Published online Mar 18, 2025. doi: 10.5312/wjo.v16.i3.103169
Revised: January 25, 2025
Accepted: February 20, 2025
Published online: March 18, 2025
Processing time: 121 Days and 15.1 Hours
Posterior lumbar interbody fusion has good clinical results, but adjacent segment disease (ASD) affects its long-term efficacy. In patients with L4-5 fusion who were followed up for more than 10 years, the ASD incidence was 33.3%. Magnetic resonance imaging (MRI) is key for ASD diagnosis, but metal artifacts from internal fixation limit its use; therefore, removing the artifacts is crucial for ASD diagnosis and treatment.
To evaluate the value of WARP MRI for patients with lumbar ASD.
In our hospital, the lumbar spines of patients with ASD were assessed via lumbar MRI, including conventional sequences and sequences for artifacts. A PACS workstation was used for image measurement, analysis, and assessment, which mainly included measurement of the internal fixation implant artifact area, evaluation of the visibility of the anatomical structures surrounding the implant, and diagnostic assessment of ASD in the section. Conventional MRI data se
A total of 30 patients with ASD after lumbar fusion and internal fixation were included in the study; the patients included 13 male and 17 female patients and were aged 66.03 ± 5.83 years. The metal artifact area of the WARP T2-tirm se
WARP sequences can significantly reduce the artifact area in the sagittal and cross-sectional images of titanium alloy spinal fixation, providing a good imaging reference for the diagnosis of ASD.
Core Tip: WARP magnetic resonance sequences can significantly reduce the artifact area in the sagittal and cross-sectional planes of titanium alloy spinal fixation, and the advantage of eliminating artifacts in the sagittal plane is more obvious. The resolution of the vertebral body, pedicle, intervertebral foramen, nerve root canal and herniated nucleus pulposus was significantly improved, which made the structures in the intervertebral space of the adjacent vertebrae clearly visible, providing a good imaging reference for the diagnosis of adjacent segment disease.
- Citation: Xu W, Xiong MY, Wang Y, Yu QF, Ye XJ, Wang SL, Li ZK. Role of WARP sequence magnetic resonance imaging with the removal of metal artifacts in the evaluation of lumbar adjacent. World J Orthop 2025; 16(3): 103169
- URL: https://www.wjgnet.com/2218-5836/full/v16/i3/103169.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i3.103169
Although posterior lumbar interbody fusion (PLIF) has achieved satisfactory clinical results, adjacent segment disease (ASD) is an important complication that affects the long-term efficacy of PLIF[1]. Over at least 10 years of follow-up, the total incidence of L4-5 segmental lumbar fusion in patients with ASD was 33.3%[2]. Diagnosis of ASD via imaging relies on magnetic resonance imaging (MRI)[3], but after lumbar fusion, patients have metal internal fixation materials implanted in their vertebral bodies. During MRI examination, these metal artifacts from metal internal fixation materials seriously affect the assessment of nerve root compression in adjacent segments and the evaluation of spinal canal stenosis, making it difficult to clearly diagnose ASD and develop treatment options. Therefore, removing metal artifacts from the lumbar spine is particularly important for the diagnosis and treatment of ASD[4,5].
Metal artifacts on MRI are usually caused by magnetic susceptibility differences between metal implants and the surrounding tissue. Traditional technologies for reducing metal artifacts require low-field intensity magnetic resonance (MR) machines, which can increase the receiver bandwidth and matrix, decrease the slice thickness, and use the short tau inversion recovery or fat suppression Dixon method according to the axis of the movement frequency coding and phase encoding direction of the metallic implants[6]. The removal of metal artifact sequences is a type of nuclear magnetic resonance (NMR) technology that can effectively reduce the area of artifacts. Many studies have investigated knee joint prostheses and dental implant prostheses via MRI, but this approach has not been reported in patients with ASD[6-9].
The WARP sequence in MRI has a strong ability to suppress artifacts. In areas where magnetic susceptibility artifacts are likely to occur, such as areas with metal implants or bones, the wavelet transform technology of the WARP sequence can effectively correct the signal distortion caused by magnetic susceptibility. For example, after spinal surgery with metal internal fixation, the WARP sequence can suppress the artifacts around the metal and more clearly display the surgical area and surrounding tissues, providing more accurate images for postoperative review and evaluation. The subjects of this study were patients with ASD after lumbar spine surgery. Research on the application of artifact-reducing MRI in such patients is relatively rare.
The purpose of this study was to evaluate the quality of WARP magnetic resonance images with metal artifact removal and conventional magnetic resonance images with metal artifact removal in the evaluation of lumbar ASD as well as role of WARP MRI in the diagnosis and treatment of ASD.
This prospective study enrolled patients with ASD who were admitted to outpatient clinics from January 1, 2024. MRI was performed at our hospital. Two kinds of sequence examinations, including routine sequence and artifact WARP removal sequence examinations, were performed simultaneously on the MRI.
