Published online Dec 18, 2025. doi: 10.5312/wjo.v16.i12.111046
Revised: July 28, 2025
Accepted: November 13, 2025
Published online: December 18, 2025
Processing time: 178 Days and 23.1 Hours
Bone defects represent a significant clinical challenge with diverse etiologies, including but not limited to tumors, trauma, necrosis, and congenital deformities, imposing substantial patient suffering and socioeconomic burdens. In recent years, novel approaches for bone defect repair have been continuously explored. Biodegradable synthetic materials, particularly those capable of gradual decom
Core Tip: Degradable magnesium (Mg) alloys have been extensively utilized in the treatment of bone defects owing to their superior mechanical properties, excellent biocompatibility, and potent osteogenic capabilities. Mg alloys enhance bone tissue regeneration via multiple mechanisms, including the bone-nerve circuit, promotion of vascular regeneration, modulation of the immune microenvironment, and upregulation of osteogenic signaling pathways. Additionally, Mg alloys have been engineered into diverse application forms, such as Mg-infused metallic scaffolds and Mg-based bone regeneration mem
- Citation: Lu JS, Han ZG, Song CY, Yang M, Huang YS, Wang KY. Biodegradable materials: Applications and advances of magnesium alloys in bone defects. World J Orthop 2025; 16(12): 111046
- URL: https://www.wjgnet.com/2218-5836/full/v16/i12/111046.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i12.111046
Bone is one of the most crucial connective tissues in the human body, offering mechanical support for muscle attachment and safeguarding internal organs[1]. Bone tissue possesses a certain capacity for repair and regeneration[2,3]. However, when the body experiences severe trauma, infection, tumors, or osteoporosis, its limited self-repair ability often leads to large-scale bone defects, significantly impacting patients’ quality of life[4,5]. Autologous bone and allogeneic bone transplantation have traditionally been the primary methods for addressing bone defects[6]. However, these approaches are associated with significant limitations, including donor site morbidity, limited availability of graft sources, and the risk of immune rejection[7,8]. Consequently, the development of novel bone implant materials has become an important focus within the field of bone regenerative medicine.
Recently, Pagani et al[9] conducted a systematic review of the application advancements of synthetic biomaterials in conjunction with fibrin within the field of bone regeneration. They provided an in-depth analysis of the significant therapeutic potential of fibrin-based biomaterials for addressing bone defects and proposed promising directions for future research[9]. The advantages of biodegradable implant materials as graft materials in bone defect repair are evident. These materials can be absorbed by the human body, thereby eliminating the necessity for secondary surgery to remove internal fixation devices[10]. It is important to highlight that magnesium (Mg) alloys exhibit mechanical properties analogous to those of natural bones and demonstrate exceptional osteogenic activity. Furthermore, they can be fully degraded within the body and are extensively utilized in the clinical management of bone diseases[11]. This advancement offers a novel direction for the utilization of Mg alloys in fracture treatment. Notably, our team has recently, for the first time, reported the successful and effective application of degradable Mg metal closure clips in achieving precise he
