Published online Mar 18, 2025. doi: 10.5312/wjo.v16.i3.101073
Revised: January 7, 2025
Accepted: February 12, 2025
Published online: March 18, 2025
Processing time: 189 Days and 15.5 Hours
A case study of multiple distinct levels of skipped thoracolumbar spine infection was reported in which 13 successful vacuum sealing drainage (VSD) surgeries were treated.
The patient underwent a total of 13 procedures within our medical facility, including five performed under local anesthesia and eight performed under general anesthesia. The source of the ailment was ultimately identified as Enterobacter cloacae. After the last procedure, the patient's symptoms were alle
The study demonstrates that the extreme lateral approach debridement combined with multiple VSD operations is a secure and successful method of treatment for recurrent spinal infection, providing an alternative to traditional surgery.
Core Tip: External approach was used to clear the infected lesion, and vacuum sealing drainage (VSD) was used to control lumbar infection. Previous studies have shown that incomplete clearance of lesions is the main cause of recurrent lumbar infections, and VSD can effectively address this issue. This case involves a jumping lesion, and Escherichia coli was isolated from the negative pressure drainage fluid of both lesions, indicating the feasibility of obtaining pathogenic microorganisms from negative pressure drainage fluid.
- Citation: Wu JJ, Chang ZQ. Treatment of refractory thoracolumbar spine infection by thirteen times of vacuum sealing drainage: A case report. World J Orthop 2025; 16(3): 101073
- URL: https://www.wjgnet.com/2218-5836/full/v16/i3/101073.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i3.101073
Infectious diseases of the spine (IDS), caused by various pathogenic microorganisms can affect various parts of the spine, such as the vertebral body, intervertebral disc, adnexa, spinal canal, and surrounding paravertebral tissues[1-3]. The incidence of IDS ranges from 1 to 2 per 100000[4] individuals. However, with aging and improvements in diagnostic technology, the incidence has increased in recent years[5]. Treatment of spinal infections is challenging, with postoperative recurrence rates of 8.3%-9.8% and mortality rates of 2%-7%[6]. Despite advances in medical technology and treatment methods, recurrence and mortality rates remain unchanged.
The high recurrence rate of IDS is often attributed to multiple factors, such as advanced age, extended disease duration, malnutrition due to prolonged consumption, impaired immunity, internal fixation, sequestration, inadequate debridement, and implantation of autologous or allogeneic bone[7,8]. Research into the causes of early mortality following surgery for spinal infections suggests that the propagation of hazardous microbes into the bloodstream can lead to bacteremia and subsequent septic shock[8].
High recurrence and mortality rates in individuals with IDS are largely attributed to the insufficient removal of pathogenic microorganisms through debridement. Additionally, the impact of the operation and implants can exacerbate the infection. This report presents a case of a patient with recurrent spinal infection who was successfully treated with a minimally invasive debridement procedure through an extreme lateral approach, combined with 13 cycles of continuous vacuum sealing drainage (VSD).
A 59-year-old Chinese man was admitted to the hospital on April 20, 2022, due to chronic lower back pain and discomfort in his right lower limb, which had persisted for the past 8 months.
The patient had low back pain symptoms 8 months ago without obvious cause, turning over pain, and the pain was significantly aggravated when the lumbar spine flexion and extension were active, and the symptoms were recurring.
This work has been reported in line with the SCARE criteria[9]. In March 2022, minimally invasive lesion debridement was performed at another hospital, although the exact surgical method was unknown. No pathogenic microorganisms were identified.
A history of hypertension for 8 years, treated with oral medication to lower blood pressure, and blood pressure control is still acceptable. Deny family history of infectious diseases and genetic history.
On physical examination, the vital signs were as follows: Body temperature, 36.1℃; blood pressure, 137/82 mmHg; heart rate, 96 beats per min; respiratory rate, 18 breaths per min. Upon admission, a physical examination revealed limited flexion and extension of the lumbar spine, along with extensive tenderness and pain upon percussion in the thoracolumbar region. The visual analogue scale (VAS) score was 8.
The laboratory results indicated the following: Red blood cell (4.24 × 109/L), hemoglobin (115 g/L), hematocrit (36.0%), C-reactive protein (CRP) (23.11 mg/L), erythrocyte sedimentation rate (74), interleukin 6 (8.19 pg/mL), serum amyloid A (54.30 mg/L), brucella agglutination tests, and T-SPOT was negative.