The inclusion criteria were as follows: (1) Patients who underwent single lumbar posterior interbody fusion and internal fixation; (2) Patients for whom lumbar surgery had been performed more than six months prior; (3) Patients who were clinically diagnosed with ASD; and (4) Patients who were no older than 80 years. The definition of ASD was as follows: Recurrent lumbar spinal stenosis or disc herniation in adjacent segments of the fusion segment, with clinical symptoms such as lower limb pain and numbness.
The exclusion criteria were as follows: (1) Patients with multilevel fusion; (2) Patients with lumbar infection; (3) Patients with lumbar tumor, and 4. patients with no internal fixation.
All the metal fixation implants that were used were titanium internal fixation systems. All the patients were informed and signed informed consent prior to the examination. Grouping: The conventional sequence MRI data were included in the control group, and the artifact sequence removal MRI data were included in the artifact sequence removal group.
MRI data were acquired via Siemens Magnetom Vida 3.0T superconducting MRI with a 32-channel spine phased-array coil. The scanning sequences included conventional T2FS sequences and WARP T2-STIR sequences in the distorted plane. The repetition time, echo time, field of view, slice thickness, slice spacing, matrix, and bandwidth of the scanning sequence are shown in Table 1.
| Items | Sagittal plane T2WI fs | Sagittal plane T2-STIR WARP | Transverse section T2WI | Transverse section T2-WARP |
| TR (ms) | 3770 | 2330 | 2880 | 2160 |
| TE (ms) | 95 | 33 | 94 | 106 |
| FOV (mm × mm) | 320 × 320 | 320 × 320 | 180 × 180 | 180 × 180 |
| Thickness (mm) | 3 | 3 | 4 | 4 |
| Interlamellar spacing (mm) | 1 | 1 | 0.4 | 0.4 |
| Matrix | 320 × 320 | 320 × 320 | 180 × 180 | 180 × 180 |
| Band width | 260 | 601 | 250 | 601 |
Two radiologists were highly qualified for image measurement, analysis, and evaluation, including internal fixation implant artifact area measurement, evaluation of the visibility of the implant surrounding anatomical structures, and evaluation of ASD sections, with a PACS workstation. Two radiologists, each with over 10 years of working experience, conducted two measurements with an interval of one week. The average value of the measurements was taken. Patient information was removed during each measurement.
Measurement of the susceptibility artifact area: The regions of interest were delineated on a Siemens Syngo Via workstation. The area of the metal artifacts on the maximum slice corresponding to the sagittal and cross-sectional T2-STIR sequences and T2-STIR-WARP optimized sequences was measured, including the metal prosthesis and the susceptibility artifacts caused by the metal prosthesis. The choice of pedicle screw enhancement is the clearest cross-sectional measurement. The MRI of each patient were measured twice and averaged.
The anatomy of the original decompression section around the implant, including the vertebra, pedicle, intervertebral nerve, nerve roots, and spinal canal, was visible via the visibility of the pedicle screw screen, and the scoring criteria were as follows: Level 0, completely invisible; Grade 1, the visible area was less than 25% of the anatomical structure; Grade 2, the visible area was between 25% and 50%; Grade 3, the visible area was between 50% and 75%; Grade 4, the visible area exceeded 75% of the expected area but was slightly affected by metal artifacts; and Grade 5, the visible area was completely visible.
The same visibility assessment method was used to assess the visibility of the intervertebral space of the upper and lower adjacent vertebrae for internal fixation, and the adjacent segment lesions were described in detail, including the type of lumbar disc herniation and the type of lumbar spinal stenosis.
SPSS 22.0 software was used for statistical analysis. A paired t test was used to compare the metal artifact areas between the two sequences, and P < 0.01 was considered to indicate a statistically significant difference. The Wilcoxon Rank Sum Test was used to evaluate the difference in the visibility of the anatomical structures around the implants. P < 0.01 was considered to indicate a statistically significant difference. Reliability evaluation was performed via kappa consistency analysis between the observers; a k value > 0.75 indicated good consistency, a k value between 0.4-0.75 indicated better consistency, and a k value < 0.4 indicated poor consistency. The measurement data are presented as the mean ± SD.
A total of 30 patients with ASD after lumbar fusion and internal fixation were included in the study. There were 13 male and 17 female patients, aged from 57 to 76 years, with an average age of 66.03 ± 5.83 years. The fusion levels were L4-5 in 16 patients, L3-4 in 8 patients, and L5-S1 in 6 patients. The average postoperative period was 12.8 ± 8.1 months.
