Published online Jun 18, 2025. doi: 10.5312/wjo.v16.i6.106804
Revised: April 1, 2025
Accepted: May 7, 2025
Published online: June 18, 2025
Processing time: 103 Days and 14 Hours
Poor musculoskeletal recovery following foot and ankle injury can result in chronic instability and persistent muscle weakness. Preliminary evidence has shown that blood flow restriction (BFR) rehabilitation can increase muscle st
To determine whether BFR is more effective than traditional rehabilitation in improving muscle strength, size, and stability after foot and ankle injury.
A systematic review and meta-analysis were performed. Articles were retrieved from MEDLINE, EMBASE, and CENTRAL databases. Included studies compared the effectiveness of BFR rehabilitation to traditional foot and ankle rehabilitation exercises. Eligible patients were those with a history of foot or ankle injury. Muscle strength, size, and dynamic balance were assessed by comparing impro
Ten studies met the inclusion criteria. Five studies were of good to excellent quality according to the PEDro scale, and 5 studies were of moderate quality as per the MINORS criteria. Two studies compared the effect of BFR and non-BFR rehabilitation on muscle strength; the overall mean difference between the BRF and non-BFR groups was 0.09 [95%CI: (0.05, 0.12), P < 0.0001]. Two studies analyzed muscle activation following BFR and non-BFR rehabilitation; the overall mean difference between the BRF and non-BFR groups was 0.09 [95%CI: (0.05, 0.12), P < 0.0001]. Data on dynamic balance was synthesized from two studies; the mean difference between the BFR and control groups was 1.23 [95%CI: (-1.55, 4.01); P = 0.39].
BFR rehabilitation is more effective than non-BFR rehabilitation at improving muscle strength and activation following foot and ankle injury. Additional studies are needed to develop a standardized BFR training protocol.
Core Tip: Blood flow restriction (BFR) involves limiting blood flow to an actively exercising muscle with the goal of inducing an acute hypoxic state. When used in combination with traditional rehabilitation exercises, BFR can improve strength and stability in patients with a history of foot or ankle injury. Our meta-analysis investigated whether rehabilitation involving BFR is more effective than traditional rehabilitation in improving muscle strength, size, and stability after foot and ankle injury.
- Citation: Balboni JM, Madhira K, Martinez V, Tung WS, Kennedy JG, Gianakos AL. Effect of blood flow restriction on muscle strength and stability following foot and ankle injury: A systematic review. World J Orthop 2025; 16(6): 106804
- URL: https://www.wjgnet.com/2218-5836/full/v16/i6/106804.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i6.106804
Preliminary studies have shown that blood flow restriction (BFR) has a measurable benefit on musculoskeletal recovery when incorporated into traditional rehabilitation protocols[1]. These findings have garnered increased attention in recent years and spurred curiosity among clinicians and patients alike. BFR may be particularly useful in foot and ankle injuries that involve muscle weakness and may play a role in preventing post-operative disuse atrophy[2]. In patients who are eager to return to sport, the use of BFR may prove invaluable in helping to accelerate recovery and restore pre-injury muscle performance[3].
By occluding blood flow to an actively exercising muscle, BFR functions to enhance metabolic stress at the cellular level. Metabolic stress describes a shift in energy metabolism in response to low oxygen levels. During exercise, cellular energy stores are depleted as substances such as lactate, phosphate, and hydrogen ions begin to accumulate[4]. The presence of these metabolites leads to cellular toxicity and muscle damage. This results in the transduction of signals that induce muscle growth through increased fast-twitch fiber recruitment, local and systemic hormone release, and cellular swelling[5]. This anabolic cascade is believed to promote increased skeletal muscle size, strength, and activation, all of which are important mediators in muscle conditioning[6].
In the setting of injury, BFR allows for earlier initiation of rehabilitation exercises by enabling the use of lighter loads to achieve similar muscular gains[7]. Patients are often unable or unwilling to tolerate heavy loads in the early phases of their rehabilitation and doing so may be contraindicated due to the risk of further injury. When rehabilitation programs are limited in their ability to introduce a heavy load or level of resistance adequate to fully activate muscle contraction and metabolic stress, improvements in muscle strength and hypertrophy are often inappreciable[8]. While loss of muscle mass is not uncommon following injury, progression to weakness and atrophy can complicate recovery[9]. BFR allows for adequate muscle stimulation without introducing excess strain on the injured joint, thereby promoting muscle preservation and expediting recovery[7].
Intensity, volume, and rest between sets are all determinants in the magnitude of metabolic stress and are therefore important components of the exercise regimen. Different training modalities can be used to maximize metabolic stress during exercise; such examples include resistance training, low-intensity resistance training plus BFR, or high-intensity interval training[4]. While there are many ways to approach physical rehabilitation, it has yet to be determined what combination is most effective following foot and ankle injury.
This systematic review and meta-analysis will compare the efficacy of BFR and non-BFR rehabilitation in patients with a history of foot or ankle injury. Outcomes to be evaluated include muscle strength, muscle size, muscle activation, and dynamic balance.
Included studies were those that examined the effect of BFR on musculoskeletal rehabilitation following foot and ankle injury, had full text, and were written in English. Eligible study designs included randomized controlled trials, cohort studies, and case series. Systematic reviews, meta-analyses, clinical commentaries, books, expert opinions, and abstracts without full text were excluded. Due to the limited number of peer-reviewed publications available on this topic, the initial search included all results generated from 1976 to 2024. The BFR group consisted of patients who engaged in traditional rehabilitation exercises while wearing the BFR device. The control (non-BFR) group consisted of patients who performed traditional rehabilitation exercises without the BFR device.
A systematic review of the literature was conducted in MEDLINE, EMBASE, and CENTRAL using the search terms ‘[(foot) OR (ankle)] AND (blood flow restriction).’ Any article containing these terms was initially retrieved for article screening. Additionally, a manual search was performed to retrieve any articles cited in reference lists that met eligibility criteria.
All articles populated by the search terms and manual reference search were retrieved from their respective databases and uploaded to Rayyan, a public software program designed to assist with blinded and independent study screening[10]. All duplicates were identified and removed by hand. Abstract and title screening was then performed by two authors independently. Once completed, selected articles underwent further full text screening. The reason for exclusion was documented for all articles during the selection process. Any discrepancies that arose were resolved by a third author. Article screening and evaluation was performed in accordance with the 2020 PRISMA guidelines[11]. Articles that met the inclusion criteria were selected for analysis.
