Revised: May 31, 2012
Accepted: July 10, 2012
Published online: July 18, 2012
This article summarizes reconstruction options available for acetabular revision following total hip arthroplasty. A thoughtful methodology to the evaluation and treatment of patients with implant failure after joint replacement is essential to guarantee accurate diagnoses, appropriate triage to reconstruction options, and optimal clinical outcomes. In the majority of patients who undergo acetabular revision, factors such as bone loss and pelvic discontinuity provide a challenge in the selection and implementation of the proper reconstruction option. With advanced evaluation algorithms, imaging techniques, and implant designs, techniques have evolved to rebuild the compromised acetabulum at the time of revision surgery. However, clinical outcomes data for these techniques continue to lag behind the exponential increase in revision hip arthroplasty cases predicted to occur over the next several years. We encourage those involved in the treatment of patients undergoing hip replacement surgery to participate in well-designed clinical studies to enhance evidence-based knowledge regarding revision acetabular reconstruction options.
- Citation: Taylor ED, Browne JA. Reconstruction options for acetabular revision. World J Orthop 2012; 3(7): 95-100
- URL: https://www.wjgnet.com/2218-5836/full/v3/i7/95.htm
- DOI: https://dx.doi.org/10.5312/wjo.v3.i7.95
Over the past several decades, total hip arthroplasty has become recognized as an effective treatment option for the reduction of pain and disability associated with advanced degenerative arthritis with successful clinical outcomes. As population factors, such as aging and obesity, drive the demand for primary hip arthroplasty, the demand for revision joint replacement will continue to rise as well. Recent data analyses have shown that in addition to the substantial increase in prevelance of primary total hip arthoplasty procedures, the rates of revision procedures are expected to rise as well[1]. Despite attempts to improve implant design and evade the previous pitfalls of early reconstruction techniques, the prevalence of revision hip arthroplasty cases has not declined[2]. In the United States, 46 000 hip revisions were performed in 2004 and this number is expected to be more than double by 2030[3].
In 2009, Bozic et al[4] released data detailing the causes of revision total hip arthroplasty. Their review demonstrated the most common causes of total hip revision, regardless of component, included instability/dislocation, mechanical loosening, and infection. Isolated acetabular component revision comprised 12.7% of all revision hip procedures and instability/dislocation was reported as the most common indication. As such, a surgeon’s pre-operative planning and understanding of suitable reconstruction options for acetabular revision is essential for the growing population of patients who will undergo total hip replacement.
There is a wide spectrum of signs of symptoms that can occur in the setting of acetabular component failure. Pain is a common presenting complaint and often times groin pain can represent acetabular component failure while thigh pain may be correlated to femoral component failure. Clinical patient presentation ultimately depends on the underlying cause, whether it be infection, polyethylene wear, instability, or aseptic loosening. The steps towards comprehensive evaluation of a painful total hip have been described in the arthroplasty literature, and these guidelines must be implemented to eliminate systemic or infectious etiologies that could preclude a definitive single-stage reconstruction of the acetabulum[5]. Leg-length discrepancy, joint deformity, location of prior incisions, functional status and baseline neurologic deficits should be detected and documented during the pre-operative evaluation as well. It is important to note that the patient population in this setting could be older with osteopenia, compromised soft tissues, and multiple medical comorbidities.
In addition to obtaining a good history and physical examination data, radiographic and advanced imaging modalities are useful in defining the extent and location of bone loss associated with acetabular component failure. Anterior-posterior pelvis radiographs and frog-leg lateral views of the involved hip can be supplemented with Judet views for evaluation of the acetabular columns. They can also give clues to the underlying cause of the component failure. Three-dimensional computed tomography is often helpful in quantifying the presence and severity of osteolytic lesions. This is especially important in the setting of prior revision hip surgery or prior irradiation where radiographs may under-estimate the amount of bone loss that is present. The information obtained from these studies assists in classifying the extent of the acetabular defects, thereby guiding treatment options. Preoperative computed tomography (CT) angiogram of iliac vessels is advised when protrusion of the failed acetabular component past Kohler’s line is substantial.