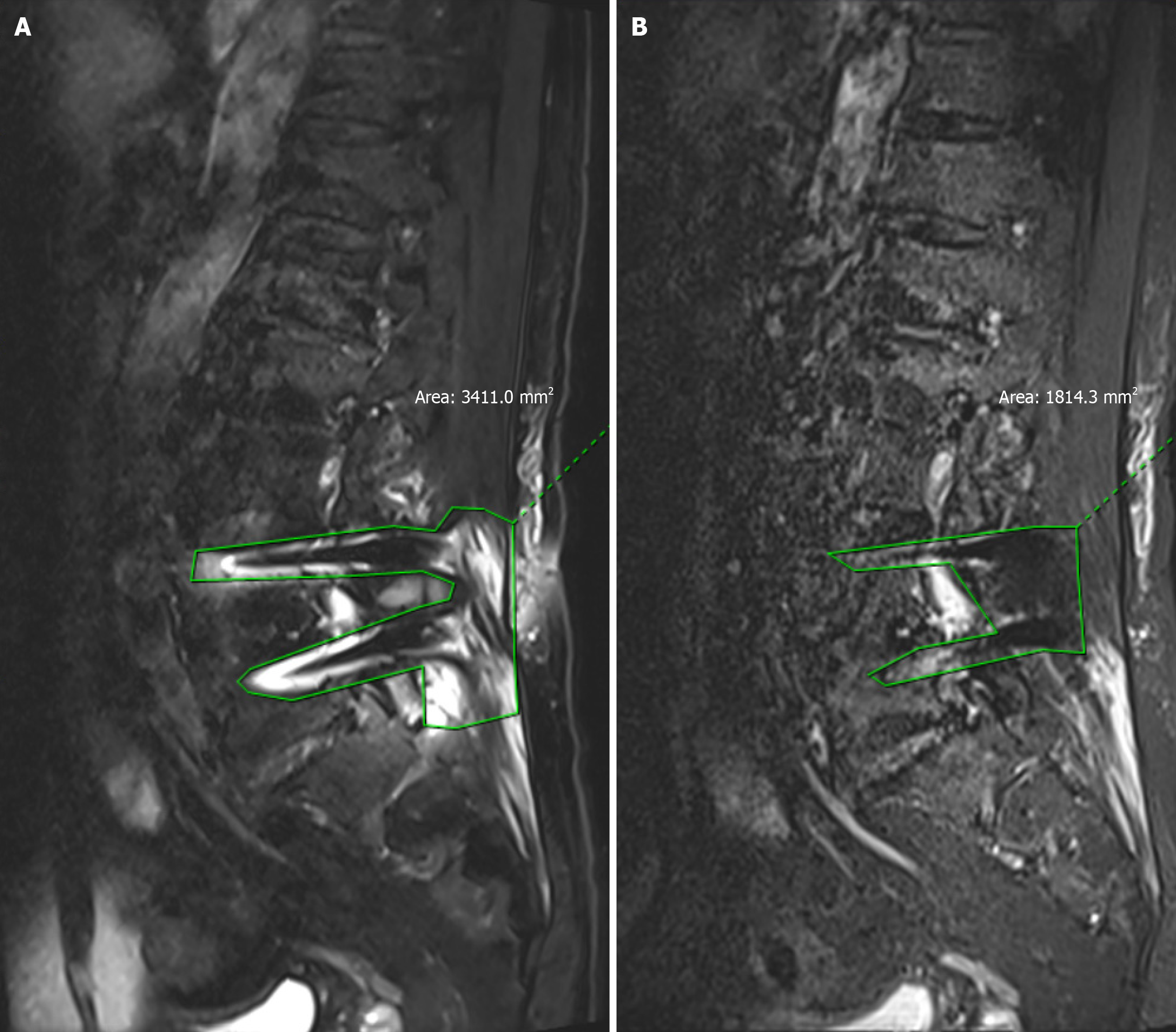
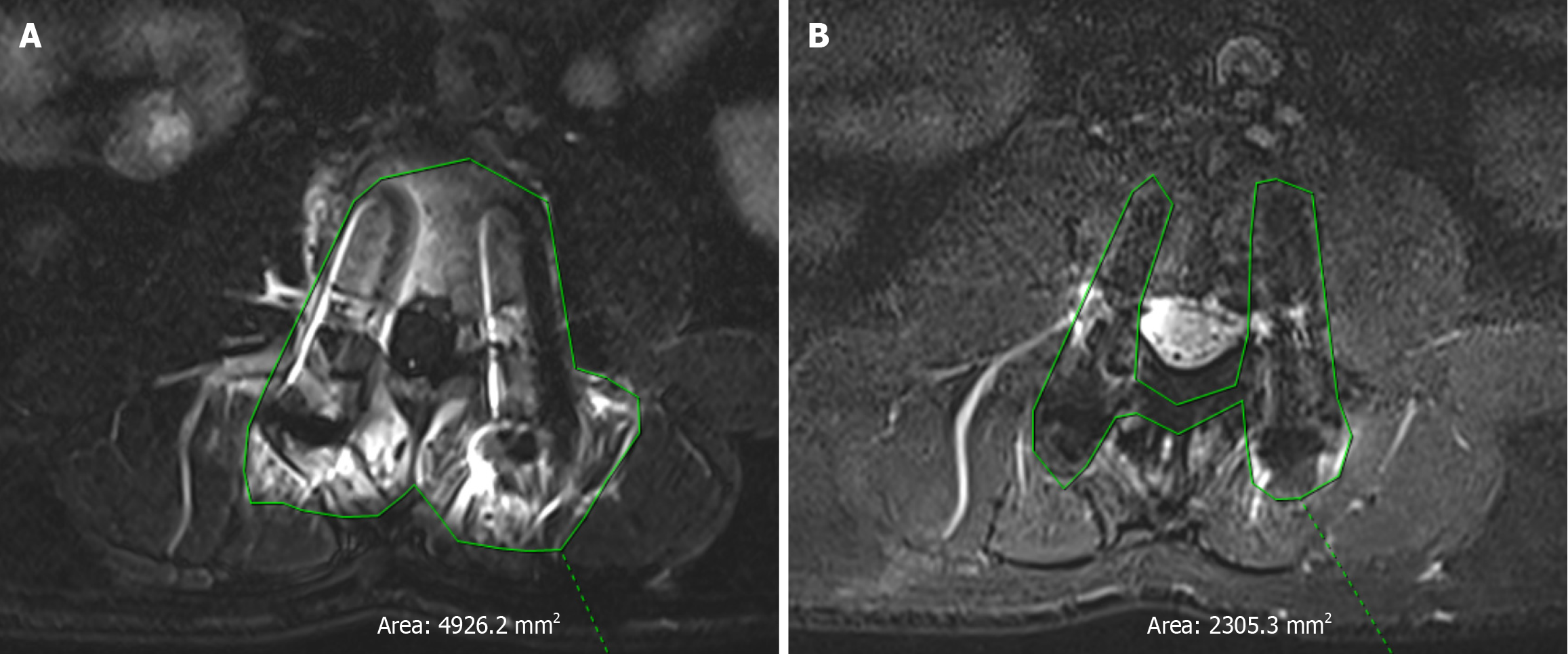
WARP T2-tirm sequence and conventional STIR sequence on the sagittal view of the metal artifact area were (20.85 ± 6.27) and (50.56 ± 8.55) cm2, t = 18.20, P = 0.000, P < 0.01, respectively, and the difference was statistically significant. The metal artifact area of the WARP T2-tirm sequence was significantly smaller than that of the conventional STIR sequence (Figures 1 and 2; Table 2).
| Grouping | Sagittal plane (cm2) | Transverse section (cm2) |
| WARP group | 20.85 ± 6.27 | 23.02 ± 6.00 |
| Control group | 50.56 ± 8.55 | 24.61 ± 4.37 |
| t | 18.20 | 21.00 |
| P value | < 0.01 | < 0.01 |
A total of 30 patients with ASD after lumbar fusion and internal fixation were included in the study. There were 13 male and 17 female patients, aged from 57 to 76 years, with an average age of 66.03 ± 5.83 years. The fusion levels were L4-5 in 16 patients, L3-4 in 8 patients, and L5-S1 in 6 patients. The average postoperative period was 12.8 ± 8.1 months.
The two imaging methods that were used in the WARP T2-tirm sequence and conventional STIR sequence at the sagittal position around the implant vertebral pedicle were found to be consistent in terms of the visibility of the intervertebral foramen and nerve root and the kappa value range of 0.713-0.809. When