All eligible studies underwent data extraction to retrieve information for study analysis. Two authors participated in data collection. Both authors were trained in the critical appraisal of clinical trials. All study characteristics were recorded in a table generated in Microsoft Excel 2024. Data to be extracted included author name, year of publication, study title, country of study, study design, sample size, BFR indication, BFR protocol, interventions, and outcome measures. Specific variables for which data were sought included muscle strength, muscle size, muscle activation, and dynamic balance.
The PEDro Scale was used to assess the quality of evidence presented in randomized control trials. The PEDro scale is a validated quality assessment tool that has been utilized in numerous systematic reviews[12]. The PEDro Scale consists of 11 items; item 1 evaluates external validity, items 2 through 9 assess internal validity, and items 10 and 11 evaluate statistical reporting. Each item is rated as yes or no which corresponds to a score of 1 or 0, respectively. The total PEDro score is calculated by finding the sum of the ratings of items 2 through 11 for a combined total score between 0 and 10[13]. A score of 0-3 is considered ‘poor’, a score of 4-5 is ‘fair’, a score of 6-8 is ‘good’, and a score between 9-10 is considered ‘excellent’. Risk of bias in the non-randomized studies was assessed in accordance with the Methodological Index for Non-Randomized Studies (MINORS)[14]. The MINORS scale is a 12-item tool. Items 1 through 8 evaluate both comparative and non-comparative studies, while items 9 through 12 evaluate comparative studies. Each item is assigned a score of either 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The total MINORS score is the sum of the individual item ratings, with comparative studies receiving a total score between 0 and 24 points and non-comparative groups receiving a total score between 0 and 16. Higher scores indicate better methodological quality[15].
Studies that evaluated similar outcomes and reported quantitative data were grouped and evaluated for heterogeneity. Meta-analyses were performed using RevMan 5.4 (Cochrane, London, England) by taking pre-intervention and post-intervention differences, as well as pooling standard deviations. A random effects model was used for all analyses performed to account for the anticipated heterogeneity between studies. The I2 statistic was used to assess heterogeneity between studies. The mean difference between BFR and control groups and their corresponding 95% confidence intervals for each outcome measure were automatically generated by RevMan. Forest plots were generated for all outcome variables analyzed. Statistical significance was set at a = 0.05.
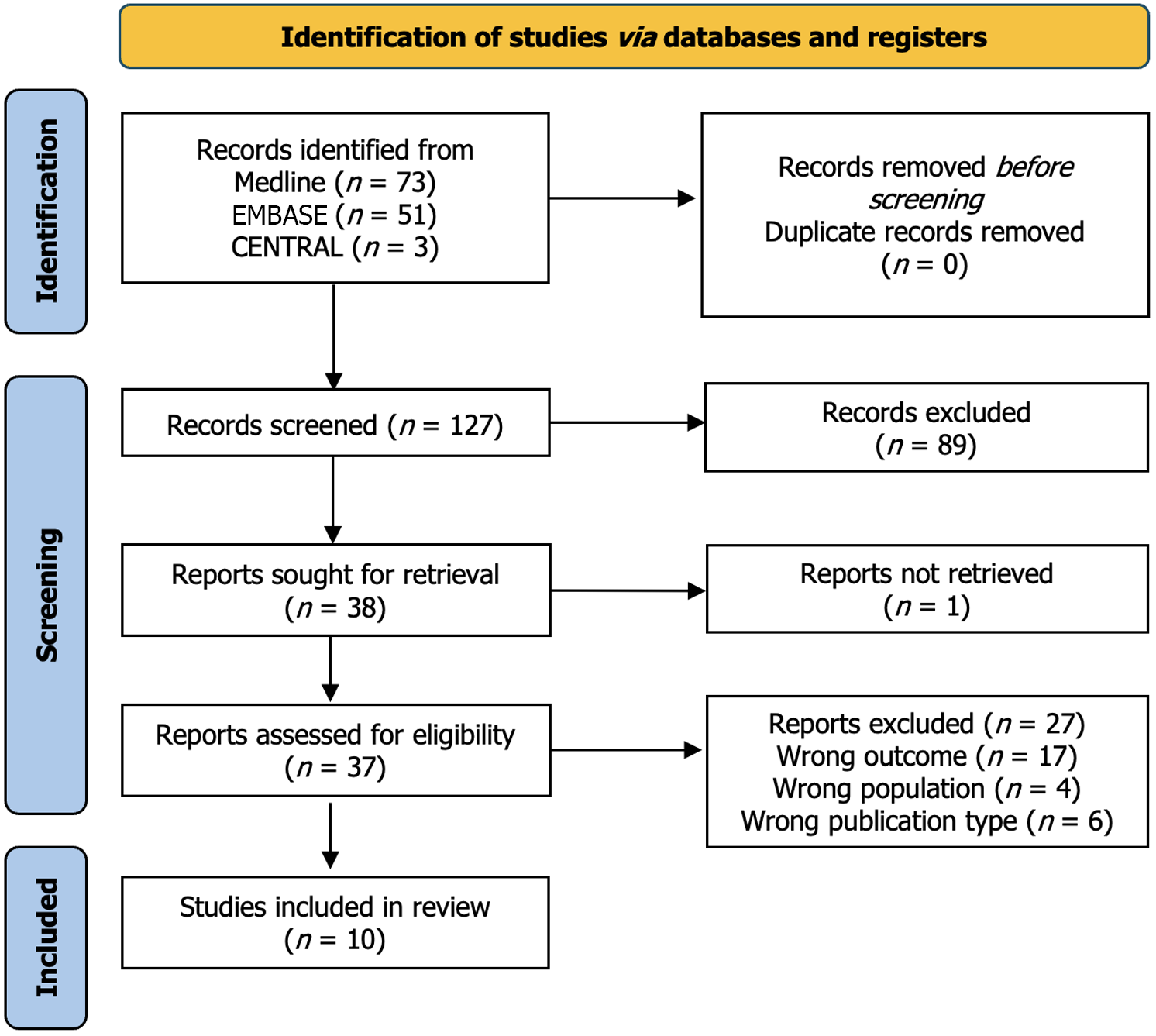
The initial search yielded a total of 127 studies. No duplicates were detected. A total of 127 articles were initially screened for eligibility by reviewing their titles and abstracts. Of these, 90 were excluded due to wrong anatomic region (47 studies), wrong study design (14 studies), and lack of the required outcome measures (29 studies). The remaining 37 studies were assessed for eligibility with the full text article; of these, 27 were excluded due to wrong outcome (17 studies), wrong population (4 studies), and wrong study design (6 studies). 10 studies were ultimately included in this review. The PRISMA diagram in Figure 1 depicts the results of the literature search and screening process.
