INTRODUCTION
Traumatic spinal cord injury (SCI) continues to impose a significant clinical and socioeconomic burden worldwide[1]. Despite remarkable advancements in emergency care, neuroprotective strategies, and rehabilitation, predicting patients’ neurological outcomes remains a critical challenge[2-5]. Conventional clinical assessments, such as the American Spinal Injury Association Impairment Scale, provide essential functional grading, while are limited in their capacity to quantify the structural integrity of residual spinal cord tissue[6]. Consequently, there is growing emphasis on the identification of reliable imaging biomarkers capable of predicting recovery trajectories with greater accuracy and objectivity.
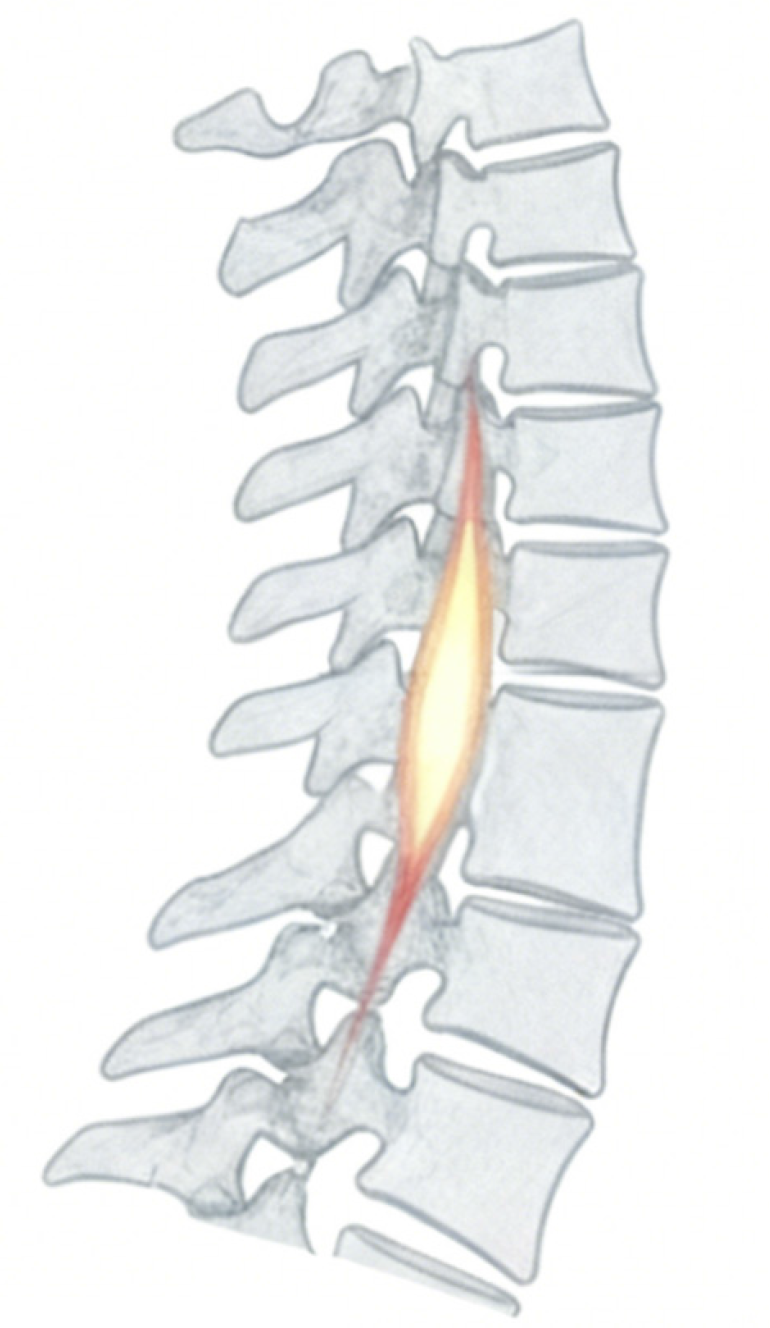
Magnetic resonance imaging (MRI), with its superior soft-tissue contrast and multiplanar capabilities, has become the cornerstone imaging modality for SCI evaluation[7]. Beyond its diagnostic role in defining the extent of cord compression, hemorrhage, and edema, MRI provides quantitative data that can aid in prognosis and develop therapeutic strategies[8,9]. Among these imaging parameters, the “tissue bridge”, defined as the continuous, visibly preserved neural tissue traversing the lesion epicenter on axial T2-weighted MRI sequences, has gained increasing recognition as an indicator of maintained neural connectivity and functional potential[10] (Figure 1). In major studies, this structure is consistently quantified by measuring its cross-sectional area or minimal sagittal width, providing an objective biomarker that contributes to ongoing efforts to define reliable MRI parameters for SCI.
