TO THE EDITOR
Kristjánsson et al[1] noted that mesenchymal stem cell (MSC) therapy for osteoarthritis (OA) offers advantages over traditional treatments like autologous chondrocyte implantation and microfracture, including lower invasiveness, long-lasting efficacy and ease of operation. MSC therapy’s effectiveness was confirmed by using visual analog scale (VAS) scores and magnetic resonance imaging (MRI)[1]. Soufan et al[2] further clarified the positive effects of MSCs on OA symptoms and knee joint structure. Adipose-tissue-derived MSCs (AD-MSCs) were most effective in the alleviation of symptoms and improvement of cartilage volume, while umbilical-cord-derived MSCs (UC-MSCs) relieved symptoms but structural effects were unclear. Generally, bone-marrow-derived MSCs (BM-MSCs) were less effective (Figure 1).
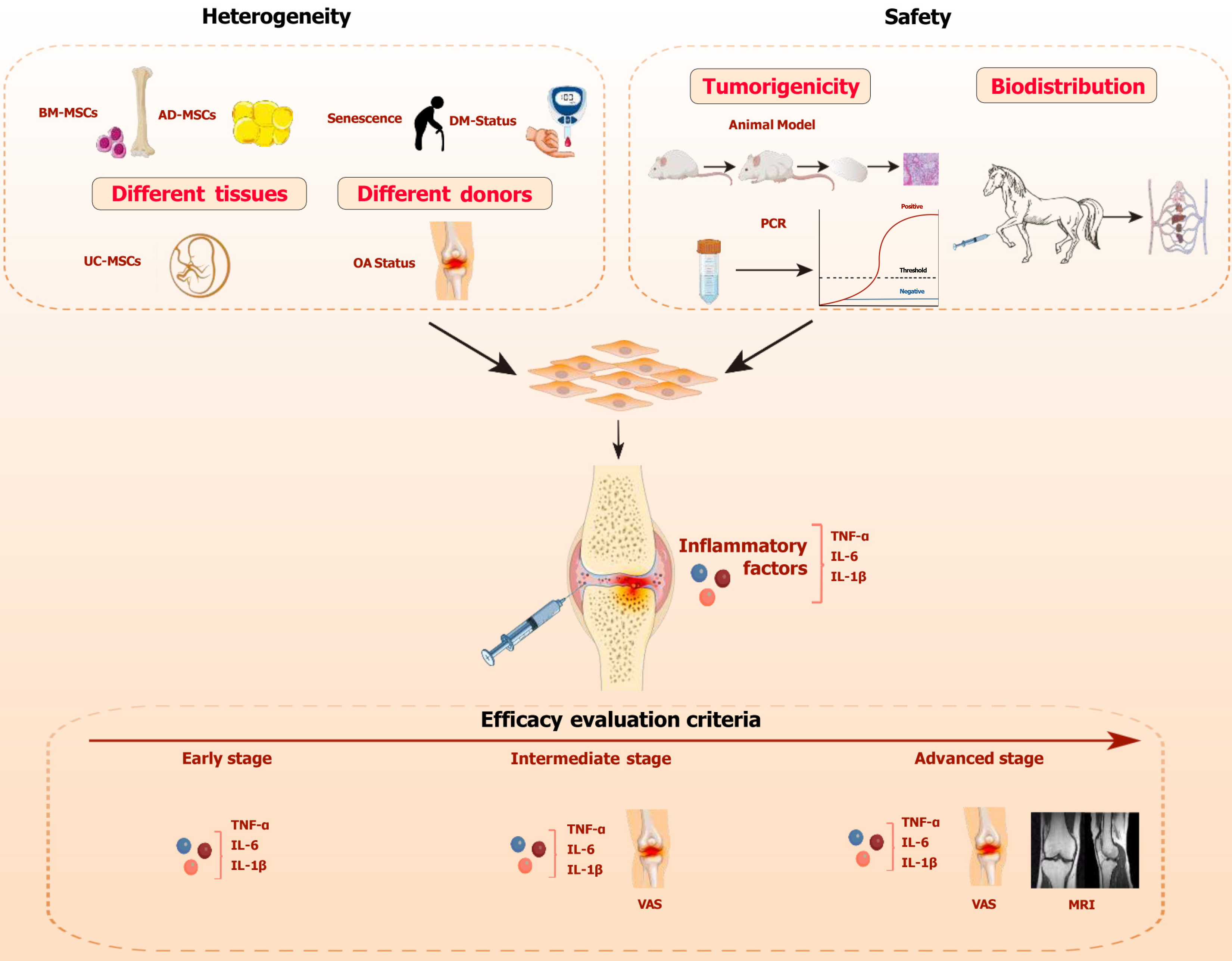
Figure 1 The key considerations for mesenchymal stem cell therapy in the treatment of osteoarthritis.