10 studies met the inclusion criteria (5 randomized controlled trials, 3 prospective studies, and 2 case series). A total of 229 patients were included, with 128 patients undergoing non-BFR rehabilitation vs 170 patients undergoing BFR rehabilitation. It is important to note that a crossover study design was utilized in 69 out of 229 total patients included in this study; these patients performed rehabilitation exercises with and without BFR. The study population was composed of 94 males and 135 females. The average age was 24.4 years (range: 20.3-33.5 years). Indications for BFR included chronic ankle instability (CAI) (124 of 229), functional ankle instability (46 of 229), medial longitudinal arch pathology (48 of 229), ankle fracture (9 of 229), and Achilles tendon rupture (2 of 229). Table 1[16-24] further illustrates characteristics of the study population.
| Ref. | Title | Study design | Country | Sample size (n) | Male (n) | Female (n) | Age (mean) | Blood flow restriction indication | Study population |
| Burkhardt et al[16] | Effects of Blood Flow Restriction on Muscle Activation During Dynamic Balance Exercises in Individuals with Chronic Ankle Instability | RCT | United States | 25 | 15 | 10 | 20.3 | Chronic ankle instability | Young adults with a history of chronic ankle instability |
| Mahmoud et al[17] | Effect of Blood Flow Restriction as a Stand-Alone Treatment on Muscle Strength, Dynamic Balance, and Physical Function in Female Patients with Chronic Ankle Instability | RCT | Saudi Arabia | 39 | 0 | 39 | 23.5 | Chronic ankle instability | Female patients with a history of chronic ankle instability |
| Okamura et al[18] | Effect of Neuromuscular Electrostimulation with Blood Flow Restriction on Acute Muscle Swelling of the Abductor Hallucis | RCT | Japan | 48 | 24 | 24 | 21 | Injuries associated with a low medial longitudinal arch | University students with abductor hallucis pathology |
| Wen et al[19] | Effect of Low-Load Blood Flow Restriction Training on Patients with Functional Ankle Instability: A Randomized Controlled Trial | RCT | China | 46 | 20 | 26 | 21.9 | Functional ankle instability | Young adults with a history of functional ankle instability |
| Werasirirat and Yimlamai[8] | Effect of Supervised Rehabilitation Combined with Blood Flow Restriction Training in Athletes with Chronic Ankle Instability: A Randomized Placebo-Controlled Trial | RCT | Thailand | 16 | 12 | 4 | 20.5 | Chronic ankle instability | Collegiate athletes with a history of chronic ankle instability |
| Clark et al[20] | Effects of Blood Flow Restriction on Balance Performance During Dynamic Balance Exercises in Individuals with Chronic Ankle Instability | Prospective | United States | 25 | 9 | 16 | 20.8 | Chronic ankle instability | Young adults with a history of chronic ankle instability |
| Killinger et al[21] | The Effects of Blood Flow Restriction on Muscle Activation and Hypoxia in Individuals with Chronic Ankle Instability | Prospective | United States | 19 | 9 | 10 | 21.8 | Chronic ankle instability | Young adults with a history of chronic ankle instability |
| Larsen et al[22] | Blood-Flow Restricted Exercise Following Ankle Fractures | Prospective | Denmark | 8 | 3 | 5 | 33 | Non-specific ankle fracture | Adults with a history of unilateral ankle fracture |
| Yow et al[23] | Blood Flow Restriction Training After Achilles Tendon Rupture | Case Series | United States | 2 | 2 | 0 | 33.5 | Achilles tendon rupture | Male soldiers with a history of left Achilles tendon rupture |
| Mortensen et al[24] | Low-Load Blood-Flow-Restricted Exercise to Prevent Muscle Atrophy and Decline in Functional Performance in a Patient Recovering from a Malleolus Fracture | Case Report | Denmark | 1 | 0 | 1 | 28 | Lateral malleolus fracture | Female with a history of lateral malleolus fracture |
The PEDro scale was used to evaluate the quality of evidence presented in the 5 randomized controlled trials; 2 of these studies received a score of ‘excellent’ and 3 of these studies received a score of ‘good’. The quality of the remaining 5 studies was evaluated according to the MINORS criteria; the results of this assessment demonstrated that all 5 studies were of ‘moderate quality.’ The results of the bias and quality assessments according to the MINORS and PEDro scale can be found in Tables 2 and 3, respectively.
| Ref. | Study design | Study type (comparative or non-comparative) | 1: clearly stated aim | 2: Inclusion of consecutive patients | 3: Prospective collection of data | 4: End points appropriate to study aim | 5: Unbiased assessment of study end point | 6: Follow-up period appropriate to study aim | 7: Less than 5% lost to follow up | 8: Prospective calculation of the study size | 9: Adequate control group | 10: Contemporary groups | 11: Baseline equivalence of groups | 12: Adequate statistical analysis | Total score |
| Clark et al[20] | Prospective | Comparative | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 22 |
| Killinger et al[21] | Prospective | Comparative | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 22 |
| Larsen et al[22] | Prospective | Comparative | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 15 |
| Yow et al[23] | Case Series | Non-Comparative | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 12 |
| Mortensen et al[24] | Case Report | Non-Comparative | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 12 |
| Ref. | 1. Eligibility criteria specified | 2. Random allocation | 3. Concealed allocation | 4. Groups similar at baseline | 5. Subject blinding | 6. Therapist blinding | 7. Assessor blinding | 8. Less than 15% dropouts | 9. Intention-to-treat analysis | 10. Between-group statistical comparisons | 11. Point measures and variability data | Total PEDro score |
| Burkhardt et al[16] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| Mahmoud et al[17] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| Okamura et al[18] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 8 |
| Wen et al[19] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| Werasirirat and Yimlamai[8] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 8 |
All patients, regardless of study group, participated in traditional rehabilitation exercises. These included isokinetic resistance training (76 out of 229 patients), dynamic balance exercises (50 out of 229 patients), and a combination of both resistance training and dynamic balance exercises (55 out of 229 patients). Patients in the BFR group differed from those in the non-BFR group only in that they wore a blood pressure cuff around their exercising limb to restrict blood flow while performing each rehabilitation exercise. Hence, the non-BFR group adhered to the traditional rehabilitation protocol involving resistance training and balance exercises, while the BFR group added BFR to the traditional rehabi
Not all studies included in this review adhered to the standard 30-15-15-15 repetition scheme for BFR. When adding BFR to traditional rehabilitation exercises, current guidelines recommend 4 sets of 30-15-15-15 repetitions (75 total repetitions)[25]. In this study, 8 studies[8,16,19-24] utilized the standard 30-15-15-15 repetition scheme, 1 study[17] utilized an alternative scheme involving 4 sets performed until muscle failure, and 1 study[18] failed to specify. BFR training was performed at a mean frequency of 2 times per week (range: 1-4 weeks) for a total duration of 3.8 weeks (range: 1-12 weeks). Limb arterial occlusion pressure ranged from 60%-80% with a mean total restriction time of 8.6 minutes (range: 5-15 minutes). The location of cuff placement for 8 out of 10 studies included in this review was the proximal thigh (inguinal fold region) of the exercising limb. Cuff placement in the remaining two studies was located 2-3 cm proximal to the patella. On average, each session involved 3 exercises (range: 1-5 exercises) performed for 4 sets for a total of 75 repetitions or until muscle failure with 29.4 seconds (range: 10-45 seconds) of rest between sets and 4 minutes (range: 1-5 minutes) of rest between exercises. Muscles targeted included tibialis anterior (145 of 229), peroneus longus (145 of 229), vastus lateralis (26 of 229) and abductor hallucis (48 of 229). Table 4 illustrates the BFR protocol used in each study included in this systematic review.