Figure 1
T2-weighted sagittal magnetic resonance imaging of the spine, where the highlighted region denotes the tissue bridge between adjacent vertebral segments.
SYNTHESIZING EVIDENCE ON TISSUE BRIDGES AND PROGNOSIS
Evidence from clinical studies
The prognostic value of tissue bridges is increasingly supported by an expanding body of literature, although critical appraisal is required due to methodological limitations. A recent longitudinal, multicenter, retrospective cohort study by Pfyffer et al[10] provided high-quality evidence, demonstrating that tissue bridge measurements in the cervical spine could be strong predictors of long-term clinical outcomes. However, the retrospective design of this and similar studies may introduce potential biases in patient selection and data collection. This study further confirms previous findings that systematically quantified tissue bridge dimensions and established their correlation with neurological recovery[11]. Taken together, these studies elucidate a consistent finding, in which patients with larger and more intact tissue bridges exhibit significantly improved motor and sensory recovery. This correlation provides a structural explanation for the varying degrees of functional recovery found in these patients. Moreover, the inverse relationship among spinal canal compromise, lesion size, and functional outcomes further emphasizes the significant role of early mechanical and ischemic damage in influencing long-term prognosis.
Methodological considerations and limitations
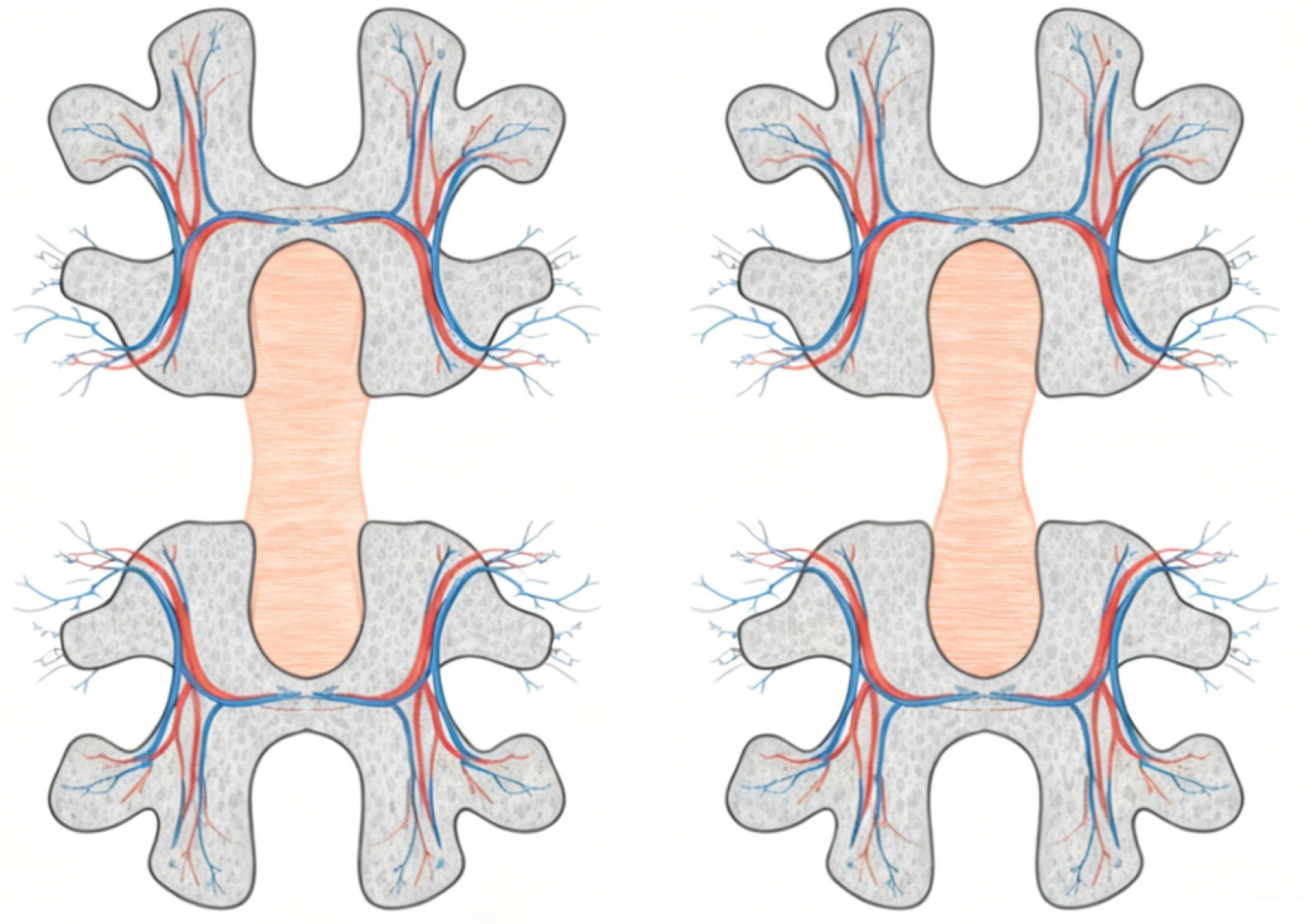
Regarding specific measurement techniques, tissue bridge quantification typically employs high-resolution T2-weighted MRI sequences. Standardized protocols involve precise delineation of the preserved neural tissue traversing the lesion epicenter, and the key parameters include cross-sectional area measured on axial views and minimal sagittal width assessed in the midsagittal plane (Figure 2). Previous research demonstrated the use of semi-automated segmentation tools to improve the reproducibility of measurements, although manual delineation remains prevalent in clinical research settings[12]. Standardized protocols typically recommend a slice thickness of ≤ 3 mm and an in-plane resolution of ≤ 0.5 × 0.5 mm2 for optimal visualization of tissue bridge anatomy.
Figure 2
Axial schematic of adjacent vertebral segments, demonstrating the comparison of intact (left) and altered (right) tissue bridge morphology together with associated vascular networks.