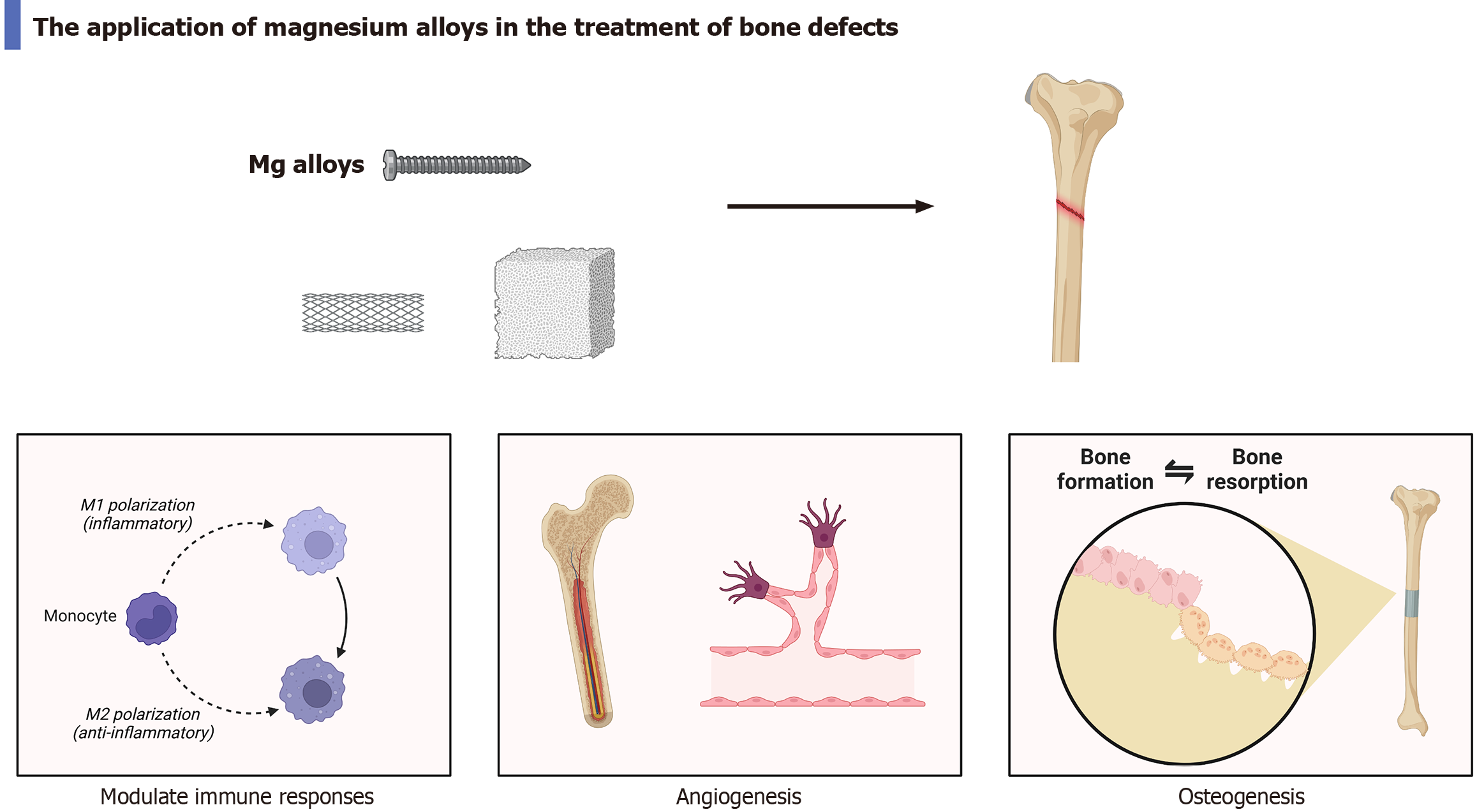
The widespread application of biodegradable medical Mg alloy materials in bone defect repair can be attributed to their superior biological properties, including a Young’s modulus closely matching that of cortical bone, excellent biocompatibility, and an appropriate natural degradation rate[13,14]. Mg alloys possess outstanding mechanical properties and are capable of providing adequate support strength for the regeneration of periosteal cells. The Young’s modulus of normal bone typically ranges from 3 GPa to 20 GPa, whereas that of Mg is approximately 45 GPa. In comparison to titanium alloys with a Young’s modulus of 110 GPa, Mg alloys exhibit a modulus closer to that of natural bone. This similarity significantly mitigates the stress shielding effect associated with internal fixation implants, thereby promoting optimal bone growth[15,16]. In addition, Mg alloys exhibit excellent biocompatibility. The primary degradation product of Mg is positively charged Mg ions, which play a critical role in bone development and reconstruction. And excessive Mg ions can be fully excreted via urine. One study has demonstrated that Mg alloys can significantly enhance the healing of tibial defects in vivo, while no adverse reactions have been reported[17]. Another study confirmed that the Mg scaffold LAE442 showed exceptional vascularization and cellular responses in vivo, thereby effectively facilitating bone healing[18]. Besides, the outstanding osteogenic properties of Mg alloys have been extensively validated in numerous studies. Zheng et al[19] found that fracture healing is modulated by the release of neuropeptides from peripheral sensory nerves, and the expression of calcitonin gene-related peptide can inhibit fibroblast differentiation while promoting callus formation. Furthermore, a Mg-containing hybrid intramedullary nail fixation system has been shown to enhance fracture healing by upregulating calcitonin gene-related peptide expression levels in vivo[19]. Vascularization serves as an essential prerequisite for bone formation. Liu et al[20] demonstrated that a Mg ion concentration of 5 mmol/L could enhance the secretion of MC3T3-E1 and platelet-derived growth factor, thereby effectively promoting angiogenesis and accelerating bone healing. Furthermore, Mg alloys are capable of directly stimulating bone formation via multiple pathways. For instance, nano-platforms incorporating Mg ions can effectively modulate the inflammatory microenvironment by inhibiting the nuclear factor kappa-B signaling pathway, thus facilitating bone remodeling[21]. Another study showed that ultra-high purity Mg and its alloy ZX00 can facilitate new bone formation by upregulating the expression levels of bone morphogenetic protein 2 and osteoprotegerin proteins[22]. In conclusion, Mg alloys exhibit multiple properties that are highly conducive to bone tissue regeneration (Figure 1). However, their excellent biological properties warrant further exploration and development to fully realize their potential in biomedical applications.