| Ref. | Limb arterial occlusion Pressure (%) | Location of tourniquet cuff | Total occlusion Time (min) | Repetitions in Set #1 | Repetitions in Set #2 | Repetitions in Set #3 | Repetitions in Set #4 | Total number of repetitions | Rest between Sets (sec) | Rest between exercises (min) | Number of exercises performed during visit | Frequency (# visits/week) | Duration of treatment Program (weeks) |
| Burkhardt et al[16] | 80% | Proximal thigh | 7.5 | 30 | 15 | 15 | 15 | 75 | 30 | 5 | 2 | 2 | 1 |
| Mahmoud et al[17] | 80% | Proximal thigh | 5 | 10 | 10 | 10 | N/A | 30 | 10 | 3 | 5 | 3 | 4 |
| Okamura et al[18] | 70% | Proximal thigh | 15 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 1 | 1 |
| Wen et al[19] | 80% | 2-3 cm proximal to patella | 7.5 | 30 | 15 | 15 | 15 | 75 | 30 | 3 | 3 | 2 | 6 |
| Werasirirat and Yimlamai[8] | 80% | Proximal thigh | N/A | 30 | 15 | 15 | 15 | 75 | 30 | N/A | 4 | 3 | 4 |
| Clark et al[20] | 80% | Proximal thigh | 8 | 30 | 15 | 15 | 15 | 75 | 30 | N/A | 4 | 2 | 1 |
| Killinger et al[21] | 80% | 2-3 cm proximal to patella | N/A | 30 | 15 | 15 | 15 | 75 | 45 | 5 | 2 | 1 | 1 |
| Larsen et al[22] | 60% | Proximal thigh | N/A | 30 | 15 | 15 | 15 | 75 | 30 | N/A | 1 | 2 | 3 |
| Yow et al[23] | 60% | Proximal thigh | N/A | 30 | 15 | 15 | N/A | N/A | 30 | 0-2 | 5 | 4 | 12 |
| Mortensen et al[24] | 80% | Proximal thigh | N/A | 30 | 15 | 15 | 15 | 75 | N/A | N/A | 2 | N/A | 5 |
| Average | 75% | 8.6 | 27.8 | 14.4 | 14.4 | 15 | 69.4 | 29.4 | 4 | 3.1 | 2.2 | 3.8 |
Meta-analyses were performed to evaluate the effect of BFR and non-BFR rehabilitation on muscle strength, size, activation, and dynamic balance. Studies were grouped for meta-analysis according to the outcome that was measured. A total of four comparisons were performed. The first comparison consisted of two randomized controlled trials[8,17] that evaluated muscle strength following BFR and non-BFR rehabilitation. Additional subgroup analyses were performed for ankle invertor, evertor, plantarflexor, and dorsiflexor muscle groups. The second comparison consisted of one randomized controlled trial[16] and one prospective study[21] that reported data on muscle activation. Subgroup analyses were performed for ankle dorsiflexor and evertor muscle groups; however, there was inadequate data to perform subgroup analyses for ankle invertor or plantarflexor muscle groups. The third comparison was synthesized from two randomized controlled trials[8,19] that reported data on dynamic balance. The fourth comparison was synthesized using the results of two randomized controlled trials[8,19] that measured the cross-sectional area of the peroneus longus following BFR and non-BFR rehabilitation. For each comparison, the overall mean difference and subgroup mean difference was calculated to determine whether there was a true difference between the BFR and non-BFR (control) condition.