In orthopaedics, classifications are judged on their reliability, reproducibility, and ability to guide treatment plans and predict outcomes. With regard to revision hip arthroplasty, classification systems for acetabular defects have been used to present the severity of bone loss that will likely be found intra-operatively, allowing for appropriate selection of reconstructive options[6]. In 1989, D’Antonio et al[7] first described what is now commonly known as the American Academy of Orthopaedic Surgeons (AAOS) classification system of acetabular abnormalities after total hip replacement. The AAOS classification system distinguishes between segmental and cavitary defects, and also subdivides the presence of pelvic discontinuity. Though widely used in the literature, this classification system does not account for the location or size of acetabular defects. A decade after the introduction of the AAOS classification system, Saleh et al[8] released results validating the Gross classification system, which quantified the extent of contained versus uncontained bone loss and implications related to use of morselized bone graft during revision reconstruction.
Perhaps the most widely cited and clinically implemented system, the Paprosky Classification was developed to establish acetabular defect type, size, and location for a collective guidance towards the selection of appropriate reconstructive options for revision surgery. Developed from a systematic review of bone loss seen in 147 failed acetabuli, this system was based on four radiographic measures obtained from an anterior-posterior radiograph of the pelvis: superior hip center migration, ischialosteolysis, the position of the implant relative to the Kohler (ilioischial) line, and teardrop osteolysis (Table 1). Unique to this methodology, defects were classified by type to indicate whether the remaining acetabular structures are completely supportive (Type I), incompletely supportive (Type II), or unsupportive (Type III) of an implanted component. In the original study, reconstructive guidelines for allograft selection were determined by the extent of remaining structural support according to defect type. Today, this classification system continues to provide a useful treatment algorithm, even with the availability of a wider variety of modular metal augmentation and reconstruction options.
| Description | ||||
| Type | Superior hip center migration | Is chialosteolysis | Kohler line | Teardrop |
| I | Minimal | None | Intact | Intact |
| IIA | Mild | Mild | Intact | Intact |
| IIB | Moderate | Mild | Intact | Intact |
| IIC | Mild | Mild | Disrupted | Moderate lysis |
| IIIA | Severe | Moderate | Intact | Moderate lysis |
| IIIB | Severe | Severe | Disrupted | Severe lysis |
Treatment of acetabular component failure and associated bone defects depends on patient characteristics, the degree and location of bone loss, the ability of the columns to support biologic fixation, and the presence of pelvic discontinuity. The ultimate goal of revision acetabular reconstruction should be to obtain stable fixation and restore the hip center[5]. The various traditional and newer revision options for the acetabular component are discussed below and outlined in Table 2.
| Acetabular revision option | Clinical pearls |
| Isolated liner exchange | The stability and orientation of the acetabular metal component should be confirmed at the time of revision, liner may be cemented if needed |
| Hemispheric porous-coated cup | May be used in conjunction with adjunct techniques of bone grafting, screw fixation recommended |
| Highly porous metal cup | Appears to be effective in achieving biologic fixation in cases of severe bone defects, augments may be used for structural support, cup-cage construct can be used to offload cup |
| Antiprotrusio cage | Useful in cases of severe bone defects or pelvic discontinuity, spans areas of healthy host bone and accommodates bone grafting deep to the cage, relies on mechanical fixation alone |
| Customized triflange implant | Requires several weeks or month to obtain implant, serves as a good salvage option in cases of catastrophic bone loss and discontinuity, may achieve biologic fixation |
The technique of isolated polyethylene liner exchange is useful in the setting of substantial polyethylene wear and osteolysis with evidence of a stable acetabular component. Previous studies have demonstrated the relationship between polyethylene wear and progressive osteolysis with compromised bone stock[9]. Liner exchange with highly cross-linked polyethylene has been shown to decrease average wear rates significantly[10].