The application of Mg alloys for bone defect filling or drug-loaded composite system fabrication represents a prominent research thrust in contemporary bone tissue engineering. Although Mg alloys have demonstrated promising potential as bone regeneration implant materials, their clinical application remains constrained by several factors, including hydrogen accumulation and the corrosion rate in physiological environments. To enhance the performance of Mg alloys effectively, a range of advanced modification technologies has been developed. Guo et al[23] fabricated a composite chitosan-Mg membrane by immersing Mg alloy in chitosan solution. This membrane demonstrated enhanced osteogenic activity in both in vivo and in vitro studies. Similarly, a subsequent study developed a dicalcium phosphate dihydrate (CaHPO4·2H2O)/MgF2 Janus membrane, which was capable of effectively promoting periosteal regeneration[24]. Furthermore, Ye et al[25] developed a three-dimensional-printed porous Mg metal scaffold and applied a Sr-containing composite coating onto its surface. This design exhibited remarkable biocompatibility and osteogenic capacity in both in vitro and in vivo experiments[25]. Besides, another study prepared Mg-doped micro-nano bioactive glass, which has the potential to promote osteogenesis through the regulation of inflammatory responses[26]. Surface modification enhances the environmental adaptability and confers specialized functionalities to Mg alloys without altering their bulk properties. For instance, calcium orthophosphate coatings significantly improve corrosion resistance and biocompatibility of Mg-based substrates[27].
Biodegradable Mg alloys exhibit substantial advantages in bone defect repair owing to their degradability, superior mechanical properties, and osteogenic activity. In the future, it will be essential to further explore the role of Mg alloys in promoting osteogenesis via mechanisms such as inflammation inhibition, antibacterial properties, and regulation of the balance between osteoblasts and osteoclasts. Additionally, it is crucial to thoroughly investigate various application forms of Mg alloys to enhance their long-term effects on the human body. Furthermore, in the future, the biological properties of Mg alloys need to be further optimized, such as improving mechanical properties and controlling the degradation rate.
| 1. | Trompet D, Melis S, Chagin AS, Maes C. Skeletal stem and progenitor cells in bone development and repair. J Bone Miner Res. 2024;39:633-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 2. | Toros T, Ozaksar K. Reconstruction of traumatic tubular bone defects using vascularized fibular graft. Injury. 2021;52:2926-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Alvarez Echazú MI, Perna O, Olivetti CE, Antezana PE, Municoy S, Tuttolomondo MV, Galdopórpora JM, Alvarez GS, Olmedo DG, Desimone MF. Recent Advances in Synthetic and Natural Biomaterials-Based Therapy for Bone Defects. Macromol Biosci. 2022;22:e2100383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Stahl A, Yang YP. Regenerative Approaches for the Treatment of Large Bone Defects. Tissue Eng Part B Rev. 2021;27:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 5. | Nele W, Martina F, Stefan R, Frank L, Georg M. Impaction bone grafting for segmental acetabular defects: a biomechanical study. Arch Orthop Trauma Surg. 2023;143:1353-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | García-Gareta E, Coathup MJ, Blunn GW. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 422] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 7. | Deng AD, Innocenti M, Arora R, Gabl M, Tang JB. Vascularized Small-Bone Transfers for Fracture Nonunion and Bony Defects. Clin Plast Surg. 2020;47:501-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Yang P, Xing J, Liu J, Luo F, Wu X, Yu B, Deng M, Xu J, Hou T. Individual Tissue-Engineered Bone in Repairing Bone Defects: A 10-Year Follow-Up Study. Tissue Eng Part A. 2020;26:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Pagani BT, Rosso MPO, Moscatel MBM, Trazzi BFM, da Cunha MR, Issa JPM, Buchaim DV, Buchaim RL. Update on synthetic biomaterials combined with fibrin derivatives for regenerative medicine: Applications in bone defect treatment: Systematic review. World J Orthop. 