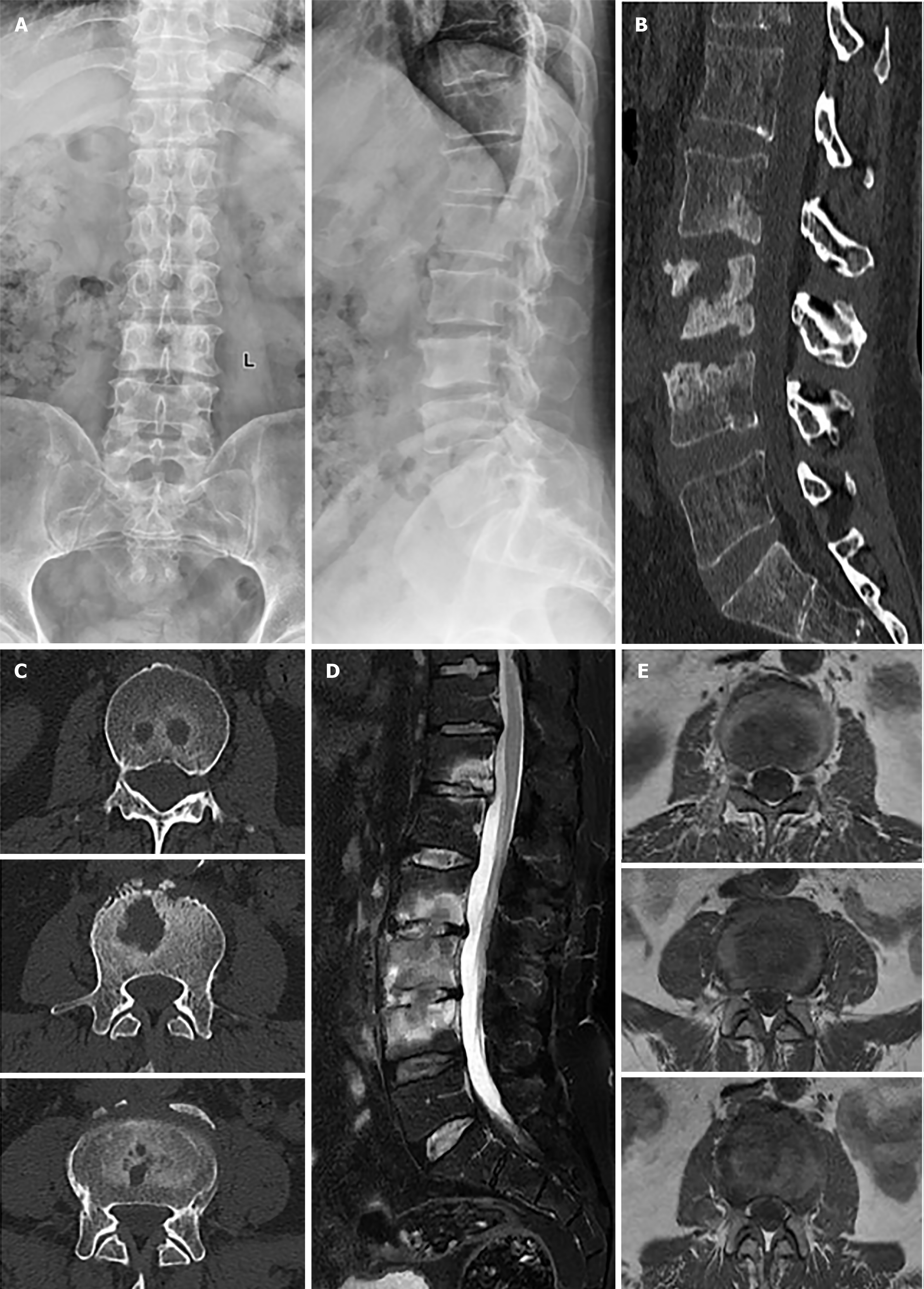
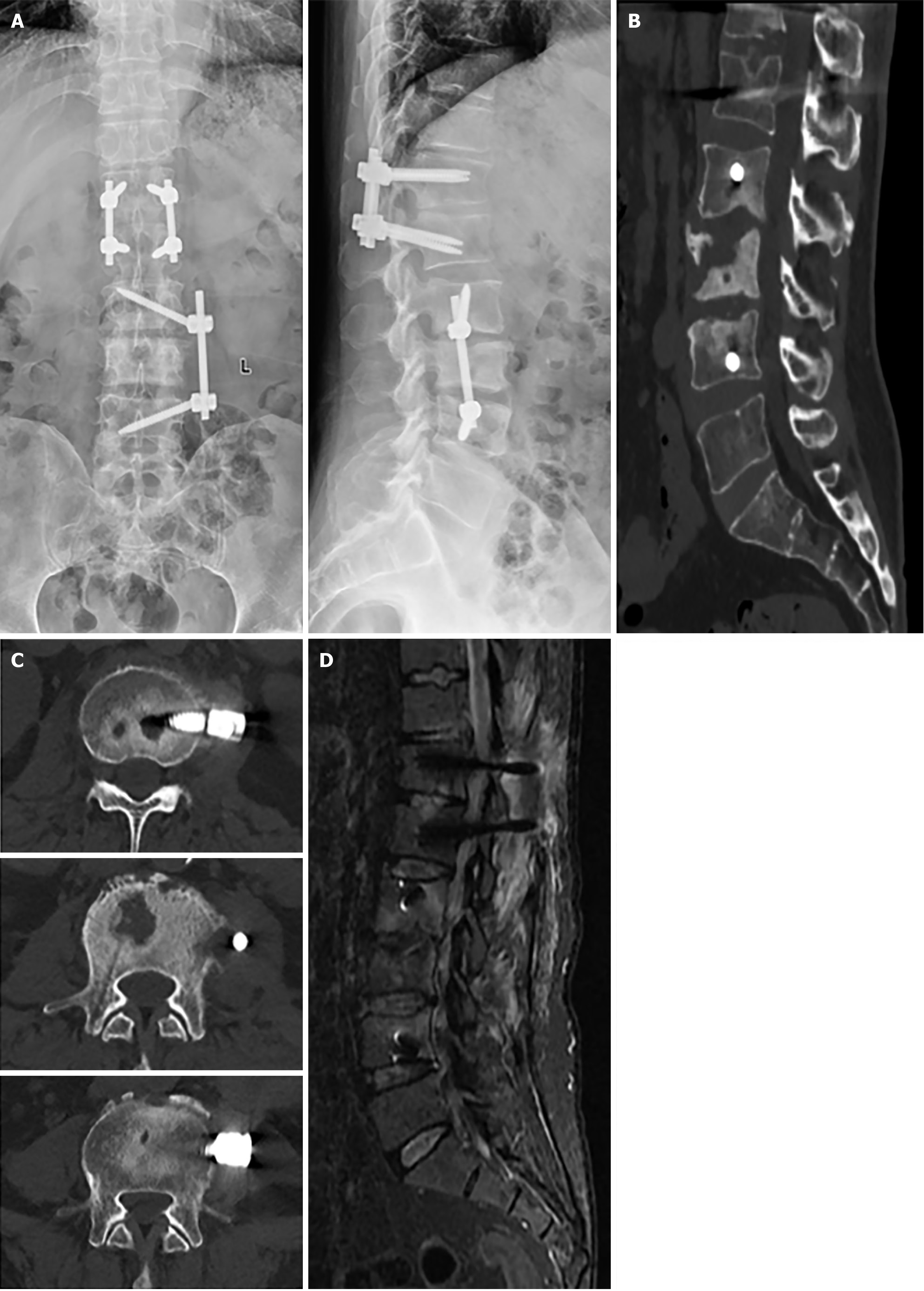
After admission, radiography, computed tomography (CT) and magnetic resonance imaging (MRI) were conducted (Figure 1). A is the anteroposterior and lateral radiography of the lumbar spine. B is the CT scan of the lumbar spine, axial CT images of the lumbar spine, demonstrating destruction of the L2-L4 vertebral bodies. D is lumbar MRI with T2-weighted lipid suppression. Axial T2-weighted image MRI showing intervertebral spaces (T12/L1, L2/3, and L3/4) A wide range of high signals is visible in T12/L1, and L2-L4.
Combined with the patient’s medical history, the final diagnosis was spine infection.
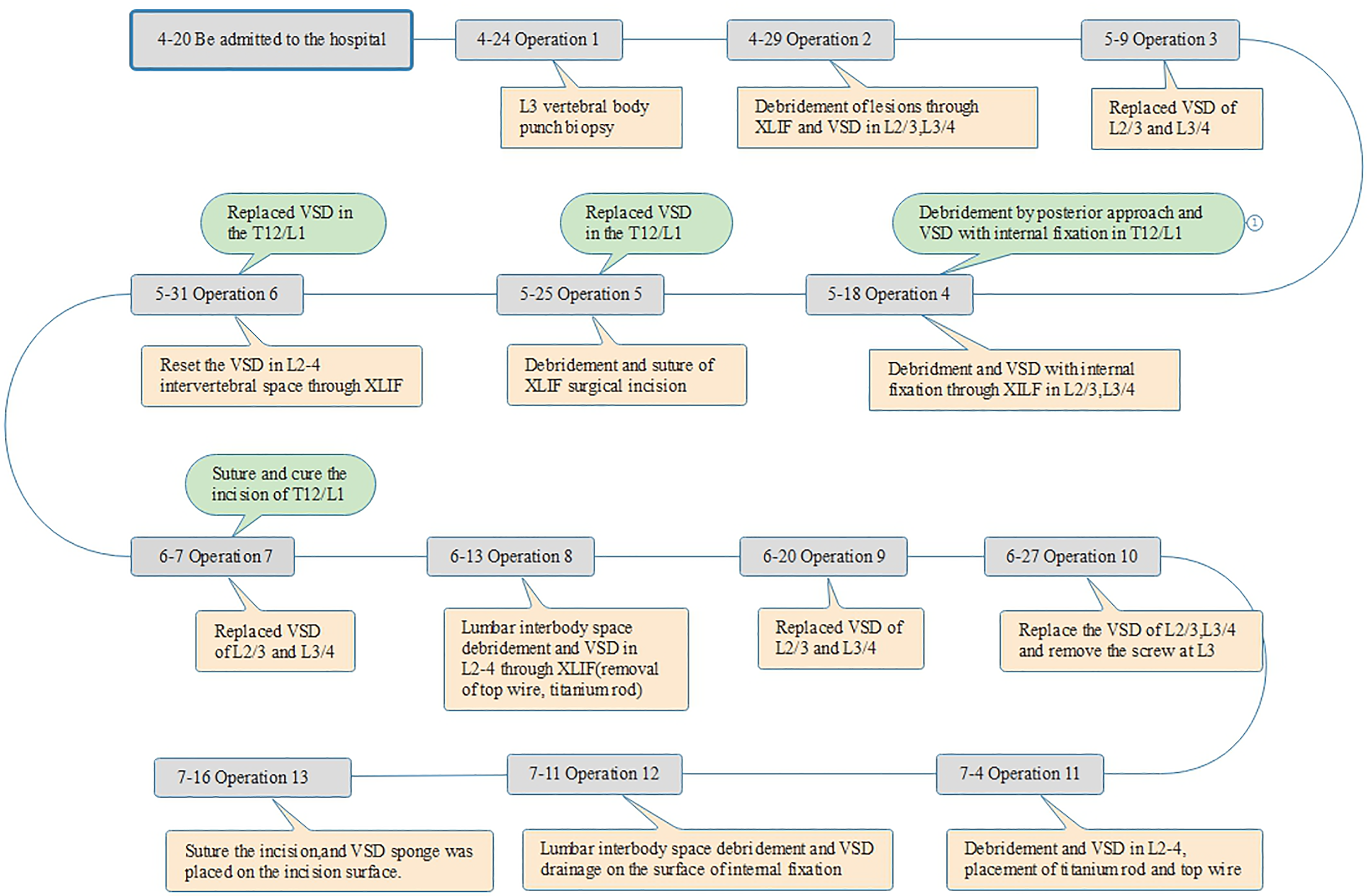
Following admission, in accordance with the patient's previous experience, a linezolid glucose injection 0.6 g was administered intravenously at a rate of 1/12 hours. The patient’s condition improved, and surgery was performed after ruling out any surgical contraindications. The non-contiguous pattern of lesions on the patient's body led to the variations in the duration of surgery. Initially, a lateral approach was applied at the L2/3/4 site, followed by a posterior approach at the T12/L1 site, as illustrated in Figure 2.