To justify isolated liner exchange, the modular metallic shell should be well-fixed and appropriately oriented[11]. This should be evaluated both pre-operatively and intra-operatively. Once the stability of the acetabular prosthesis is confirmed and liner exchange is contemplated, it is then important to consider the adequacy of the locking mechanism between the liner and the metallic shell. If the locking mechanism is compromised, one may consider cementing a new liner into the fixed metallic shell to prevent micromotion between the two surfaces for primary fixation. The clinical track-record and historical performance of the implant should be considered along with the available liner and head size options offered by that particular component.
Historically, treatment options for acetabular component instability or malposition included use of cemented acetabular all-polyethylene prostheses implanted with the same techniques that had been employed for the primary arthroplasty procedure. The results of the cemented revision procedures were poor, resulting from mechanical failure secondary to poor cement interdigitation and fixation leading to excessive micromotion within the acetabular bed[12]. In contrast, hemispheric metal cups with porous coating and associated techniques have been developed that encourage bone in-growth and held the promise of durable biologic fixation.
Cementess hemispherical porous-coated implants are the most commonly used implants for acetabular reconstruction in North America. With supportive and viable host bone and a reliable in growth surface, the cups address most revision problems encountered. Initial stability is provided with a press-fit and screw fixation. Cavitary defects are addressed with morselized bone graft.
These components are acceptable for patients who have not shown evidence of hip center migration or significant pelvic discontinuity (Paprosky types I, IIA and IIB), which can be assessed pre-operatively as well as intra-operatively[5]. It is generally accepted that at least 50% of the bone stock must be present to support the cup. Internal fixation with screws is also advocated to supplement the in-growth of the press-fit component. When there are focal superior segmental or cavitary defects identified at that time of revision, modular metallic augments, structural allograft, or morselized impacted allograft[13] may be added to supplement the acetabular bed. Care must be taken to maintain the appropriate orientation of the revision cup despite the presence of augments in the dome. Park et al[14] recently published long-term data from their cohort of patients who underwent revision hip arthroplasty with use of a cememntlessacetabular shell. In this group, survivorship, with revision of the shell for aspetic loosening or evidence of loosening as the endpoint, was 95% at 20-year follow-up.
Jumbo cups following allografting for focal defects also have a role in acetabular revision surgery. There is no universally-acceted definition of what diameter defined the jumbo cup. Jumbo cups are loosely defined by the ratio of component size to the pelvis and the hip joint, as compared to the size of the original implant[15]. These jumbo cups offer numerous advantages in regards to maximizing the contact area between the cup and host bone when a larger reamer is necessary to establish rim contact. The larger components can also accommodate larger femoral heads, reducing the rate of dislocation. A large mismatch between a large shell and a small femoral head may increase the rate of impingement and reduce the soft tissue constraints to dislocation and is to be avoided. Another potential disadvantage with the jumbo cup comes with displacement of the femoral head hip center into a lateral inferior position, which has been reported. Nevertheless, satisfactory mid-term results and survivorship are documented with use of acetabular jumbo cups in revision arthroplasty, with survival rates as high 92% at 14 years[16,17].
High hip center placement of an uncementedacetabular hemispheric component is another option when there is a defect in the superolateral dome or posterior column that precludes the standard placement of a hemispheric shell in a more anatomic location. To accommodate the defect, the shell is placed in a more superior position. Accordingly, it is often necessary to do concomitant procedures at the time of revision to ensure that soft tissue tension and appropriate leg lengths are restored. As superior placement of the hip center can also be associated with lateralization of the component, there have been some reports of increased dislocation or loosening rates with high hip center placement[16]. A high hip center is also disadvantageous from a biomechanical standpoint and will typically result in a limp. Hip stability may be compromised due to the small head size and bony impingement. A long term follow-up study by Hendricks et al, however, reported survivorship of 89% after 15 years in their cohort of patients who underwent high placement of noncementedacetabular components[18].
A traditional contraindication to use of a cementless hemisphere revision component was recent pelvis irradiation, although preliminary reports of successful results using newer porous metal technology suggests that this recommendation may need to be revisited. The presence of pelvic discontinuity and severe bone loss (Paprosky type IIC or III) may warrant the use of techniques that can provide more stability for the implant in the setting of compromised bone stock.