However, the retrospective design of this and similar studies may introduce potential biases in patient selection and data collection. A key consideration is the heterogeneity in injury levels and severity across study populations, which may affect the generalizability of the results. Although recent studies have improved the methodology for evaluating tissue bridges as a biomarker[13,14], the variability in MRI acquisition parameters across different centers and scanners presents a potential source of measurement error, which may limit the consistency and cross-site comparability of tissue bridge quantification. This heterogeneity highlights the need for standardized imaging protocols that specify optimal sequence parameters, including recommended field strengths, slice thickness, and spatial resolution to ensure consistent quantification across different platforms. While the emphasis on objective, quantifiable parameters represents a critical advancement over subjective radiological assessments and establishes a foundation for reproducible models, their effective integration into clinical practice necessitates the explicit recognition and systematic resolution of these limitations. Addressing issues of study design, technical standardization, and patient heterogeneity is therefore crucial for a balanced interpretation of the current evidence and for guiding the future research required to validate and standardize this promising biomarker.
CLINICAL AND TRANSLATIONAL IMPLICATIONS
Practical considerations for clinical implementation
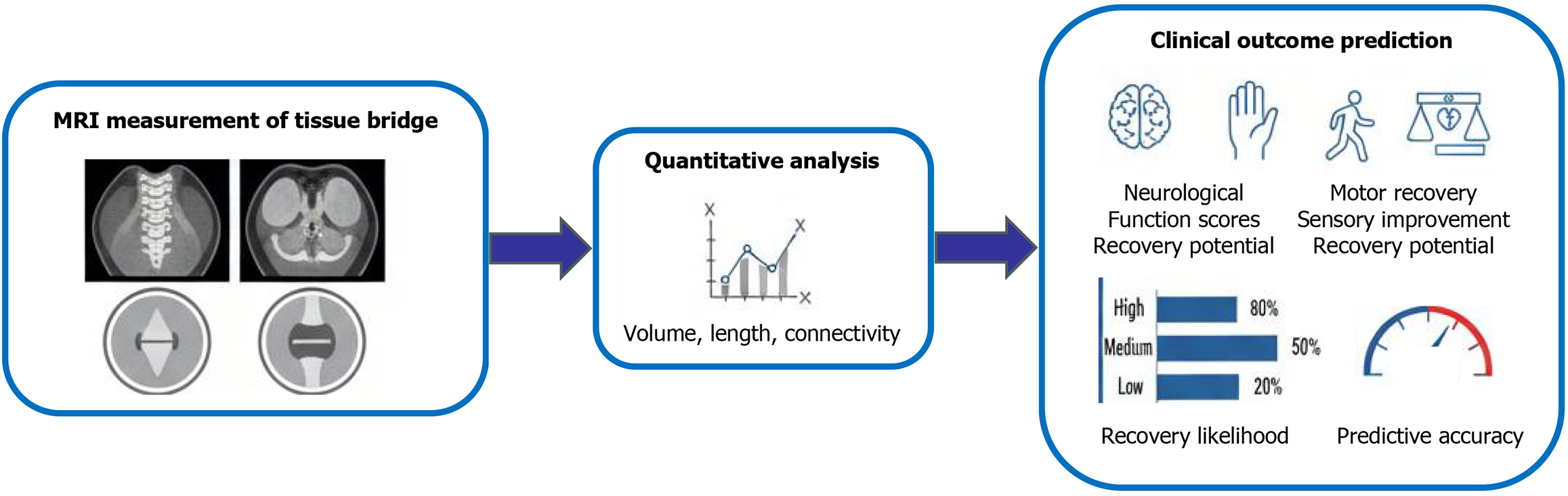
The translation of tissue bridge analysis into clinical practice holds remarkable promise, as supported by emerging quantitative evidence. For successful integration into routine workflows, key practical considerations must be addressed. These include the development of user-friendly software for semi-automated segmentation, the establishment of standardized imaging protocols to minimize inter-scanner variability, and providing specialized training for radiologists and clinicians to ensure consistent measurement techniques (Figure 3).
Figure 3 Flowchart depicting the workflow of tissue bridge assessment - from magnetic resonance imaging-based measurement (sagittal/axial imaging) through quantitative analysis (volume, length, connectivity) to the prediction of clinical outcomes (e.g., neurological function, motor/sensory recovery potential) and associated prognostic metrics.
MRI: Magnetic resonance imaging.
Clinical applications
Firstly, early identification of patients with substantial preserved tissue bridges could inform more aggressive and targeted neurorehabilitation protocols[15,16]. For instance, Pfyffer et al[10] demonstrated that tissue cross-sectional area is a strong independent predictor of long-term motor recovery, and reported correlation coefficients (r) ranged from 0.60 to 0.75, with lower limb motor scores at one year. Secondly, incorporating this biomarker into clinical trial design can significantly improve patient