The dashed box highlights three key factors to consider: The heterogeneity of mesenchymal stem cell (MSCs), the safety of MSCs, and the standards for evaluating their efficacy. Heterogeneity includes tissue heterogeneity and donor heterogeneity. Safety encompasses tumorigenicity and biodistribution. The horizontal red arrow indicates the progression timeline of osteoarthritis, with different efficacy evaluation standards applied at each stage. MSC: Mesenchymal stem cell; OA: Osteoarthritis; BM-MSC: Bone-marrow-mesenchymal stem cell; AD-MSC: Adipose-tissue-derived mesenchymal stem cell; UC-MSC: Umbilical-cord-derived mesenchymal stem cell; IL: Interleukin; TNF-α: Tumor necrosis factor-alpha; VAS: Visual analog scale; MRI: Magnetic resonance imaging.
Efficacy evaluation criteria
The two articles represent significant advancements in OA regenerative medicine, introducing innovative treatments and research perspectives. It is worth noting that both authors utilized subjective scales and imaging changes in knee joints (such as changes in cartilage volume observed via MRI) as indicators to assess the efficacy of MSC therapy. However, subjective scales (such as the VAS), commonly used to assess OA severity, are affected by patients’ subjective perception, leading to considerable variability. Additionally, imaging follow-up can reflect therapeutic effects through changes in cartilage structure, but OA is not only a degenerative joint disease, but also a chronic inflammatory condition driven by systemic inflammation[3]. In the progression of OA, high levels of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 are key drivers of chondrocyte apoptosis, cartilage matrix degradation, cartilage collapse, and synovial infiltration[4]. MSCs produce prostaglandin E2, which inhibits the transcriptional activation of nuclear factor kappa-B (NF-κB), reducing the release of pro-inflammatory cytokines, while secreting anti-inflammatory factors such as TNF-α-stimulated gene 6 protein and IL-1 receptor antagonist to alleviate the inflammatory response[5]. The research indicated that the reduction in mRNA expression of IL-10, IL-1β, and TNF-α is associated with the alleviation of OA symptoms[6]. Similarly, Zhuang et al[7] discovered that indole-3-propionic acid could reduce the expression of inflammatory cytokines such as IL-6, TNF-α, inducible nitric oxide synthase, and cyclooxygenase-2 in joints with OA, alleviating symptoms. MSCs may inhibit OA progression by modulating inflammation and immune responses, rather than mainly relying on the cartilage differentiation potential of MSCs[4]. The increase in cartilage volume observed in OA patients after MSC therapy may be indirectly caused by MSCs through the inhibition of inflammatory cytokines in the joint cavity and immune modulation (such as MSCs’ regulation of macrophages to polarize from M1 to M2, which initiates of tissue regeneration and repair[8]). Therefore, imaging changes may not serve as a timely and effective indicator in a short period (such as 12 months). In conclusion, in the early clinical stage of OA (e.g., Kellgren-Lawrence grade 0), patients may present with minimal or no noticeable symptoms, experiencing only transient discomfort. For such cases, we recommend assessing treatment efficacy by monitoring trends in inflammatory markers, including TNF-α, IL-1β, and IL-6. If there is a downward trend or the values of inflammatory markers remain within the same range, it indicates that the treatment has effectively slowed the progression of OA and may even have reversed the inflammation in the joint cavity. However, if inflammatory markers continue to rise, it suggests that the treatment is ineffective and the disease is progressing. When the patient experiences persistent and significant pain, it signifies that the disease has progressed to the intermediate stage, at which point imaging exams often do not reveal significant changes. In the intermediate stage, characterized by prominent clinical symptoms, a combination of inflammatory markers and the VAS should be use for evaluation. Pain is the primary symptom at this stage, and VAS should be the main evaluation metric. However, since VAS is a subjective scoring system, it is prone to patient bias, so inflammatory markers should be used as secondary indicators. When patients are unable to perceive significant changes in pain, the trend in inflammatory markers can be used to assess the treatment efficacy. For advanced-stage patients, who exhibit clear radiological changes, assessment should integrate imaging examinations alongside inflammatory markers and VAS to provide a comprehensive evaluation. In imaging assessments, changes in cartilage volume are considered the gold standard for evaluating efficacy. However, changes in cartilage cannot be observed in the short term. Therefore, combining VAS and inflammatory markers can offer a more timely evaluation of disease progression and treatment effectiveness (Figure 1).
