. Am J Physiol Heart Circ Physiol. 2000;278:H521-H529. [PubMed] |
| 109. | Arnoczky SP, Tian T, Lavagnino M, Gardner K, Schuler P, Morse P. Activation of stress-activated protein kinases (SAPK) in tendon cells following cyclic strain: the effects of strain frequency, strain magnitude, and cytosolic calcium. J Orthop Res. 2002;20:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 110. | Sackin H. Mechanosensitive channels. Annu Rev Physiol. 1995;57:333-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 194] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 111. | Diamond SL, Sachs F, Sigurdson WJ. Mechanically induced calcium mobilization in cultured endothelial cells is dependent on actin and phospholipase. Arterioscler Thromb. 1994;14:2000-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. Int Orthop. 2007;31:783-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 113. | Eliasson P, Andersson T, Aspenberg P. Rat Achilles tendon healing: mechanical loading and gene expression. J Appl Physiol. 2009;107:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 114. | Buckwalter JA, Cruess RL. Healing of the musculoskeletal tissues. In: Rockwood CA Jr, Green DP (editors) Fractures in adults. J.B. Lippincott, Philadelphia 1991; 203-205. |
| 115. | Hibino N, Hamada Y, Sairyo K, Yukata K, Sano T, Yasui N. Callus formation during healing of the repaired tendon-bone junction. A rat experimental model. J Bone Joint Surg Br. 2007;89:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med. 2002;36:239-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 262] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 117. | Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795-1803. [PubMed] |
| 118. | Larson RV, Simonian PT. Semitendinosus augmentation of acute patellar tendon repair with immediate mobilization. Am J Sports Med. 1995;23:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Lindy PB, Boynton MD, Fadale PD. Repair of patellar tendon disruptions without hardware. J Orthop Trauma. 1995;9:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 120. | Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63:932-937. [PubMed] |
| 121. | Nebelung W, Becker R, Urbach D, Röpke M, Roessner A. Histological findings of tendon-bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch Orthop Trauma Surg. 2003;123:158-163. [PubMed] |
| 122. | Newsham-West R, Nicholson H, Walton M, Milburn P. Long-term morphology of a healing bone-tendon interface: a histological observation in the sheep model. J Anat. 2007;210:318-327. [PubMed] |
| 123. | Singha BI, Sinhaa S, Singha S, Shrivastavaa R, Mandaliab VI. Stress fracture patella following patella tendon repair. Injury Extra. 2004;35:13-16 Available from: http://www.sciencedirect.com/science/article/pii/S1572346103000084. |
| 124. | Massoud EI. Repair of fresh patellar tendon rupture: tension regulation at the suture line. Int Orthop. 2010;34:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 125. | Massoud EI. Repair of fresh open tear of Achilles tendon Tension regulation at the suture line. Foot Ankle Surg. 2011;17:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 126. | Bourne RB, Bitar H, Andreae PR, Martin LM, Finlay JB, Marquis F. In-vivo comparison of four absorbable sutures: Vicryl, Dexon Plus, Maxon and PDS. Can J Surg. 1988;31:43-45. [PubMed] |
| 127. | Singha BI, Sinhaa S, Singha S, Shrivastavaa R, Mandaliab VI. Stress fracture patella following patella tendon repair. Injury Extra. 2004;35:13-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 128. | Greenwald D, Shumway S, Albear P, Gottlieb L. Mechanical comparison of 10 suture materials before and after in vivo incubation. J Surg Res. 1994;56:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 146] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 129. | Cetti R, Christensen SE, Ejsted R, Jensen NM, Jorgensen U. Operative versus nonoperative treatment of Achilles tendon rupture. A prospective randomized study and review of the literature. Am J Sports Med. 1993;21:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 377] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 130. | Rettig AC, Liotta FJ, Klootwyk TE, Porter DA, Mieling P. Potential risk of rerupture in primary achilles tendon repair in athletes younger than 30 years of age. Am J Sports Med. 2005;33:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 131. | Häggmark T, Eriksson E. Hypotrophy of the soleus muscle in man after achilles tendon rupture. Discussion of findings obtained by computed tomography and morphologic studies. Am J Sports Med. 1979;7:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 1.6] [Reference Citation Analysis (9)] |
| 132. | Bruns J, Kampen J, Kahrs J, Plitz W. Achilles tendon rupture: experimental results on spontaneous repair in a sheep-model. Knee Surg Sports Traumatol Arthrosc. 2000;8:364-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 133. | Akeson WH, Woo SL, Amiel D, Coutts RD, Daniel D. The connective tissue response to immobility: biochemical changes in periarticular connective tissue of the immobilized rabbit knee. Clin Orthop Relat Res. 1973;356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 138] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 134. | Akeson WH, Amiel D, Mechanic GL, Woo SL, Harwood FL, Hamer ML. Collagen cross-linking alterations in joint contractures: changes in the reducible cross-links in periarticular connective tissue collagen after nine weeks of immobilization. Connect Tissue Res. 1977;5:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 122] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 135. | Yamamoto E, Hayashi K, Yamamoto N. Mechanical properties of collagen fascicles from stress-shielded patellar tendons in the rabbit. Clin Biomech (Bristol, Avon). 1999;14:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
P- Reviewer Song GB S- Editor Song XX L- Editor Roemmele A E- Editor Wu HL