P < 0.001 according to the Wilcoxon Rank Sum Test, the differences were considered to be statistically significant. The WARP T2-tirm sequence clearly revealed the vertebral body, pedicle, and intervertebral foramen around the implants, whereas the STIR sequence clearly revealed the nerve roots in the intervertebral foramen (Table 3).
| Grouping | Ver vertebral body | Vertebral pedicle | Intervertebral nerve foramen | Nerve root |
| WARP group | 3.03 ± 0.67 | 1.97 ± 0.56 | 3.63 ± 0.61 | 2.80 ± 0.48 |
| Control group | 1.87 ± 0.63 | 0.73 ± 0.58 | 3.00 ± 0.45 | 2.10 ± 0.48 |
| t | 24.85 | 19.37 | 32.36 | 31.671 |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
In all 30 patients, all adjacent segments of the WARP T2-tirm sequence could be clearly observed (Grade 4 or higher), whereas it was difficult to determine the conventional STIR sequence because of the presence of metal artifacts. The WARP T2-sequences included sequences from 18 patients that clearly revealed upper ASD and sequences from 12 patients that revealed the presence of ASD. There were 15 patients diagnosed with lumbar disc herniation and 15 patients diagnosed with lumbar spinal stenosis. The WARP sequence data combined with clinical symptoms were used for the physical examination of ASD in 26 patients, including 15 patients who underwent reoperation (Table 4).