Heterogeneity
MSCs have high heterogeneity under the influence of factors like donor age, metabolic status, and pathophysiological conditions, affecting therapeutic outcomes. Kristjánsson et al[1] confirmed the efficacy and safety of MSC, but they did not address heterogeneity’s impact on clinical outcomes. Soufan et al[2] highlighted tissue source the heterogeneity, noting AD-MSCs’ superior efficacy over UC-MSCs and BM-MSCs, guiding clinical source selection[2]. Furthermore, the heterogeneity of different donors is also crucial when MSC products are selected. In aging organisms, the function of MSCs themselves deteriorates, and their stemness gradually diminishes. Additionally, within the joint cavity, senescent cells accumulate and release the senescence-associated secretory phenotype, which induces early senescence of MSCs in an NF-κB-dependent manner, thereby affecting MSC function[9]. Other studies have found that in elderly patients, MSCs are influenced by age-driven OA inflammation and the nutritional microenvironment, leading to a decline in chondrogenic differentiation potential and exacerbating cartilage degradation[10]. In diabetic patients, high blood glucose levels reduce the survival time of MSCs in the body. Hyperglycemia can inhibit MSC interactions through the AMPK pathway, weakening their anti-inflammatory capabilities[11]. The accumulation of advanced glycosylation end products in diabetic patients not only inhibits MSC differentiation and proliferation but also induces stem cell apoptosis[12]. Furthermore, in OA patients with specific genetic backgrounds, their MSCs may overexpress OA-related genes, which leads to a significant reduction in the differentiation potential and anti-inflammatory capacity of the MSCs[13] (Figure 1). OA patients are often old people and have multiple comorbidities, which complicates the selection of clinical research subjects and MSCs in MSC therapy. In MSC therapy, it is necessary to standardize research and selection of MSC product. To ensure consistency and reliability in clinical trials, it is essential to select patients with comparable age, gender, and comorbidity profiles, while also using MSCs derived from the same tissue and donor source. In clinical treatment, minimizing heterogeneity is particularly critical to improving the efficacy of MSCs in elderly OA patients with underlying comorbidities. Gene transduction of autologous MSCs in vitro holds promise for reducing heterogeneity. Piñeiro-Ramil et al[14] demonstrated that the transduction of simian virus 40 large T antigen and human telomerase reverse transcriptase enabled primary MSCs from elderly donors to become immortalized MSCs, overcoming issues such as the decline in proliferative capacity associated with donor aging[14]. Additionally, heterologous MSCs can also be sourced from completely healthy individuals. Studies have shown that UC-MSCs, which exhibit limited heterogeneity that can be further reduced during in vitro expansion, possess stronger immunomodulatory potential and are less likely to elicit alloimmune responses, making them the preferred choice for heterologous MSC therapy[15,16]. It is worth noting that even within the same batch of MSCs, different subtypes may vary significantly in their proliferation and differentiation potential. Induced pluripotent stem cell-derived MSC exhibit superior consistency in this regard, making them a promising direction for future MSC therapy development[17]. Moreover, MSC heterogeneity is closely associated with differences in cell cycle distribution. Specifically, heterogeneity is linked to the entry of MSCs into the G2/M phase, with a higher proportion of cells entering G2/M phase showing greater heterogeneity, which may represent a breakthrough for addressing the challenges posed by MSC heterogeneity[15]. Both autologous and heterologous MSCs experience telomere shortening, DNA damage accumulation, and increased adipogenic differentiation during in vitro expansion, significantly affecting their therapeutic efficacy in clinical applications[18]. Immortalized MSCs, generated through transduction technologies, can minimize these expansion-related effects and are ideal candidates for in vitro tissue engineering models[14]. In addition to in vitro expansion, the preservation of MSCs is also a crucial step for their clinical translation. Cryopreservation is currently the only effective long-term method to maintain stem cell viability and functionality, with slow freezing and vitrification being the primary techniques[19]. Both methods face certain limitations, as cryopreservation can impact cell function, particularly during thawing, leading to a decline in biological characteristics and immunomodulatory capacity[20]. These issues urgently require further research and optimization.