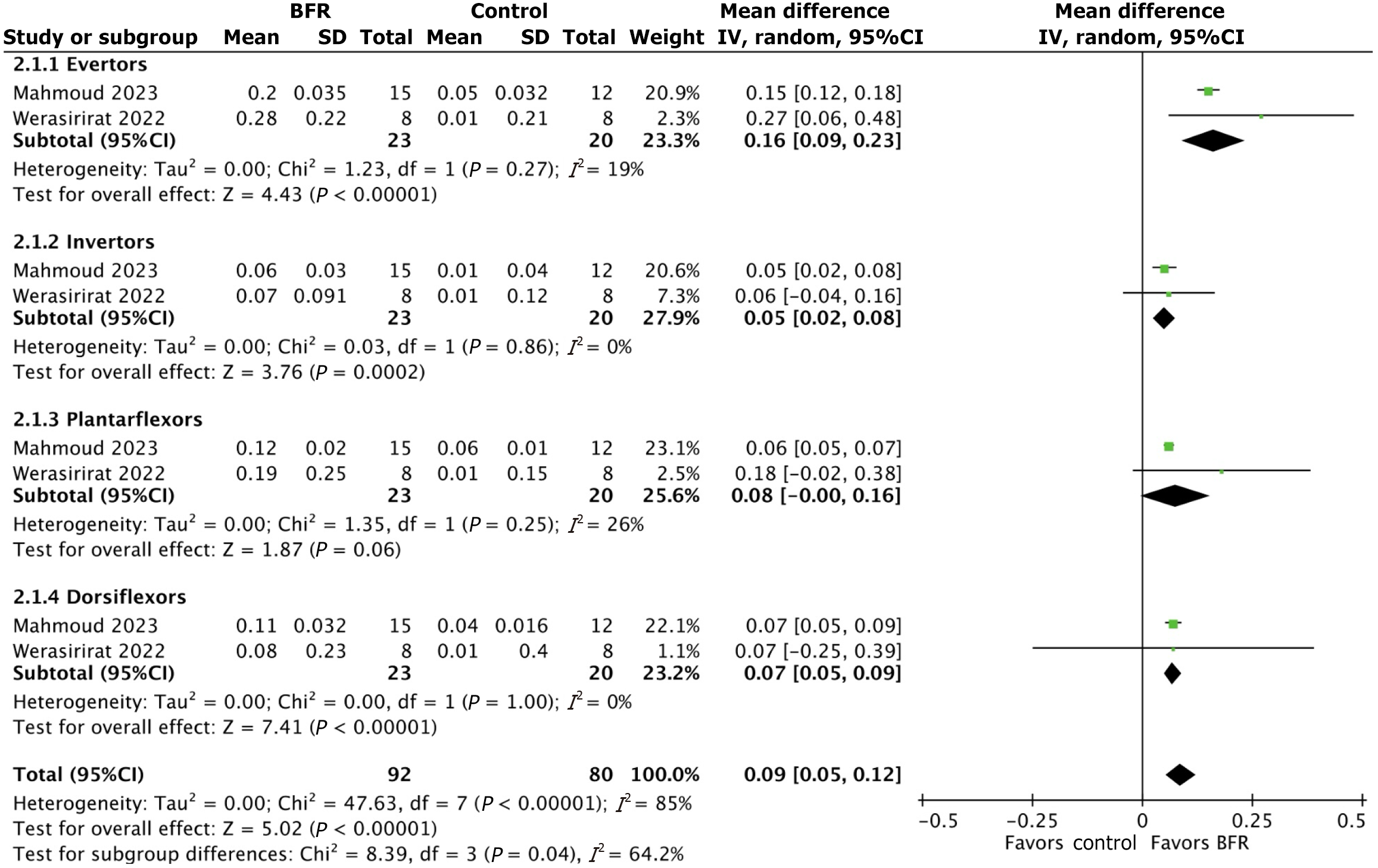
Comparison 1: Effect of BFR and non-BFR rehabilitation on muscle strength: Data on muscle strength was synthesized from two randomized controlled trials[8,17]. Average peak torque to body weight ratio was measured at an angular velocity of 60°/s for ankle dorsiflexor, plantarflexor, invertor, and evertor muscles. For ankle dorsiflexors, pre- and post-training average peak torque to body weight ratio values were 0.40 and 0.50, respectively in the BFR group and 0.39 and 0.42, respectively in the control group. The subgroup mean difference in average peak torque to body ratio for ankle dorsiflexors between the BFR and control groups was 0.07 [95%CI: (0.05, 0.09); P value < 0.0001]. For ankle plantarflexors, pre- and post-training average peak torque to body weight ratio values were 0.41 and 0.55, respectively in the BFR group and 0.41 and 0.45, respectively in the control group. The subgroup mean difference in average peak torque to body ratio for ankle plantarflexors between the BFR and control groups was 0.08 [95%CI: (0.00, 0.16); P value = 0.06]. For ankle invertor muscles, pre- and post-training average peak torque to body weight ratio values were 0.23 and 0.30, respectively in the BFR group and 0.22 and 0.23, respectively in the control group. The subgroup mean difference in average peak torque to body ratio for ankle invertors between the BFR and control groups was 0.05 [95%CI: (0.02, 0.08); P value = 0.0002]. For ankle evertors, pre- and post-training average peak torque to body weight ratio values were 0.25 and 0.48, respectively in the BFR group and 0.26 and 0.29, respectively in controls (Figure 2). The subgroup mean difference in average peak torque to body ratio for ankle evertors between the BFR and control groups was 0.16 [95%CI: (0.09, 0.23); P value < 0.0001]. The overall mean difference across all subgroups was 0.09 [95%CI: (0.05, 0.12), P value < 0.0001]. A case series performed by Yow et al[23] in 2018 describes two patients who incorporated BFR into their traditional rehabilitation program after Achilles tendon rupture. Pre- and post-training peak torque values were 5.3 and 33.0, respectively in patient one and 22.9 and 35.6, respectively in patient two.