In recent years, highly porous metal components have become popular options for both primary and revision arthroplasty procedures. Tantalum implants (Trabecular Metal, Zimmer, Inc, Warsaw, Indiana, United States) were developed to provide increased porosity and a trabecular bone-like configuration to allow for rapid and extensive bone in growth along with good initial stability in bone. Some designs incorporate a locking mechnism for the polyethylene insert, whereas others require a cemented polyethylene liner, which allows for placement of the shell in the areas of large defects and compensatory orientation of the cemented liner to re-establish femoral head coverage and hip stability.
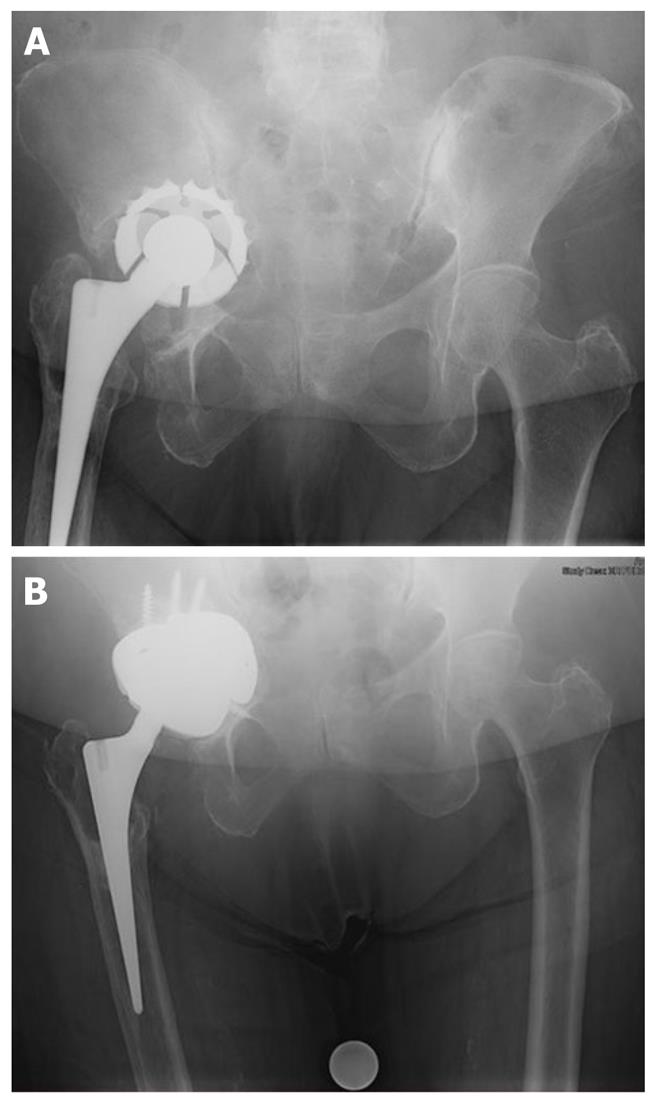
Modular revision systems that use porous metal augments have been developed. These augments are assembled intraoperatively based upon the defects encountered and act like structural bone graft substitutes (Figure 1). The cup may also be supplemented with a cage fixed into the ilium (the so-called “cup-cage” construct) to offload the porous metal cup to allow time for bony in growth and cup stabilization.
A large published series by Skytta et al[19] reviewed the surgical short-term results of 827 revisions performed with the Trabecular Metal acetabular shell. After 3 year follow-up, the overall survivorship was 92% with the rates of aseptic loosening documented as 2%. In another retrospective series comparing titanium and tantalum cups, similar results in hip revision cases with minor bone deficiencies (Paprosky I, IIA, and IIB) were demonstrated[20]. However, the performance of the two implants differed significantly in the cases associated with severe bone loss (Paprosky IIC and III), with 12% of tantalum cups and 24% of titanium cups demonstrating evidence of loosening and failure. To investigate this distinction further, Fernandes et al[21] performed a retrospective review to evaluate the outcome of TM acetabular components used in revision cases with major bone deficiency. They demonstrated satisfactory mid-term results with only 1/46 patients showing evidence of loosening over an average follow-up of 50 mo.