Safety
To date, more than 14000 MSC-related clinical studies have been registered on ClinicalTrials.gov. The European Union has approved MSCs for treating Crohn’s disease-related enterocutaneous fistulas, while MSC therapies have been approved for graft-versus-host disease in South Korea, Japan, and Canada[17]. Cartistem (MEDIPOST), containing allogeneic UC-MSCs and hyaluronic acid hydrogel, is the only MSC product approved globally for musculoskeletal disorders[21]. Clinical results indicate that after receiving Cartistem treatment, patients show the formation of hyaline-like cartilage. Significant improvements were observed in the International Cartilage Repair Society scores, VAS scores, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores, and International Knee Documentation Committee scores of the patients[22]. However, its limitations include the need for invasive open arthrotomy and defect drilling, which increase surgical complexity and trauma, and although short-term outcomes are promising, long-term follow-up indicates that there is no significant advantage in clinical and radiological improvements compared to traditional surgical approaches[21,23]. However, based on various existing clinical studies, it is undeniable that intra-articular injection of MSC has demonstrated long-term therapeutic efficacy. In a prospective double-blinded, randomized controlled Phase IIb clinical trial, the experimental group receiving intra-articular MSC injections showed significant improvement in WOMAC scores after six months of treatment, and notably delayed cartilage damage[24]. In another study comparing the efficacy of intra-articular MSC injections with hyaluronic acid injections, after 12 months of treatment, patients who received MSC injections exhibited significantly greater cartilage volume than those treated with hyaluronic acid[25]. Despite the growing use of MSCs, safety concerns still persist in clinical treatments. The authors of the two articles did not focus much on safety issues. According to the International Society for Stem Cell Research’s “Guidelines for Stem Cell Research and Clinical Translation”, preclinical studies on tumorigenicity and biodistribution should be done before MSC clinical trials to ensure safety and efficacy[26]. However, there is a lack of a standardized approach for assessing the safety and efficacy of MSC products. The main risk is their potential to induce tumor formation[27]. While short-term safety is reported in some clinical trials, long-term tumorigenic potential is not evaluated in most studies. In a preclinical evaluation of the safety and tumorigenicity of allogeneic human UC-MSCs administered at an ultra-high dose over a 26-week period, no evidence of tumor formation was observed[28]. Similarly, in another study with a longer follow-up period of 96 weeks investigating AD-MSCs for the treatment of OA, no tumor formation was detected[29]. Although these studies with longer follow-up periods have not observed tumor formation, the current body of research remains limited, and the evidence is not yet sufficient to draw definitive conclusions. Further studies with larger sample sizes and extended follow-up durations are necessary to comprehensively evaluate the long-term safety and potential tumorigenicity of MSC-based therapies. This is particularly important for ensuring the safe clinical translation of MSCs in regenerative medicine. Animal models are considered the gold standard for assessing tumorigenicity. Galpayage Dona et al[30] transplanted human embryonic stem cells into NODSCID-Gamma mice and assessed tumorigenicity by evaluating tumor size in the hind limbs, confirming their tumorigenic potential. Despite their high accuracy, animal models are impractical for routine clinical tumorigenicity testing due to long procedures. Kuroda et al[31] proposed to detect LIN28, a pluripotency and cancer stem cell gene, by using PCR to assess the safety and tumorigenic potential of stem cell products in about 2-3 hours, offering a practical alternative to animal models[31] (Figure 1). Additionally, whether MSCs are administered locally or systemically, biodistribution studies are essential to ensure both safety and efficacy. MSCs may persist or proliferate in vivo, so understanding their biodistribution is crucial for safety and dosage. Prolonged retention increases tumor risk, while migration to non-target sites may cause embolism or organ damage. Exogenous MSCs may also be recognized as foreign by the immune system. There are currently many tracking methods for the biodistribution of MSCs, such as PCR, optical imaging, flow cytometry, and MRI. Among them, fluorescent nanodiamonds are considered a promising MSC tracking technology due to their high sensitivity, background-free imaging, and the ability to avoid any negative impact on the biological activity of MSCs[27]. Thäte et al[32] found that MSCs were initially accumulated in the lungs and tissue persistence was low 24 hours after administration, while Zhuang et al[27] demonstrated that MSCs migrated toward injured sites using magnetic modulation for imaging[32,33]. Due to the blood-joint barrier, the biological distribution of MSCs following intra-articular injection differs from intravenous administration. In a rat OA model, after intra-articular injection of human AD-MSCs, the human FOXP2 gene was detected exclusively in joint tissues, indicating a localized distribution of MSCs via intra-articular injection[34]. This suggests that immune responses in the joint are typically confined to the intra-articular environment. Therefore, understanding immune activation within the joint cavity following MSC injection is critical. Joswig et al[35] performed multiple intra-articular injections of allogeneic or autologous MSCs in horses, observing significant immune activation after the second allogeneic MSC injection (Figure 1). In another study comparing the biological distribution of human BM-MSCs via intravenous and intra-articular routes in mice, it was found that MSCs were present at lower concentrations in peripheral blood following intra-articular injection, but persisted for a longer duration compared to intravenous injection. This raises concerns about the potential adverse effects of prolonged low-level MSC presence in peripheral blood, warranting further investigation[36]. In future studies, we must evaluate the safety and efficacy of stem cell therapies, with a focus on tumorigenicity and biodistribution of MSCs.