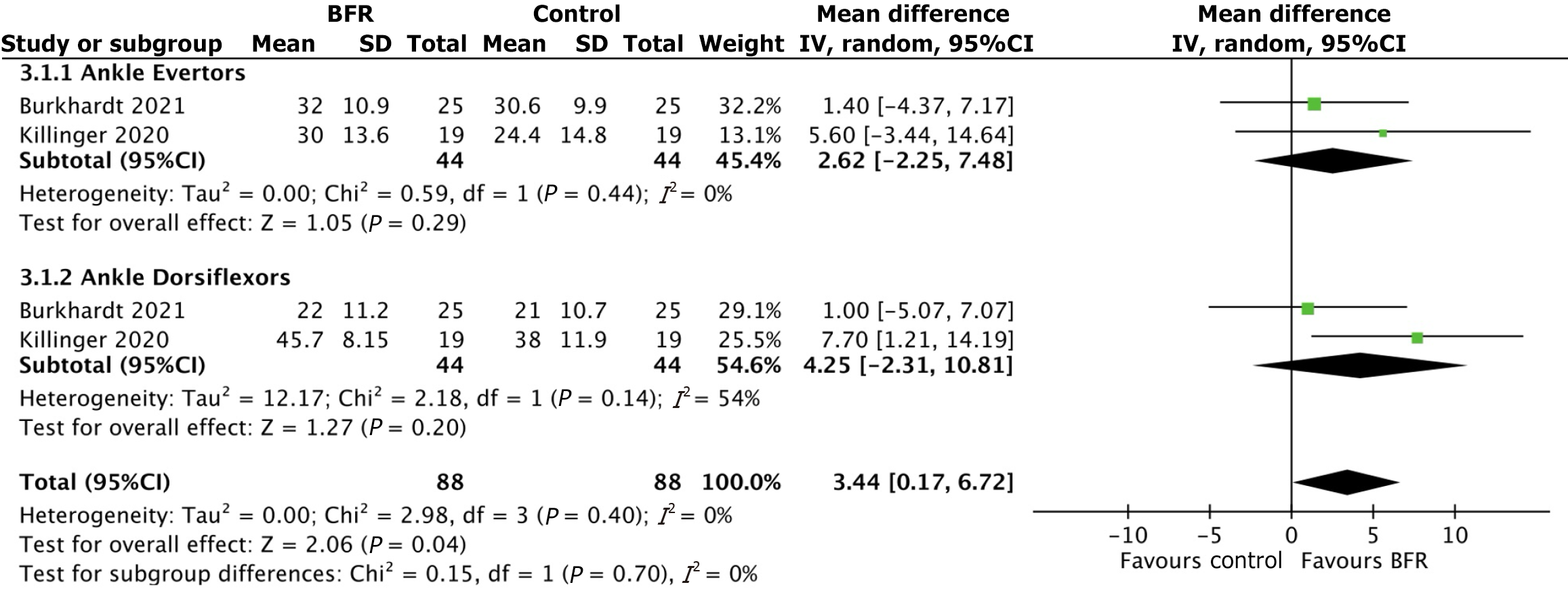
Comparison 2: Effect of BFR and non-BFR rehabilitation on muscle activation: Data on muscle activation was synthesized from one randomized controlled trial[16] and one prospective study[21]. Percent muscle activation was measured in ankle dorsiflexor and evertor muscles with and without BFR. Results of ankle dorsiflexors showed a mean activation of 32.2% after BFR compared to 28.3% in controls. The subgroup mean difference in muscle activation for ankle dorsiflexors between the BFR and control groups was 2.62 [95%CI: (-2.25, 7.48); P value = 0.29]. Mean muscle activation in ankle evertors was 31.1% after BFR compared to 27.9% in controls. The subgroup mean difference in muscle activation for ankle evertors between the BFR and control groups was 4.25 [95%CI: (-2.31, 10.81); P value = 0.20]. The overall mean difference across all subgroups was 3.44 [95%CI: (0.17, 6.72); P value = 0.04]. Percent activation of the vastus lateralis and soleus with and without BFR was reported in one study[16]. Vastus lateralis showed 38.5% activation after BFR compared 27.1% in controls. Soleus muscle activation was 36.1% after BFR compared to 31.5% in controls. Figure 3 further depicts the results of this analysis.
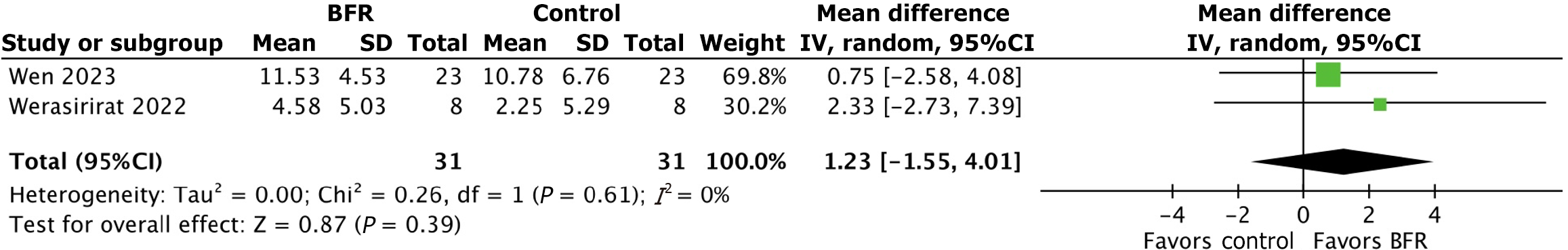
Comparison 3: Effect of BFR and non-BFR rehabilitation on dynamic balance: Data on dynamic balance was synthesized from two randomized controlled trials[8,19]. Y-Balance test scores were used in to assess improvements in dynamic balance with and without BFR. Balance scores were evaluated before and after training in both the BFR and control groups. Average pre- and post-training Y-Balance test scores were 87.1 and 95.5, respectively in the BFR group compared to 87.3 and 94.7, respectively in controls. The mean percent change in pre-and post-training Y-balance test score was 9.7% in the BFR group compared to 8.6% in the control group. The mean difference in Y-Balance test scores between the BFR and control groups was 1.23 [95%CI: (-1.55, 4.01); P value = 0.39]. Figure 4 further depicts the results of this analysis.
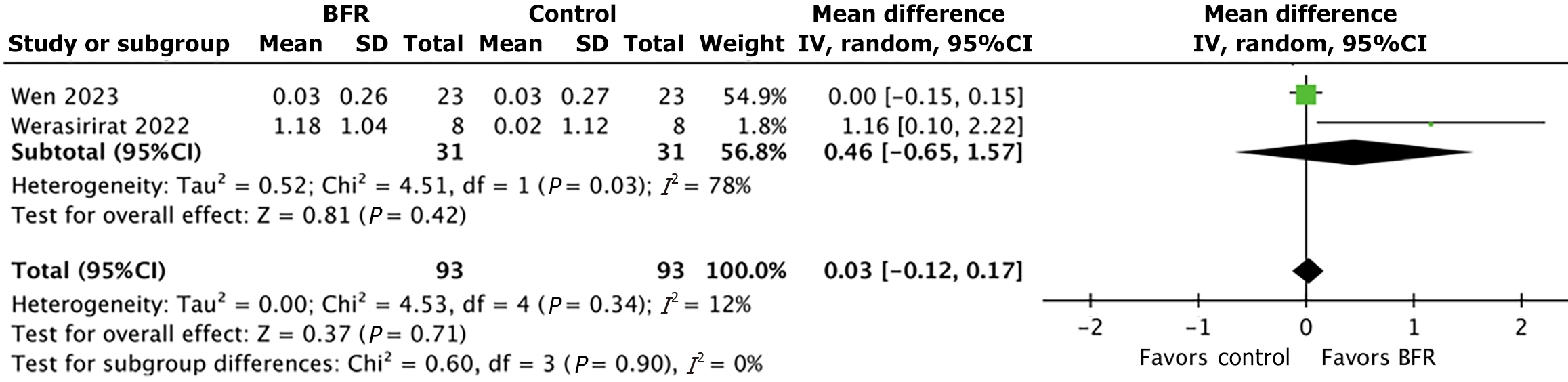
Comparison 4: Effect of BFR and non-BFR rehabilitation on muscle cross-sectional area: The percent change in muscle cross-sectional area was calculated using pre- and post-training measurements following BFR and non-BFR rehabilitation for abductor hallucis, peroneus longus, tibialis anterior, and triceps surae. One study[18] measured the abductor hallucis muscle; the abductor hallucis exhibited a 3.3% increase in cross-sectional area in the BFR group compared to a 1.7% increase in controls. One study[19] measured the tibialis anterior muscle; the tibialis anterior showed a 1.84% increase in cross-sectional area in the BFR group compared to a 1.33% increase in controls. One study[19] measured the triceps surae muscle; the triceps surae showed a 0.46% increase in cross-sectional area in the BFR group compared to a 0.42% increase in controls. The peroneus longus was the focus of two studies[8.19], and exhibited a mean increase in cross-sectional area of 7.1% in the BFR group compared to 1.8% in the control group. The subgroup mean difference in peroneus longus cross-sectional area between the BFR and control groups was 0.46 [95%CI: (-0.65, 1.57); P value = 0.42]. The overall mean difference between all muscle groups was 0.03 [95%CI: (-0.12, 0.17); P value = 0.42]. Figure 5 further depicts the results of this analysis.