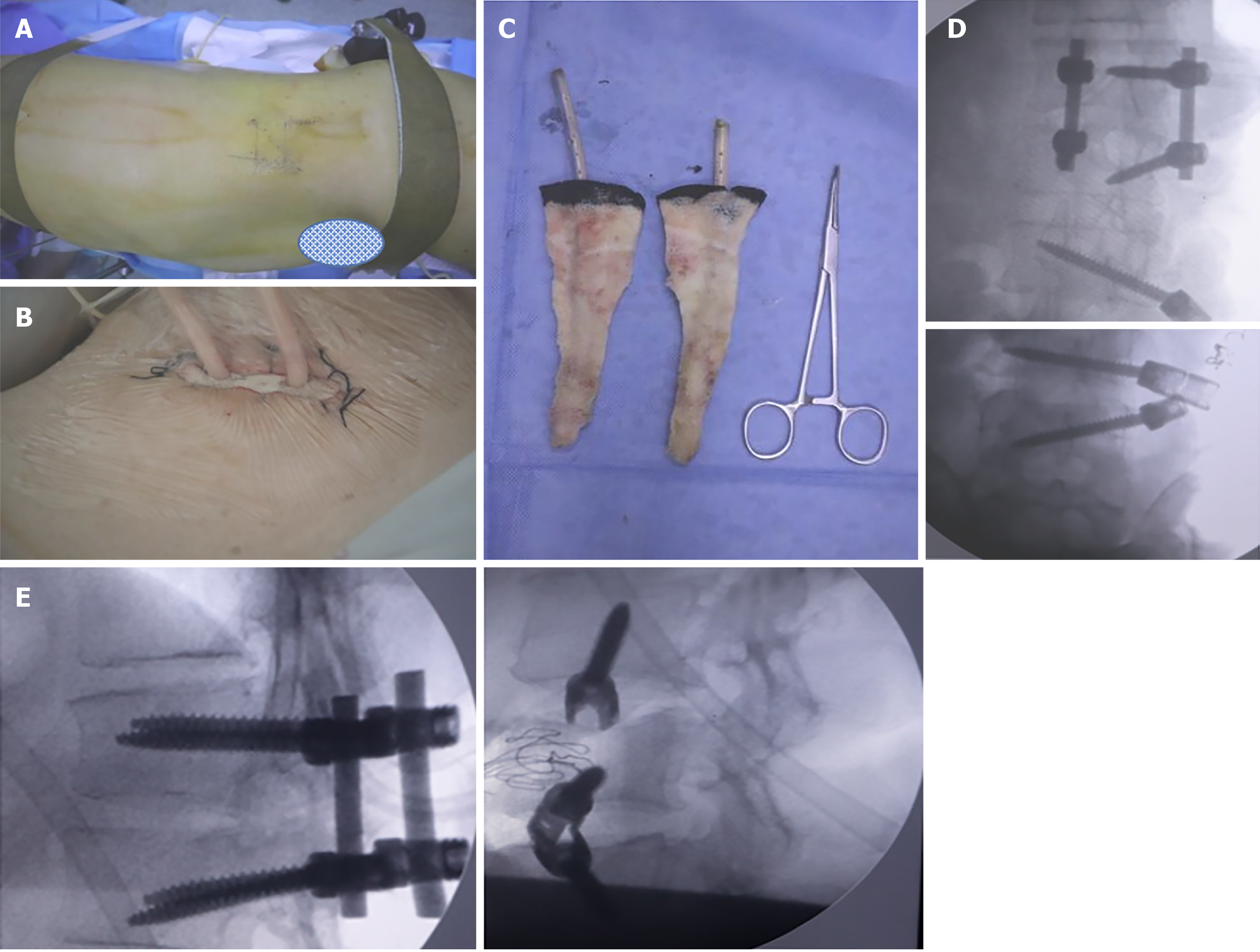
On April 24, 2022, an L3 vertebral body punch biopsy was performed under local anesthesia, and the bacterial culture yielded negative results. The lesions at L2/3 and L3/4 were debrided using an extreme lateral approach and VSD while the patient was under general anesthesia on April 29, 2022. The bacterial culture yielded negative results after the procedure, as shown in Figure 3A and B. After 10 days, the VSD was replaced. On May 18, two surgeries were performed: One in the T12/L1 area, where a posterior approach was employed for debridement and internal fixation of the VSD under general anesthesia. The left side was accessed using the Wiltse approach, while the right side underwent TLIF decompression and lesion debridement. The other two levels (L2/3 and L3/4) underwent debridement and VSD with internal fixation through an extreme lateral approach (utilizing titanium rods and top-wire insertion), as shown in Figure 3C-E.