| Grouping | Canalis spinalis | Herniation of the nucleus pulposus |
| WARP group | 4.33 ± 0.55 | 4.67 ± 0.48 |
| Control group | 2.30 ± 0.75 | 2.33 ± 0.96 |
| t | 43.42 | 53.31 |
| P value | < 0.001 | < 0.001 |
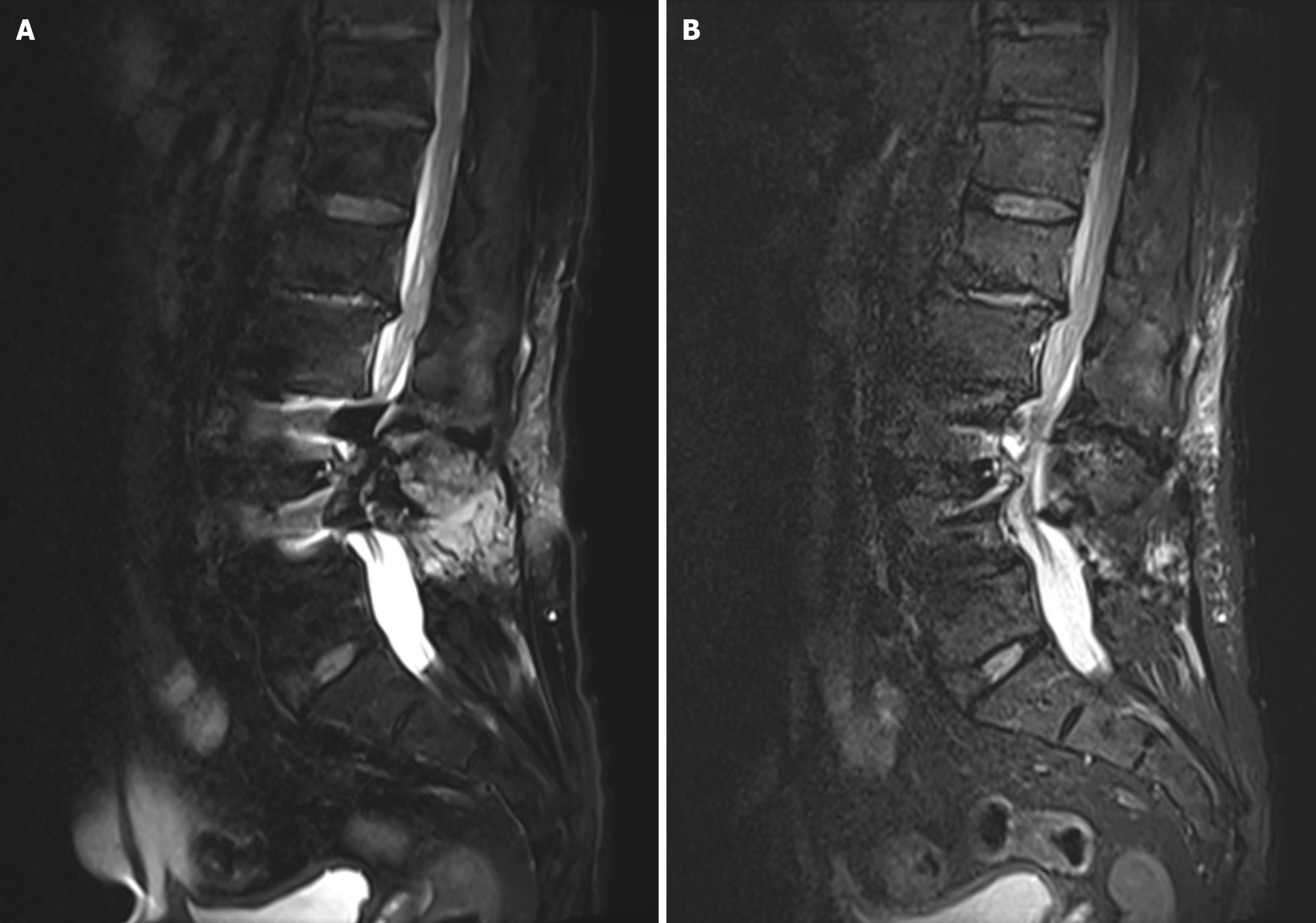
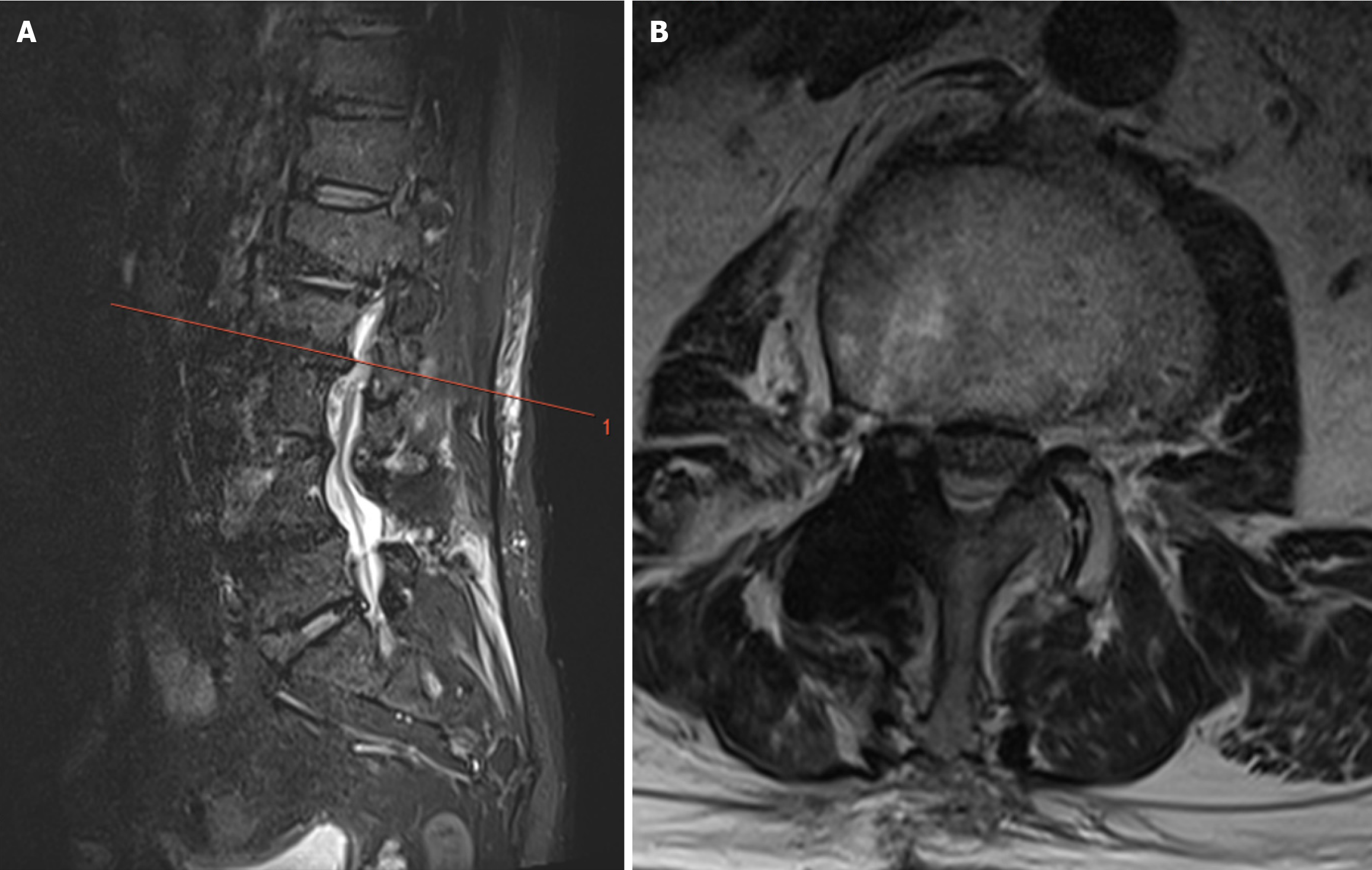
A 71-year-old male presented with radicular pain and numbness in the right lower limb 2 years after L3-4 PLIF surgery. Compared with conventional MRI sequences, the use of a deartifacting sequence clearly revealed the spinal canal and the herniated intervertebral disc in the adjacent vertebrae, with a significant reduction in the range of metal artifacts. In the cross-sectional view, clear images of the spinal canal and the herniated nucleus pulposus were observed, which could help in the diagnosis of ASD (Figures 3 and 4).
ASD is characterized by a degenerative process that occurs adjacent to a previously fused spine segment, with new onset of clinical symptoms such as radiculopathy, myelopathy, or instability. The etiology is related to the natural history of the disease process, increased biomechanical stress at adjacent segments, clinical factors specific to the individual patient, intraoperative factors, and malalignment. Treatment is usually nonoperative, but surgical intervention can be indicated[3]. ASD was defined as follows: Radiological ASD (R-ASD), which was defined as described by Nakashima from plain X-rays and MRI. Conditions characterized by narrowing of the disc height by > 3 mm, posterior opening > 5°, and progress of slippage > 3 mm in comparison with preoperative lateral flexion radiographs were considered R-ASDs[10]. MRI was also used to evaluate the postoperative progression of disc degeneration according to Pfirrmann’s classification, and the progression of spinal canal stenosis was evaluated according to Imagama’s classification. However, the artifacts caused by internal metal fixation during magnetic resonance examination seriously affect the imaging-based diagnosis of ASD. A study evaluating magnetic resonance artifacts after total cervical disc replacement revealed that stainless steel implants resulted in the worst deformation (Prestige ST), and the adjacent segment or the index segment cannot be fully displayed under these conditions[11].