The armamentarium for treatment large bone defects (Paprosky IIC or III) has traditionally included antriprotrusio cages. These expansile implants are indicated for cases in which stability cannot be obtained with an uncementedhemispheric cup or in situations where the remaining host bone is too compromised to achieve biologic fixation of a porous implant. Antiprotrusio cages provide a larger contact area between the remaining host bone and the implants, which potentially reduces the likelihood of implant migration. Current implant designs also allow for concomitant treatment of bone defects with either morselized or bulk allograft materials protected by the cage construct. Use of antiprotrusio cages requires wide surgical exposure as they span the acetabular defects or area of discontinuity. Solid fixation into the posterior column is essential and, in the most severe cases, additional internal fixation with posterior column plating may be warranted.
In the setting of combined segmental and cavitary defects, impaction bone grafting with compressed particulate graft used in conjunction with an antiprotrusiocage construct has shown successful clinical results. With this construct, the healthy host bone is bridged by the cage implant, which protects the grafted area while consolidation and reconstitution of the acetabular bed occurs[22]. In one original study, a review of patients with combined segmental and cavitary defects (Paprosky III) treated with an antiprotrusio cage and allograft demonstrated radiographic remodeling of the graft behind the cages at 5-year follow-up[23]. The rate of aspetic failure was 12% in this group, which exceeded the historial results with these implants. The increased rate of loosening and need for revision in these cases, compared with other acetabular reconstruction options, is likely multifactorial and includes the increased severity of discontinuity and defect found in the patients for whom use of a cage is indicated. This construct does rely on mechanical fixation alone with no potential for long-term biologic incorporation.
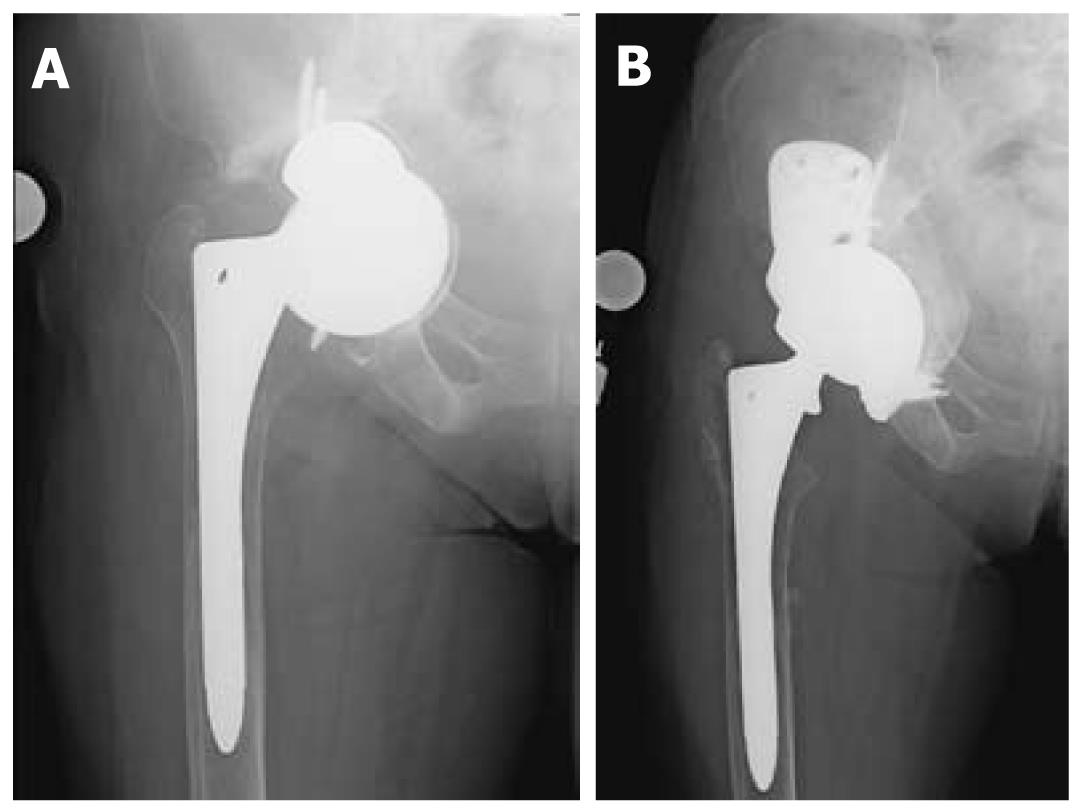
Custom triflangeacetabular prostheses are indicated for the treatment of massive acetabuluar bone loss and pelvic discontinuity, situations where the amount of bone loss exceeds the limits of defect-matching technqiues (Figure 2)[16]. They are also considered as reconstructive options when the host bone stock has been compromised by radiation. The implant is customized from data obtained from 3-dimensional CT reconstruction imaging, which details the degree and location of bone loss as well as the orientation of the pelvic dissociation. Accordingly, the time needed to design, manufacture, and sterilize these prostheses can take up to several months and must be taken into consideration during the pre-operative planning process. Modern triflange cups encorporate porous ingrowth surfaces to encourage biologic fixation to host bone. The high cost of these implants and lack of intraoperative modularity is a consideration. Many surgeons consider the use of the custom triflange implant as a final salvage procedure when there is catastrophic bone loss.