Four days after the operation, a bacterial culture of the drainage fluid from the extreme lateral VSD revealed Enterobacter cloaca (++++). Based on the drug sensitivity test, the antibiotic was changed to imipenem/cilastatin sodium (1 g intravenous infusion administered every 12 hours). Debridement and suturing of the extreme lateral surgical incision were performed under local anesthesia on May 25, along with VSD was replacement at T12/L1. Five days after the suturing, the patient developed fever, with a temperature reaching 40.5℃. Blood culture results were negative. Upon examination, pus was found exuding from the extreme lateral surgical incision, and a bacterial culture of the pus yielded Enterobacter cloacae (++). Additionally, the negative-pressure drainage fluid from T12/L1 was sent for bacterial culture, which revealed Enterobacter cloacae (++). On May 31, the VSD was replaced in the T12/L1 and reset at the L2-4 intervertebral space under local anesthesia. The antibiotics were adjusted to include imipenem/cilastatin sodium (1 g every 12 hours) and piperacillin/tazobactam sodium (4.5 g every 8 hours).
On June 7, the suturing of T12/L1 and VSD replacement of L2-4 were performed under local anesthesia. The T12/L1 incision healed, and Enterobacter cloacae (+++) was detected in the L3/4 intervertebral space. On June 13, lumbar interbody space debridement and VSD at L2-4 (including the removal of the top wire, titanium rod, and screw at L3) were performed under general anesthesia. On June 20, VSD replacement at L2-4 was conducted under general anesthesia. VSD replacement was performed again on June 27, as depicted in Figure 4A.
In the third consecutive test, the bacterial culture of the fluid obtained through the VSD was negative. On July 4, debridement and VSD placement in L2-4 were performed, followed by the placement of a titanium rod and top wire under general anesthesia, as shown in Figure 4B. On July 11, lumbar interbody space debridement and VSD drainage were performed on the internal fixation surfaces. On July 16, the incision was sutured under local anesthesia, and a VSD sponge was placed on the incision site. Postoperatively, the incision healed successfully, as shown in Figure 4C and D.