stratification. Quantitative measures, such as a tissue bridge volume greater than 25 mm3, have been associated with a 3.5-fold higher probability of achieving independent ambulation, thereby creating more homogeneous cohorts and increasing the statistical power required for detecting therapeutic efficacy of neuroprotective or regenerative interventions[9,17]. This approach promotes a deeper integration of quantitative imaging into the multidisciplinary management of SCI, bridging the fields of radiology, neurosurgery, and rehabilitation medicine.
Technological integration and automated analysis
From a translational perspective, incorporating tissue bridge metrics into automated image analysis pipelines may enhance workflow efficiency and objectivity[18]. Artificial intelligence and machine learning algorithms are poised to further refine these predictions and mitigate measurement variability. Recent models integrating baseline tissue bridge width and clinical scores have achieved high predictive accuracy for motor outcomes, in which area-under-the-curve values have exceeded 0.85[19-23]. This multi-parametric approach moves beyond qualitative assessment, ultimately enabling the prediction of personalized recovery trajectories and supporting more informed clinical decision-making. Future research should concentrate on validating these automated tools across a range of clinical settings and scanner platforms to ensure their robustness and broad generalizability.
ETHICAL AND CLINICAL DECISION-MAKING CONSIDERATIONS
The prognostic power of tissue bridge analysis introduces important ethical and clinical communication considerations. Effectively conveying this information to patients and their families is of great importance, as it plays a significant role in shaping expectations concerning rehabilitation goals and long-term prognoses. While the identification of a preserved tissue bridge may require more favorable counseling and the allocation of intensive rehabilitative interventions, clinicians must exercise caution to avoid deterministic conclusions that may inadvertently diminish hope or hinder patient engagement in their recovery process. Conversely, when conveying a poor prognosis in cases with minimal preserved tissue, it is imperative that the information be communicated in a manner that sustains motivation while establishing realistic expectations. The ethical application of this biomarker requires a detailed approach to communication, highlighting it as a probabilistic indicator to guide personalized care planning, rather than as an unequivocal predictor. This strategy promotes informed shared decision-making, while protecting the therapeutic alliance and preserving the possibility of meaningful recovery, irrespective of the injury’s severity.