2025;16:106181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Liu C, Ren Z, Xu Y, Pang S, Zhao X, Zhao Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning. 2018;2018:9216314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Zhou Y, Zhang A, Wu J, Guo S, Sun Q. Application and Perspectives: Magnesium Materials in Bone Regeneration. ACS Biomater Sci Eng. 2024;10:3514-3527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 12. | Han Q, Zhang X, Wang W, Wang K, Wang T. The threat of hemorrhage from pelvic fractures: Clinicians seeking new solutions based on biomedical Mg implant. J Magnesium Alloys. 2025;13:1476-1479. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Fan L, Chen S, Yang M, Liu Y, Liu J. Metallic Materials for Bone Repair. Adv Healthc Mater. 2024;13:e2302132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 14. | He X, Li Y, Zou D, Zu H, Li W, Zheng Y. An overview of magnesium-based implants in orthopaedics and a prospect of its application in spine fusion. Bioact Mater. 2024;39:456-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 15. | Sadat Hashemi T, Jaiswal S, McCarthy HO, Levingstone TJ, Dunne NJ. Biofunctionalisation of porous additively manufactured magnesium-based alloys for Orthopaedic applications: A review. Biomater Adv. 2025;169:214170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Lu Y, Deshmukh S, Jones I, Chiu YL. Biodegradable magnesium alloys for orthopaedic applications. Biomater Transl. 2021;2:214-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Levorova J, Duskova J, Drahos M, Vrbova R, Vojtech D, Kubasek J, Bartos M, Dugova L, Ulmann D, Foltan R. In vivo study on biodegradable magnesium alloys: Bone healing around WE43 screws. J Biomater Appl. 2018;32:886-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Kleer-Reiter N, Julmi S, Feichtner F, Waselau AC, Klose C, Wriggers P, Maier HJ, Meyer-Lindenberg A. Biocompatibility and degradation of the open-pored magnesium scaffolds LAE442 and La2. Biomed Mater. 2021;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Zheng N, Xu J, Ruan YC, Chang L, Wang X, Yao H, Wang J, Zhang R, Xue Q, Tang N, Ong T, Schilcher J, O'Keefe RJ, Qin L. Magnesium facilitates the healing of atypical femoral fractures: A single-cell transcriptomic study. Mater Today (Kidlington). 2022;52:43-62. [DOI] [Full Text] |
| 20. | Liu W, Guo S, Tang Z, Wei X, Gao P, Wang N, Li X, Guo Z. Magnesium promotes bone formation and angiogenesis by enhancing MC3T3-E1 secretion of PDGF-BB. Biochem Biophys Res Commun. 2020;528:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Weng Z, Ye J, Cai C, Liu Z, Liu Y, Xu Y, Yuan J, Zhang W, Liu L, Jiang J, Cheng X, Wang X. Inflammatory microenvironment regulation and osteogenesis promotion by bone-targeting calcium and magnesium repletion nanoplatform for osteoporosis therapy. J Nanobiotechnology. 2024;22:314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 22. | Okutan B, Schwarze UY, Habisch H, Iskhakova K, Ćwieka H, Ribeiro-Machado C, Moosmann JP, Blanchet C, Brcic I, Santos SG, Madl T, Zeller-Plumhoff B, Weinberg AM, Wieland DCF, Sommer NG. Biodegradable ultrahigh-purity magnesium and its alloy ZX00 promote osteogenesis in the medullary cavity and glycogenolysis in the liver. Acta Biomater. 2025;195:599-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Guo Y, Yu Y, Han L, Ma S, Zhao J, Chen H, Yang Z, Zhang F, Xia Y, Zhou Y. Biocompatibility and osteogenic activity of guided bone regeneration membrane based on chitosan-coated magnesium alloy. Mater Sci Eng C Mater Biol Appl. 2019;100:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Han Y, Wang X, Wei P, Zhang D, Gao M, Yu Z, Wang Q, Tan L, Tian Y. Biodegradable Magnesium alloy Janus membrane with surface-selective osteoinduction and soft tissue healing properties in guided bone regeneration. Acta Biomater. 2025;195:582-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Ye J, Miao B, Xiong Y, Guan Y, Lu Y, Jia Z, Wu Y, Sun X, Guan C, He R, Xiong X, Jia H, Jiang H, Liu Z, Zhang Y, Wei Y, Lin W, Wang A, Wang Y, Meng H, Xu W, Yuan G, Peng J. 3D printed porous magnesium metal scaffolds with bioactive coating for bone defect repair: enhancing angiogenesis and osteogenesis. J Nanobiotechnology. 2025;23:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (1)] |
| 26. | Dai K, Zhao F, Zhang W, Chen D, Hang F, Zou X, Chen X. 3D-printed magnesium-doped micro-nano bioactive glass composite scaffolds repair critical bone defects by promoting osteogenesis, angiogenesis, and immunomodulation. Biomed Mater. 2024;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Dorozhkin SV. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014;10:2919-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/