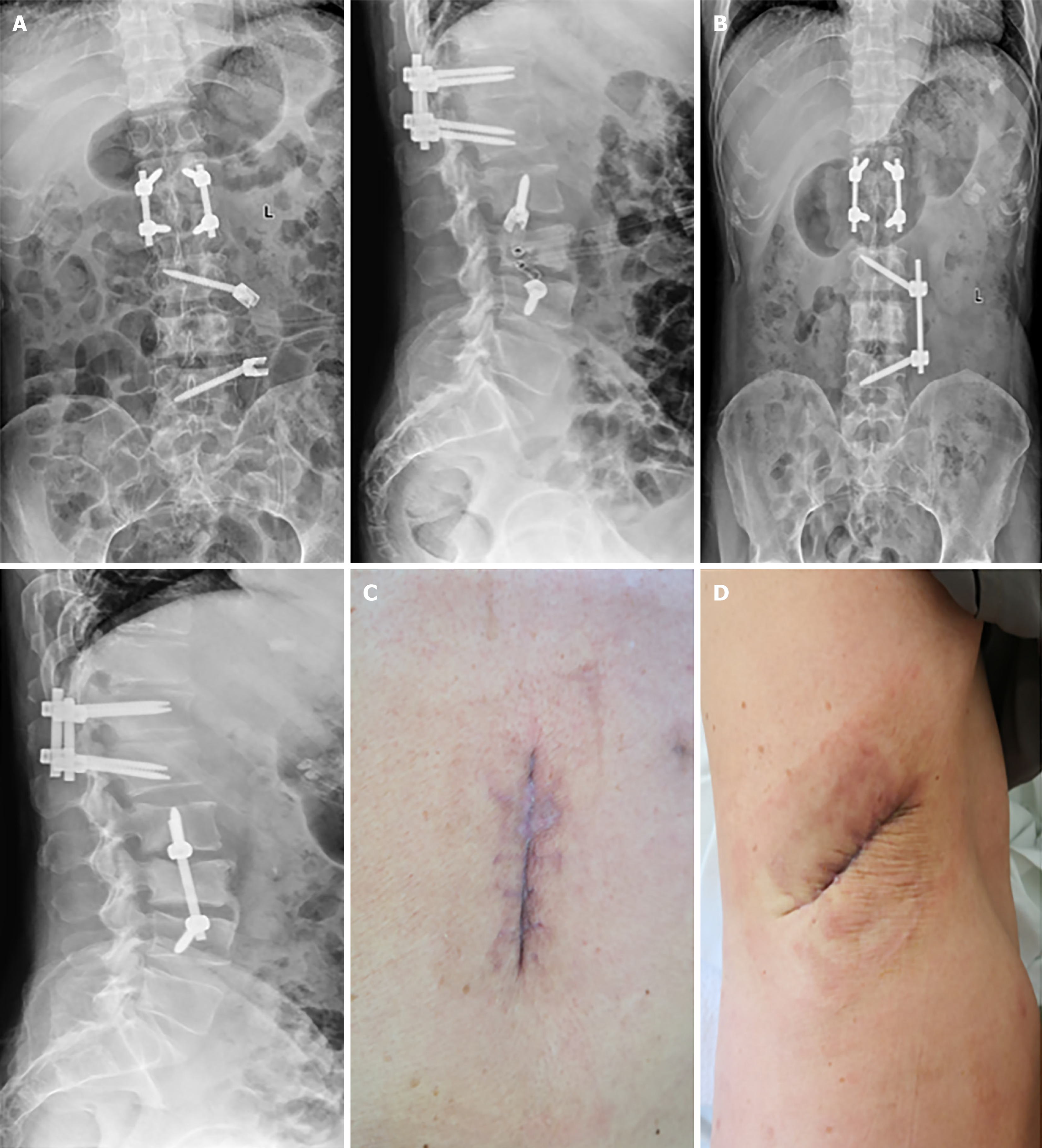
The VAS score decreased from 8 to 0 at the time of discharge, and the Japanese Orthopaedic Association score increased from 8 to 19 and 27 three months postoperatively. Imaging techniques, such as radiography, CT, and MRI, were reviewed, as depicted in Figure 5.
Although the majority of IDS infections are treatable with conservative methods successfully, some cases still require surgical intervention[10]. Surgery is indicated for various reasons[11], such as neurological dysfunction, kyphosis, spinal instability, intractable pain, large abscess, ineffective conservative treatments. Surgical intervention should be considered following standard conservative treatment. The purpose of surgical treatment is to completely debride the infection, confirm the pathogen, maintain or restore spinal stability, restore spinal canal volume, and relieve nerve compression. Conventional surgical techniques typically involve anterior, posterior, or combined anterior and posterior approaches with or without internal fixation, which may be performed in single or multiple stages. The selection of the surgical technique should be based on the patient’s condition and other factors. In this case, the patient was diagnosed with a noncontiguous infection. After comprehensive consideration, the surgical plan was subdivided into various sections and steps: The treatment of the lesions in the T12/L1 space involved posterior VSD and internal fixation, with the left side being approached by the Wiltse method and the right side by TLIF decompression and lesion removal. Lesions in the L2/3 and L3/4 spaces were managed with VSD and internal fixation through an extreme lateral approach. The extreme lateral approach offers a distinct advantage as it directly accesses the vertebral space of the lesion without damaging the facet joints, which are essential for maintaining the stability of the posterior column and overall structural integrity of the spine[12]. Therefore, an internal fixation device can be implanted in the second stage. In addition, compared with other approaches, the extreme lateral approach to debride the lesion can facilitate the placement of a larger and more suitable VSD sponge, resulting in a more comprehensive drainage effect. At the same time, in comparison to the traditional anterior and posterior approaches, the extreme lateral approach was found to be safer owing to fewer associated complications[13]. Karikari et al[14] conducted a study on the clinical efficacy of extreme lateral interbody fusion in a one-stage treatment of 13 cases of lumbar infection, yielding positive results. Similarly, Wang et al[15] analyzed the efficacy of 22 cases of lumbar tuberculosis treated using an extreme lateral approach combined with lateral or posterior percutaneous screw internal fixation and found it to be successful. Cui et al[16] presented a case report that further demonstrated the effectiveness of the extreme lateral approach in treating lumbar spine infections. Based on the patient's condition, the extreme lateral approach was determined to be a minimally invasive and effective surgical technique for the treatment of spinal infections.