CRITICAL APPRAISAL AND FUTURE DIRECTIONS
Current challenges and standardization needs
While the evidence supporting the role of tissue bridges in SCI prognosis is compelling, several considerations and future directions warrant attention[24,25]. To advance the field, there is a clear need for standardized, validated protocols to define and measure tissue bridges across different MRI scanners, as well as across various injury levels (cervical vs thoracic). The foundational research by Schwab and Brösamle[26], which demonstrated the regenerative potential of lesioned corticospinal tract fibers in adult rats under specific experimental conditions, provides significant mechanistic insights that underlie clinical observations of preserved neural pathways. This basic science foundation is strongly supported by clinical evidence, particularly in Pfyffer et al’s research[24], which concluded that tissue bridges could predict recovery following both traumatic and ischemic thoracic SCIs. This pivotal study not only validates the prognostic value of tissue bridges beyond the cervical region but also highlights their potential applicability across different injury levels and etiologies. Consequently, prospective studies with larger, more diverse cohorts are needed to confirm generalizability across a wider spectrum of injury severities and to establish robust normative values.
Multimodal integration and composite prognostic models
The integration of tissue bridge assessment with other advanced MRI techniques represents a critical area of advancement in SCI research and clinical management. Modalities, such as diffusion tensor imaging, which assesses white matter integrity, magnetization transfer imaging, which is sensitive to myelin content, and functional MRI, which measures brain and spinal cord activity, provide complementary insights into both microstructural and functional integrity, extending beyond macroscopic structural analysis[27-30]. A multimodal imaging approach will likely yield a more comprehensive understanding of post-injury plasticity and repair mechanisms, potentially bridging the gap between the regenerative potential suggested by animal models and the structural preservation evident in human patients.
Moreover, the future of SCI prognosis is likely to rely on the development of composite models that integrate multiple biomarkers. One of the primary advantages of tissue bridges is their ability to provide an objective, quantifiable measure of structural preservation, complementing more traditional prognostic factors. In contrast to conventional indicators, such as the American Spinal Injury Association Impairment Scale grade or somatosensory evoked potentials, tissue bridges present a distinct structural correlate that directly visualizes the anatomical pathways through which recovery may occur. While clinical examinations remain fundamental for assessing neurological function, and electrophysiological studies provide information on conduction integrity, tissue bridges uniquely quantify the physical pathway through which such conduction may be noteworthy. Combining imaging biomarkers, such as tissue bridges, with molecular indicators, electrophysiological data, and detailed clinical scores will promote the development of composite prognostic models. A particularly promising direction involves the integration of imaging features with biofluid biomarkers, such as neurofilament light chain and glial fibrillary acidic protein, providing complementary information on ongoing axonal injury and astroglial pathology. The combination of structural MRI data (tissue bridges) with such molecular markers of neuronal damage can significantly enhance prognostic accuracy by capturing both the structural substrate for recovery and the dynamic biological processes following SCI. Such tools can significantly enhance the ability of clinicians to monitor disease progression, evaluate therapeutic responses, and optimize rehabilitation on an individual basis. The convergence of evidence from foundational experimental research and rigorous clinical validation paves the way for these more sophisticated, personalized approaches to SCI management.
CONCLUSION
The identification of MRI-based tissue bridge biomarkers as predictors of neurological recovery represents a critical advancement in precision prognostication for traumatic SCI. By quantifying the extent of preserved spinal cord tissue, clinicians can transcend descriptive imaging and embrace objective, data-driven decision-making. The study could exemplify how translational imaging research could bridge the gap between structural assessment and clinical outcome prediction. Ongoing efforts in this domain, combining advanced imaging techniques, computational analytics, and interdisciplinary collaboration, may be crucial for enhancing the quality of life and promoting functional independence for patients with SCI.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade B, Grade C
Creativity or Innovation: Grade C, Grade C
Scientific Significance: Grade B, Grade C
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Kalyoncu Aycenk A, MD, Assistant Professor, Türkiye; Shen Y, MD, Professor, China S-Editor: Bai SR L-Editor: A P-Editor: Zhao YQ