In 2007, DeBoer et al[24] published the results of their study of 30 hips with failed hip arthroplasty and pelvic discontinuity treated with custom manufactured acetabular prostheses. The authors found definite radiographic healing of the discontinuity without evidence of implant migration or screw breakage at the mean ten-year follow-up. They documented a marked improvement in Harris hip scores and stability of the implant over the years of follow-up. The dislocation rate was 16%, however no revisions were required in their study group. Christie et al[25] also published results from a retrospective review of 76 hips reconstructed with custom triflange prostheses. In their group, re-operation occurred in 7.8% of their patients for dislocation, but no triflange components had to be removed. Almost all patients showed radiographic evidence of remodeling and there was marked improvement in the Harris hip scores.
Contraindications to use of custom triflange prostheses include urgent clinical situations that do not allow for the wait period required to manufacture the customized implant. In addition, given the complexity of the prosthesis, the technical challenge and the extensive surgical exposure required for its implantation, custom triflange prostheses should not be used in cases where defect-matching techniques can be employed and less complex and costly acetabular reconstruction options are suitable.
The goals of acetabular revision are to extract failed implants with minimal host tissue and bone destruction, implant an acetabular prosthesis that will provide durable function and lasting pain relief, and to address osseous defects or dissociation by effectively restoring bone stock. From acetabular bone loss, polyethylene wear and osteolysis, to catastrophic pelvic discontinuity, there is now a spectrum of reconstruction options that allows for consideration of patient factors and the condition of the acetabular bed to guide the treatment algorithm. Requirements for a successful and durable long-term result include supportive host bone and stable implants. With the improvements being made in currently available biomaterials and implant designs, there is still a significant amount of research that needs to be done in the form of well-designed clinical studies to ensure that we are providing the optimal services to the growing number of patients that will stand in need of revision reconstruction in the future.