Numerous studies have demonstrated the beneficial effects of VSD in the management of various types of acute and chronic surgical infections and infected wounds[17]. Although the literature[18] has suggested the use of VSD for treating spinal site infections, no related studies have reported on the treatment of IDS. However, we applied an innovative approach by inserting a VSD sponge into the intervertebral space at the site of infection to drain the residual tissue exudate and secretions from the lesion and eliminate bacterial adhesion tissues. Simultaneously, the VSD sponge was successful in eliminating bacterial biofilm[19] and had a positive outcome in the management of IDS. In this case, the VSD sponge was regularly changed through the extreme lateral approach to manage the deep infection. After three debridement and VSD sessions, fresh granulation tissue was removed, and the incision was closed with sutures. On the fifth postoperative day, pus discharge was observed from the incision, and bacterial culture revealed Enterobacter cloacae, indicating recurrence of infection. The patient underwent three debridement and VSD procedures via the extreme lateral approach, during which antibiotics were administered constantly. Fresh granulation tissue was observed intraoperatively in the intervertebral space; however, recurrence occurred after the incision was sutured. Following eight procedures using the VSD technique, the patient was successfully treated. The recurrence of the infection in this case may be attributed to two factors: Intravertebral implantation and internal fixation of L3, and incomplete elimination of the bacteria in the lesion. We recommend using VSD until the infection is sufficiently controlled for incision closure, instead of implanting an internal fixation on the affected vertebra. Based on this case and our successful treatment experience, we suggest that the surgical incisions can be closed when the CRP level drops below 25 and more than half of its peak value[8], when the granulation tissue in the intervertebral space is fresh, and when bacterial cultures from the intervertebral space yield negative results on three consecutive occasions.
Identifying pathogenic microorganisms is a critical step in the diagnosis of illnesses, as well as in the selection of the most effective antibiotics for treatment. Current conventional methods for obtaining pathogenic bacteria, such as blood culture and tissue specimen culture (including lesion puncture biopsy, lesion drainage fluid culture, and lesion of interoperation), are not effective in providing satisfactory positive rates in the process of etiological diagnosis. One of the most significant elements in determining the results of laboratory tests for etiology is the timing of antibiotic administration, which has a significant impact on the positive rate of pathogen culture. For example, the early application of antibiotics has the greatest impact on the positive rate of pathogen cultures[20]. Sampling of different tissues from different locations may have influenced the test results. Kim et al[21] proposed that the success rate of pathogen culture in soft tissue specimens is higher. To determine the presence of bacteria, drainage fluid and tissues were obtained through percutaneous puncture vertebral biopsy and multiple debridement procedures, and the results were ultimately negative. Moreover, during the fever and chills, the patient's blood was tested multiple times; however, the results were inconclusive. However, we devised a creative approach by placing a VSD sponge in the intervertebral space, which enabled us to reserve drainage fluid for bacterial culture. Detection of Enterobacter cloaca in the two jump lesions at T12/L1 and L3/4 verified the reliability of the bacterial culture results. According to a study conducted by Wang et al[22], the effectiveness of implanting a VSD sponge in the intervertebral space to obtain pathogens was 71.4%, which is consistent with previous investigations. Compared with traditional culture methods, such as blood culture (no more than 25%), percutaneous vertebral body biopsy (39.7%), and intervertebral disc soft tissue biopsy (52.9%)[21], the rate of bacteria detected through negative-pressure drainage fluid is significantly higher. Evidence suggests that inserting a VSD sponge into the intervertebral space and using negative-pressure suction to acquire pathogenic bacteria are more effective, resulting in a higher success rate.