The findings of this study demonstrate that BFR results in greater improvements in muscle strength, size, and activation compared to traditional foot and ankle rehabilitation alone. This study utilized peak torque to body weight ratio to assess muscle strength before and after training in both the BFR and control groups for ankle dorsiflexor, plantarflexor, invertor, and evertor muscles. Results revealed greater muscular strength gains after BFR compared to controls in all four muscle groups studied. While the exact mechanism by which BFR improves muscle strength is not fully understood, the authors of Killinger et al[21] believe that significant muscle activation during BFR may be primarily responsible.
Previous studies have found that the hypoxic intramuscular environment induced by BFR results in increased muscle activation via stimulation of group III and IV muscle afferents[26]. This study evaluated muscle activation before and after training in both the BFR and control groups. Our results were consistent with the current literature; both ankle dorsiflexors and evertors exhibited greater muscle activation with BFR compared to controls[21]. These findings reaffirm a positive association between muscle strength and percent muscle activation. What remains unclear is whether the observed improvements in muscle strength are due to greater muscle activation, muscle hypertrophy, or a combination of both. Muscle cross-sectional area was measured before and after BFR to determine whether a similar relationship exists between muscle strength and muscle size.
The percent change in muscle cross-sectional was greater with BFR compared to controls for all muscles studied, including abductor hallucis, tibialis anterior, triceps surae, and peroneus longus. Moreover, two previous studies[27,28] found that BFR is more effective than traditional rehabilitation in improving the strength of the quadriceps muscle and increasing thigh circumference. Collectively, these findings suggest that increased muscle strength following BFR may be due to not only enhanced muscle activation, but also greater muscle hypertrophy.
Delayed activation of peroneus longus, tibialis anterior, and gastrocnemius has been identified in patients with CAI and likely plays a role in predisposing these patients to repeat injury[6]. Weakness of the peroneal tendons in particular is a known risk factor for lateral ankle sprain (LAS), and studies have shown that patients with a history of LAS have reduced peroneus longus cross-sectional area compared to those without LAS[8,9]. By increasing the thickness of these muscles and improving muscle activation, BFR may augment musculoskeletal rehabilitation and improve stability in CAI patients[7].
Two studies, consisting of 9 patients in total, investigated the role of BFR in patients with foot or ankle fracture. A 2022 study conducted by Larsen et al[22] reported that early use of BFR in patients with unilateral ankle fracture was well tolerated and not associated with any serious adverse events. Among 8 total patients included in their study, 7 reported delayed-onset muscle soreness and 1 reported peripheral paresthesia, however all adverse events lasted less than 24 hours. In a subsequent case study published by Mortensen et al[24] in 2023, a patient with a lateral malleolus fracture successfully completed all BFR training sessions without any exacerbations in pain. Radiographs obtained from this patient before and after training showed no evidence that the BFR protocol adversely affected the bone healing process.
Miller et al[29] investigated the systemic consequences of BFR and identified no adverse effects on the cardiovascular or pulmonary systems. However, evidence remains mixed with other studies reporting signs of increased cardiac stress, including elevated blood pressure and reduced stroke volume[30]. At present, the most common adverse effects that have been reported include subcutaneous hemorrhage, rhabdomyolysis, numbness, bruising, and delayed onset muscle soreness[22,31]. BFR remains contraindicated in patients with a history of clotting disorders, deep vein thrombosis, hypertension, diabetes, and peripheral vascular disease[31]. While serious side effects appear to be minimal when BFR is performed in adherence with recommended guidelines, the adverse effects of BFR are still being explored due to only recent integration into clinical practice.
Selection of the appropriate BFR protocol is important to optimize training benefits and prevent injury. BFR appears to provide the greatest benefit when combined with low-load resistance exercise, which is generally defined as 20%-40% of 1-repetition maximum[32]. Current guidelines recommend 4 sets of 30-15-15-15 repetitions (75 total repetitions) using a cuff pressure between 40-80% of the total limb occlusion pressure, although lower pressures may be needed in patients with smaller limbs or to avoid discomfort[16].
Limitations inherent to this analysis include the lack of a standardized BFR protocol and the comparison of studies with very different sample sizes. While 7 out of 10 studies included in this review utilized the 30-15-15-15 repetition scheme with a limb arterial occlusion pressure of 80% for a total of 75 repetitions, heterogeneity among BFR protocols in the remaining 3 studies may affect outcomes. To enhance comparability across research, future studies should utilize a BFR protocol that aligns with current clinical concepts, such as the frequently cited 30-15-15-15 protocol used by the majority of studies included in this review[16,31]. Additional limitations of this study include the paucity of literature available on this topic. Although the inclusion of case reports was deemed necessary, the lack of a control group makes it difficult to contextualize the data presented in these two studies. To address this limitation, all results were weighted according to sample size when pooling data from multiple studies. Nonetheless, additional randomized-controlled trials are needed to improve the overall power and generalizability of these results.