The metal artifacts in functional MRI are usually composed of differences in metal magnetic susceptibility between the implant and the surrounding tissue caused by[6] the inevitable artifacts in MRI. WARP sequences have been used for imaging after hip replacement and oral and dental material implantation. Previous studies have shown that the WARP sequence can be used for imaging metal-on-metal hip surface reconstruction at 1.5T and 3T, and the image quality around the implant is good[12]. Another study revealed that, according to quantitative and qualitative image standards, the STIR warp and T1 warp sequences are obviously better, but only the number of STIR images with clinically relevant artifacts is lower[13]. Related studies suggest that when MRI is performed after orthodontic surgery, orthodontic appliances are usually kept in the mouth, which also produces many artifacts that affect the assessment of the disease. WARP has been shown to have a more significant effect in vitro than in vivo[14]. However, studies of WARP sequences after lumbar internal fixation are rare. One study used nonmagnetic carbon-enhanced PEEK implants and titanium spine implants to compare metal artifacts via MRI. This study revealed that prosthesis artifacts were significantly reduced when C-FRP screws were used. The use of an MRI artifact reduction sequence can reduce the number of artifacts related to the implant. This effect was stronger in titanium screw constructs than in C-FRP screw constructs[15].
Compared with conventional MRI, this method allows the removal of artifact magnetic resonance data for the diagnosis of ASD, making the figure line clearer. The sagittal metal artifact area of the T2-tirm sequence was significantly smaller than that of the STIR sequence [(20.85 ± 6.27) cm² vs (50.56 ± 8.55) cm², P < 0.01]. Although there were significant differences in the cross-section, 23.02 ± 6.00 cm2and 24.61 ± 4.37 cm2 (P < 0.01), the difference in size of the cross-section was lower than that of the sagittal plane. Vertebral body image structure; subjective observation of artifacts in the vertebral body, vertebral body, pedicle, intervertebral neural foramen, nerve root, and spinal canal; and highlighting of the image quality of the nucleus pulposus via conventional magnetic resonance (NMR) (P < 0.01). MRI with artifact removal has significant advantages in the evaluation of ASD after lumbar surgery by imaging. This study focused on adjacent intervertebral spaces in 30 patients. In all 30 patients, all the adjacent segments were visible (Grade 4 or higher) via the MR sequences with artifacts removed, while it was difficult to visualize these segments in the conventional STIR sequences because of the presence of metal artifacts. The results revealed that 26 patients were diagnosed with ASD via the sequence with artifact removal combined with clinical symptoms and physical examination, and 15 of the patients underwent reoperation. Specific lesions can be assessed by removing artifact sequence segment types and pathological changes to analyze the causes of ASD due to disc herniation and lumbar spinal stenosis, which can lead to the use of different treatment methods; for example, in patients with lumbar disc disease caused by ASD, the lateral lumbar road endoscopic technique can be chosen. If lumbar vertebral canal stenosis is caused by ASD, we can use UBE technology. The removal of artifacts to allow clinicians to choose the appropriate sequence for decision-making related to the treatment of ASD has great clinical application prospects and value.
ASD following lumbar spine surgery is a relatively common postoperative complication. In the process of diagnosing ASD, MRI serves as a crucial objective basis. However, the presence of lumbar internal fixators results in significant internal fixation artifacts. These artifacts severely interfere with the observation of the adjacent vertebral region, often obscuring the intervertebral discs and intraspinal structures of the adjacent vertebrae. This makes it difficult for doctors to determine whether the nucleus pulposus has herniated or determine the specific location of the herniation, greatly increasing the diagnostic difficulty and thus having a direct negative impact on the formulation and implementation of the patient's treatment plan. The results of this study indicate that the application of WARP sequence MRI technology can effectively eliminate internal fixation artifacts and clearly display the intervertebral discs and intraspinal structures of adjacent vertebrae. When nucleus pulposus herniation occurs in the intervertebral foramen region, lumbar lateral-approach transforaminal endoscopic discectomy is usually selected as the treatment plan. This surgical method has advantages such as minimal trauma and rapid recovery, and it can accurately address lesions in the intervertebral foramen region. When the nucleus pulposus has central-type herniation, interbody fusion surgery becomes a more appropriate choice. This surgical approach can effectively stabilize the spinal structure and resolve the problems associated with central-type herniation. WARP-sequence MRI technology significantly improves image clarity, providing doctors with more accurate and detailed imaging information. This technology plays a pivotal role in the development of surgical plans and the clinical decision-making process, strongly promoting the precision and scientific nature of the treatment of ASD after lumbar spine surgery.
This study has the following advantages. First, this study focused on ASD, and the results can help spinal surgeons diagnose ASD; this approach has not been previously reported. In this study, the examination time was optimized according to the previous MRI parameters to shorten the examination time. The examination time of the artifact reduction MRI was 8 minutes, and that of conventional MRI was 5 minutes. In addition, this study performed a cross-sectional artifact removal MRI examination, which has not been previously reported. On the other hand, this study has several limitations. First, the size of metal artifacts in the literature is always associated with the presence of significant metal materials[16]. In this study, the methods used for lumbar spinal internal fixation with titanium alloy were not the same, but this did not affect the results. Second, this was a single-center study, the number of individuals involved in the study was limited, and the sample size was small; however, the statistical results of this study were reliable.