Our findings suggest that VSD is an effective option for patients with prolonged and recurrent spinal infections, as it may reduce the risk of recurrence. VSD allows for precise detection of pathogenic microorganisms in negative-pressure drainage fluid, and supports the closure of surgical incisions following three consecutive negative bacterial cultures. However, this study’s limitations must be acknowledged, as it is a single case report and cannot be generalized to demonstrate the efficacy of treatment for spinal infections. Additionally, the postoperative follow-up period was short, highlighting the need for extended monitoring and statistical analyses to evaluate mid- and long-term complications and recurrence.
| 1. | Lener S, Hartmann S, Barbagallo GMV, Certo F, Thomé C, Tschugg A. Management of spinal infection: a review of the literature. Acta Neurochir (Wien). 2018;160:487-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 2. | Tsantes AG, Papadopoulos DV, Vrioni G, Sioutis S, Sapkas G, Benzakour A, Benzakour T, Angelini A, Ruggieri P, Mavrogenis AF; World Association Against Infection In Orthopedics And Trauma W A I O T Study Group On Bone And Joint Infection Definitions. Spinal Infections: An Update. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Liu X, Zheng M, Sun J, Cui X. A diagnostic model for differentiating tuberculous spondylitis from pyogenic spondylitis on computed tomography images. Eur Radiol. 2021;31:7626-7636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Duarte RM, Vaccaro AR. Spinal infection: state of the art and management algorithm. Eur Spine J. 2013;22:2787-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 5. | Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med. 2020;8:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 275] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 6. | Bydon M, De la Garza-Ramos R, Macki M, Naumann M, Sciubba DM, Wolinsky JP, Bydon A, Gokaslan ZL, Witham TF. Spinal instrumentation in patients with primary spinal infections does not lead to greater recurrent infection rates: an analysis of 118 cases. World Neurosurg. 2014;82:e807-e814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Sobottke R, Seifert H, Fätkenheuer G, Schmidt M, Gossmann A, Eysel P. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008;105:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Chen SH, Lee CH, Huang KC, Hsieh PH, Tsai SY. Postoperative wound infection after posterior spinal instrumentation: analysis of long-term treatment outcomes. Eur Spine J. 2015;24:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Sohrabi C, Mathew G, Maria N, Kerwan A, Franchi T, Agha RA; Collaborators. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg. 2023;109:1136-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2850] [Cited by in RCA: 2627] [Article Influence: 875.7] [Reference Citation Analysis (2)] |
| 10. | Scheyerer MJ, Herren C, Kühne C, Neufang J, Pieroh P, von der Höh NH. Surgical Treatment Strategies for Pyogenic Spondylodiscitis of the Thoracolumbar Spine. Z Orthop Unfall. 2022;160:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, Holtom PD, Huddleston PM 3rd, Petermann GW, Osmon DR. Executive Summary: 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin Infect Dis. 2015;61:859-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 978] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 13. | Ma XY, Yang HZ, Zou XB, Wang BB, Yang JC, Xia H, Wu ZH. Treatment of primary spondylodiscitisby: one-stage extreme lateral debridement, bone autograft and continuously closed irrigation combined with posterior internal fixation. Zhongguo Jizhu Jisui Zazhi. 2018;28:726. [DOI] [Full Text] |
| 14. | Karikari IO, Nimjee SM, Hardin CA, Hughes BD, Hodges TR, Mehta AI, Choi J, Brown CR, Isaacs RE. Extreme lateral interbody fusion approach for isolated thoracic and thoracolumbar spine diseases: initial clinical experience and early outcomes. J Spinal Disord Tech. 2011;24:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Wang QY, Huang MG, Ou DQ, Xu YC, Dong JW, Yin HD, Chen W, Rong LM. One-stage extreme lateral interbody fusion and percutaneous pedicle screw fixation in lumbar spine tuberculosis. J Musculoskelet Neuronal Interact. 2017;17:450-455. [PubMed] |
| 16. | Cui H, Chang Z, Yu X. Treatment of lumbar brucella spondylitis with negative pressure wound therapy via extreme lateral approach: A case report. Front Surg. 2022;9:974931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51:301-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (3)] |
| 18. | Zeng J, Sun X, Sun Z, Guan J, Han C, Zhao X, Zhang P, Xie Y, Zhao J. Negative Pressure Wound Therapy Versus Closed Suction Irrigation System in the Treatment of Deep Surgical Site Infection After Lumbar Surgery. World Neurosurg. 2019;127:e389-e395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Lianhua Y, Yunchao H, Guangqiang Z, Kun Y, Xing L, Fengli G. The effect of iatrogenic Staphylococcus epidermidis intercellar adhesion operon on the formation of bacterial biofilm on polyvinyl chloride surfaces. Surg Infect (Larchmt). 2014;15:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Wang YC, Wong CB, Wang IC, Fu TS, Chen LH, Chen WJ. Exposure of Prebiopsy Antibiotics Influence Bacteriological Diagnosis and Clinical Outcomes in Patients With Infectious Spondylitis. Medicine (Baltimore). 2016;95:e3343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Kim CJ, Kang SJ, Choe PG, Park WB, Jang HC, Jung SI, Song KH, Kim ES, Kim HB, Oh MD, Park KH, Kim NJ. Which tissues are best for microbiological diagnosis in patients with pyogenic vertebral osteomyelitis undergoing needle biopsy? Clin Microbiol Infect. 2015;21:931-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Wang J, Yang Y, Xing W, Xing H, Bai Y, Chang Z. Safety and efficacy of negative pressure wound therapy in treating deep surgical site infection after lumbar surgery. Int Orthop. 2022;46:2629-2635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/