BFR is an innovative tool with valuable applications in foot and ankle injury rehabilitation. Compared to traditional rehabilitation alone, BFR is more effective in improving muscle strength, size, and activation. Additional studies are needed to better understand the physiologic mechanisms responsible for these changes and to develop a standardized training protocol.
| 1. | Wortman RJ, Brown SM, Savage-Elliott I, Finley ZJ, Mulcahey MK. Blood Flow Restriction Training for Athletes: A Systematic Review. Am J Sports Med. 2021;49:1938-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 2. | Saraf A, Goyal M, Goyal K. Blood Flow Restriction Training-An Overview and Implication in New Generation Physical Therapy: A Narrative Review. J Lifestyle Med. 2022;12:63-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 3. | Arriel RA, Rodrigues JF, Souza HLR, Meireles A, Leitão LFM, Crisafulli A, Marocolo M. Ischemia-Reperfusion Intervention: From Enhancements in Exercise Performance to Accelerated Performance Recovery-A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | de Freitas MC, Gerosa-Neto J, Zanchi NE, Lira FS, Rossi FE. Role of metabolic stress for enhancing muscle adaptations: Practical applications. World J Methodol. 2017;7:46-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45:187-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 356] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 6. | Brandner CR, Warmington SA, Kidgell DJ. Corticomotor Excitability is Increased Following an Acute Bout of Blood Flow Restriction Resistance Exercise. Front Hum Neurosci. 2015;9:652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Cognetti DJ, Sheean AJ, Owens JG. Blood Flow Restriction Therapy and Its Use for Rehabilitation and Return to Sport: Physiology, Application, and Guidelines for Implementation. Arthrosc Sports Med Rehabil. 2022;4:e71-e76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 8. | Werasirirat P, Yimlamai T. Effect of supervised rehabilitation combined with blood flow restriction training in athletes with chronic ankle instability: a randomized placebo-controlled trial. J Exerc Rehabil. 2022;18:123-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 9. | Lobo CC, Morales CR, Sanz DR, Corbalán IS, Marín AG, López DL. Ultrasonography Comparison of Peroneus Muscle Cross-sectional Area in Subjects With or Without Lateral Ankle Sprains. J Manipulative Physiol Ther. 2016;39:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 14304] [Article Influence: 1430.4] [Reference Citation Analysis (1)] |
| 11. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51068] [Article Influence: 10213.6] [Reference Citation Analysis (2)] |
| 12. | de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1498] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 13. | Cashin AG, McAuley JH. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J Physiother. 2020;66:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 601] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 14. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 6125] [Article Influence: 266.3] [Reference Citation Analysis (0)] |
| 15. | Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1492] [Cited by in RCA: 1523] [Article Influence: 138.5] [Reference Citation Analysis (0)] |
| 16. | Burkhardt M, Burkholder E, Goetschius J. Effects of Blood Flow Restriction on Muscle Activation During Dynamic Balance Exercises in Individuals With Chronic Ankle Instability. J Sport Rehabil. 2021;30:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Mahmoud WS, Radwan NL, Ibrahim MM, Hasan S, Alamri AM, Ibrahim AR. Effect of blood flow restriction as a stand-alone treatment on muscle strength, dynamic balance, and physical function in female patients with chronic ankle instability. Medicine (Baltimore). 2023;102:e35765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Okamura K, Hamaguchi M, Ueno Y, Kida T. Effect of Neuromuscular Electrostimulation With Blood Flow Restriction on Acute Muscle Swelling of the Abductor Hallucis. J Sport Rehabil. 2024;33:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Wen Z, Zhu J, Wu X, Zheng B, Zhao L, Luo X, Wu Z. Effect of Low-Load Blood Flow Restriction Training on Patients With Functional Ankle Instability: A Randomized Controlled Trial. J Sport Rehabil. 2023;32:863-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Clark K, Trickett J, Donovan L, Dawson J, Goetschius J. Effects of Blood Flow Restriction on Balance Performance During Dynamic Balance Exercises in Individuals With Chronic Ankle Instability. J Sport Rehabil. 2024;33:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Killinger B, Lauver JD, Donovan L, Goetschius J. The Effects of Blood Flow Restriction on Muscle Activation and Hypoxia in Individuals With Chronic Ankle Instability. J Sport Rehabil. 2020;29:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Larsen P, Platzer OJ, Lollesgaard L, Pedersen SK, Nielsen PK, Rathleff MS, Bandholm T, Jensen ST, Elsoe R. Blood-flow restricted exercise following ankle fractures - A feasibility study. Foot Ankle Surg. 2022;28:726-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Yow BG, Tennent DJ, Dowd TC, Loenneke JP, Owens JG. Blood Flow Restriction Training After Achilles Tendon Rupture. J Foot Ankle Surg. 2018;57:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Mortensen L, Mechlenburg I, Langgård Jørgensen S. Low-Load Blood-Flow-Restricted Exercise to Prevent Muscle Atrophy and Decline in Functional Performance in a Patient Recovering From a Malleolus Fracture. A Case Report. Clin J Sport Med. 2023;33:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Patterson SD, Hughes L, Warmington S, Burr J, Scott BR, Owens J, Abe T, Nielsen JL, Libardi CA, Laurentino G, Neto GR, Brandner C, Martin-Hernandez J, Loenneke J. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front Physiol. 2019;10:533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 493] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 26. | Yoshida M, Aoki N, Taniguchi K, Yoshida M, Katayose M. Kinematic analysis of the ankle joint on the side-hop test in subjects with ankle sprains. Transl Sports Med. 2018;1:265-272. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Tennent DJ, Hylden CM, Johnson AE, Burns TC, Wilken JM, Owens JG. Blood Flow Restriction Training After Knee Arthroscopy: A Randomized Controlled Pilot Study. Clin J Sport Med. 2017;27:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 28. | Ohta H, Kurosawa H, Ikeda H, Iwase Y, Satou N, Nakamura S. Low-load resistance muscular training with moderate restriction of blood flow after anterior cruciate ligament reconstruction. Acta Orthop Scand. 2003;74:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Miller BC, Tirko AW, Shipe JM, Sumeriski OR, Moran K. The Systemic Effects of Blood Flow Restriction Training: A Systematic Review. Int J Sports Phys Ther. 2021;16:978-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Anderson AB, Owens JG, Patterson SD, Dickens JF, LeClere LE. Blood Flow Restriction Therapy: From Development to Applications. Sports Med Arthrosc Rev. 2019;27:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Lorenz DS, Bailey L, Wilk KE, Mangine RE, Head P, Grindstaff TL, Morrison S. Blood Flow Restriction Training. J Athl Train. 2021;56:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 32. | Scott BR, Loenneke JP, Slattery KM, Dascombe BJ. Exercise with blood flow restriction: an updated evidence-based approach for enhanced muscular development. Sports Med. 2015;45:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/