WARP magnetic resonance sequences can significantly reduce the artifact area in sagittal and cross-sectional images of titanium alloy spinal fixation, and the advantage of eliminating artifacts in the sagittal plane was more obvious. The resolution of the vertebral body, pedicle, intervertebral foramen, nerve root canal, and herniated nucleus pulposus was significantly improved, which made the structures in the intervertebral space of the adjacent vertebrae clearly visible, providing a good imaging reference for the diagnosis of ASD.
| 1. | Okuda S, Nagamoto Y, Matsumoto T, Sugiura T, Takahashi Y, Iwasaki M. Adjacent Segment Disease After Single Segment Posterior Lumbar Interbody Fusion for Degenerative Spondylolisthesis: Minimum 10 Years Follow-up. Spine (Phila Pa 1976). 2018;43:E1384-E1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Jeong TS, Son S, Lee SG, Ahn Y, Jung JM, Yoo BR. Comparison of adjacent segment disease after minimally invasive versus open lumbar fusion: a minimum 10-year follow-up. J Neurosurg Spine. 2022;36:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 3. | McDonald CL, Alsoof D, Glueck J, Osorio C, Stone B, McCluskey L, Diebo BG, Daniels AH, Basques BA. Adjacent Segment Disease After Spinal Fusion. JBJS Rev. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 4. | Kohyama S, Yoshii Y, Okamoto Y, Nakajima T. Advances in Bone Joint Imaging-Metal Artifact Reduction. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Yuan C, Zhou J, Wang L, Deng Z. Adjacent segment disease after minimally invasive transforaminal lumbar interbody fusion for degenerative lumbar diseases: incidence and risk factors. BMC Musculoskelet Disord. 2022;23:982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 6. | Choo HJ, Lee SJ, Lee YH. [Metallic Artifacts on MR Imaging and Methods for Their Reduction]. Taehan Yongsang Uihakhoe Chi. 2020;81:41-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Cortes AR, Abdala-Junior R, Weber M, Arita ES, Ackerman JL. Influence of pulse sequence parameters at 1.5 T and 3.0 T on MRI artefacts produced by metal-ceramic restorations. Dentomaxillofac Radiol. 2015;44:20150136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Kumar NM, de Cesar Netto C, Schon LC, Fritz J. Metal Artifact Reduction Magnetic Resonance Imaging Around Arthroplasty Implants: The Negative Effect of Long Echo Trains on the Implant-Related Artifact. Invest Radiol. 2017;52:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Lindgren K, Anderson MB, Peters CL, Pelt CE, Gililland JM. The Prevalence of Positive Findings on Metal Artifact Reduction Sequence Magnetic Resonance Imaging in Metal-on-Metal Total Hip Arthroplasty. J Arthroplasty. 2016;31:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Nakashima H, Kawakami N, Tsuji T, Ohara T, Suzuki Y, Saito T, Nohara A, Tauchi R, Ohta K, Hamajima N, Imagama S. Adjacent Segment Disease After Posterior Lumbar Interbody Fusion: Based on Cases With a Minimum of 10 Years of Follow-up. Spine (Phila Pa 1976). 2015;40:E831-E841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Fayyazi AH, Taormina J, Svach D, Stein J, Ordway NR. Assessment of Magnetic Resonance Imaging Artifact Following Cervical Total Disc Arthroplasty. Int J Spine Surg. 2015;9:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Lazik A, Landgraeber S, Schulte P, Kraff O, Lauenstein TC, Theysohn JM. Usefulness of metal artifact reduction with WARP technique at 1.5 and 3T MRI in imaging metal-on-metal hip resurfacings. Skeletal Radiol. 2015;44:941-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 13. | Sutter R, Ulbrich EJ, Jellus V, Nittka M, Pfirrmann CW. Reduction of metal artifacts in patients with total hip arthroplasty with slice-encoding metal artifact correction and view-angle tilting MR imaging. Radiology. 2012;265:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Zachriat C, Asbach P, Blankenstein KI, Peroz I, Blankenstein FH. MRI with intraoral orthodontic appliance-a comparative in vitro and in vivo study of image artefacts at 1.5 T. Dentomaxillofac Radiol. 2015;44:20140416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Osterhoff G, Huber FA, Graf LC, Erdlen F, Pape HC, Sprengel K, Guggenberger R. Comparison of metal artifact reduction techniques in magnetic resonance imaging of carbon-reinforced PEEK and titanium spinal implants. Acta Radiol. 2022;63:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Johannsen KM, de Carvalho E Silva Fuglsig JM, Hansen B, Wenzel A, Spin-Neto R. Magnetic resonance imaging artefacts caused by orthodontic appliances and/or implant-supported prosthesis: a systematic review. Oral Radiol. 2023;39:394-407. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/