| 1. | Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 643] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 2. | Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Dunbar MJ, Howard A, Bogoch ER, Parvizi J, Kreder HJ. Orthopaedics in 2020: predictors of musculoskeletal need. J Bone Joint Surg Am. 2009;91:2276-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1284] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 5. | Bozic KJ, Rubash HE. The painful total hip replacement. Clin Orthop Relat Res. 2004;420:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Sporer SM. How to do a revision total hip arthroplasty: revision of the acetabulum. J Bone Joint Surg Am. 2011;93:1359-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | D'Antonio JA, Capello WN, Borden LS, Bargar WL, Bierbaum BF, Boettcher WG, Steinberg ME, Stulberg SD, Wedge JH. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res. 1989;243:126-137. [PubMed] |
| 8. | Saleh KJ, Holtzman J, Gafni ASaleh L, Jaroszynski G, Wong P, Woodgate I, Davis A, Gross AE. Development, test reliability and validation of a classification for revision hip arthroplasty. J Orthop Res. 2001;19:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Naudie DD, Engh CA. Surgical management of polyethylene wear and pelvic osteolysis with modular uncemented acetabular components. J Arthroplasty. 2004;19:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Terefenko KM, Sychterz CJ, Orishimo K, Engh CA. Polyethylene liner exchange for excessive wear and osteolysis. J Arthroplasty. 2002;17:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Lombardi AV, Berend KR. Isolated acetabular liner exchange. J Am Acad Orthop Surg. 2008;16:243-248. [PubMed] |
| 12. | Petera P, Rubash H. Revision Total Hip Arthoplasty: The Acetabular component. J Am Acad Orthop Surg. 1995;3:15-21. |
| 13. | Lee JM, Nam HT. Acetabular revision total hip arthroplasty using an impacted morselized allograft and a cementless cup: minimum 10-year follow-up. J Arthroplasty. 2011;26:1057-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Park DK, Della Valle CJ, Quigley L, Moric M, Rosenberg AG, Galante JO. Revision of the acetabular component without cement. A concise follow-up, at twenty to twenty-four years, of a previous report. J Bone Joint Surg Am. 2009;91:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Ito H, Matsuno T, Aoki Y, Minami A. Acetabular components without bulk bone graft in revision surgery: A 5- to 13-year follow-up study. J Arthroplasty. 2003;18:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Wedemeyer C, Neuerburg C, Heep H, von Knoch F, von Knoch M, Löer F, Saxler G. Jumbo cups for revision of acetabular defects after total hip arthroplasty: a retrospective review of a case series. Arch Orthop Trauma Surg. 2008;128:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Brown T, Cui Q, Mihalko W, Saleh K; Arthritis and Arthoplasty: The Hip. Elsevier Health Sciences; 2009. . |
| 18. | Hendricks KJ, Harris WH. High placement of noncemented acetabular components in revision total hip arthroplasty. A concise follow-up, at a minimum of fifteen years, of a previous report. J Bone Joint Surg Am. 2006;88:2231-2236. [PubMed] |
| 19. | Skyttä ET, Eskelinen A, Paavolainen PO, Remes VM. Early results of 827 trabecular metal revision shells in acetabular revision. J Arthroplasty. 2011;26:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Jafari SM, Bender B, Coyle C, Parvizi J, Sharkey PF, Hozack WJ. Do tantalum and titanium cups show similar results in revision hip arthroplasty? Clin Orthop Relat Res. 2010;468:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Davies JH, Laflamme GY, Delisle J, Fernandes J. Trabecular metal used for major bone loss in acetabular hip revision. J Arthroplasty. 2011;26:1245-1250. [PubMed] |
| 22. | Oakes DA, Cabanela ME. Impaction bone grafting for revision hip arthroplasty: biology and clinical applications. J Am Acad Orthop Surg. 2006;14:620-628. [PubMed] |
| 23. | Berry DJ, Müller ME. Revision arthroplasty using an anti-protrusio cage for massive acetabular bone deficiency. J Bone Joint Surg Br. 1992;74:711-715. [PubMed] |
| 24. | DeBoer DK, Christie MJ, Brinson MF, Morrison JC. Revision total hip arthroplasty for pelvic discontinuity. J Bone Joint Surg Am. 2007;89:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Christie MJ, Barrington SA, Brinson MF, Ruhling ME, DeBoer DK. Bridging massive acetabular defects with the triflange cup: 2- to 9-year results. Clin Orthop Relat Res. 2001;393:216-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty. 1994;9:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 794] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
Peer reviewer: Andor Sebestyén, MD, MBA, PhD, Associate Professor, National Health Insurance Fund Administration, South-Transdanubian Regional Health Insurance Fund, Nagy Lajos kir. u. 3, Pécs, 7623, Hungary
S- Editor Yang XC L- Editor A E- Editor Yang XC