Published online Nov 18, 2025. doi: 10.5312/wjo.v16.i11.110276
Revised: June 12, 2025
Accepted: October 11, 2025
Published online: November 18, 2025
Processing time: 164 Days and 9.5 Hours
Minimally invasive lumbar interbody fusion (LIF) procedures have evolved ra
To investigate the comparative effectiveness of RA vs conventional LIF tech
A systematic review and meta-analysis was conducted in accordance with PRISMA 2020 and Cochrane guidelines. Databases searched included PubMed, EMBASE, Web of Science, Scopus, and the Cochrane Library (through May 2025). Eligible studies were randomized controlled trials and observational studies comparing RA with fluoroscopy - or navigation-guided LIF (transforaminal lumbar interbody fusion, lateral lumbar interbody fusion, oblique lumbar interbody fusion) in adults. Two reviewers independently extracted data and assessed risk of bias. The Grading of Recommendations Assessment, Development and Evaluation framework was used to evaluate certainty of evidence. Meta-analyses were performed where data were sufficiently homogeneous.
Twenty-two studies were included, encompassing a total of 2313 patients - 1046 who underwent RA-guided procedures and 1267 who received comparator techniques. Meta-analyses showed that RA significantly improved perfect pedicle screw placement [pooled odds ratio = 2.93; 95% confidence interval (CI): 1.40-6.14; I2 = 78.2%] and reduced intraoperative blood loss (pooled standardized mean difference = -0.28; 95%CI: -0.47 to -0.08; I2 = 0%). Operative time did not significantly differ between groups (pooled standardized mean difference = 0.01; 95%CI:
RA LIF improves pedicle screw placement accuracy and reduces blood loss and surgeon radiation exposure while maintaining similar clinical outcomes and safety profiles to conventional techniques. These findings support the integration of RA into spine surgery but highlight the need for high-quality multicenter randomized controlled trials and cost-effectiveness studies to guide broader implementation.
Core Tip: This article confirms that robot-assisted lumbar interbody fusion significantly improves pedicle screw placement accuracy and reduces intraoperative blood loss, with no significant difference in operative time. Additionally, robot-assisted techniques offer clear advantages in reducing surgeon radiation exposure and adjacent segment degeneration. Despite these benefits, long-term clinical and fusion outcomes were comparable to those of conventional methods. These findings support the clinical precision and perioperative efficiency of robotic systems while highlighting the need for high-quality multicenter trials and cost-effectiveness analyses to inform broader implementation in spine surgery.
- Citation: Ardila CM, Ángel-Estrada S, González-Arroyave D. Robot-assisted vs conventional lumbar interbody fusion: A systematic review and meta-analysis of perioperative, radiographic, and clinical outcomes. World J Orthop 2025; 16(11): 110276
- URL: https://www.wjgnet.com/2218-5836/full/v16/i11/110276.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i11.110276
The evolution of minimally invasive techniques in lumbar interbody fusion (LIF), including transforaminal lumbar interbody fusion (TLIF), lateral lumbar interbody fusion (LLIF), and oblique lumbar interbody fusion (OLIF) approaches, has transformed the management of degenerative spinal pathologies by reducing soft tissue disruption, blood loss, and recovery time[1,2]. Central to these procedures is the accurate placement of pedicle screws, which ensures spinal stability and optimal biomechanical load distribution[3]. Traditional fluoroscopy-guided (FG) methods, while widely adopted, are associated with significant radiation exposure, a steep learning curve, and variable screw placement accuracy[4,5]. In response, robot-assisted (RA) navigation systems - such as the Tianji Robot (TiR) and Mazor X Stealth - have been de
Recent systematic reviews confirm RA’s superiority over freehand and FG techniques in screw placement accuracy[8,9]. Most recently, Guan et al[10] conducted a focused systematic review and meta-analysis comparing RA-TLIF and FG-TLIF. Their results demonstrated significantly higher “perfect” and “clinically acceptable” screw placement accuracy with RA, as well as a 74% reduction in proximal facet joint violation (FJV). Although they also reported reduced estimated blood loss (EBL) and radiation dose with RA, they found no consistent advantage in surgical duration, noting the limited superiority of RA in terms of overall efficiency and revision rates. Importantly, their analysis was limited to TLIF procedures, was based predominantly on cohort studies, and did not include navigation-guided techniques as comparators.
While these systematic reviews provide valuable insights, critical gaps persist. First, existing reviews - including those by McKenzie et al[8], Perdomo-Pantoja et al[9], and Guan et al[10] - predominantly focus on TLIF, neglecting RA’s applica
Although RA demonstrates advantages in TLIF, its broader utility across LIF techniques lacks systematic evaluation. For example, standalone LLIF with RA-assisted posterior fixation in a single lateral position presents unique technical challenges, including reduced pedicle screw placement accuracy and restricted alignment correction[6]. Similarly, OLIF combined with RA navigation, though theoretically advantageous for minimizing paravertebral muscle injury, lacks robust clinical validation[2]. Furthermore, while both RA and navigation reduce radiation exposure, their relative merits in operative efficiency, learning curves, and cost-effectiveness remain unclear[3,7].
Patient-reported outcomes (PROs) further complicate this landscape. Although RA-TLIF shows promising short-term recovery benefits[4], long-term data on functional improvement and screw-loosening rates - critical for assessing durability - are limited[15]. Additionally, navigation systems, which predate RA, offer comparable accuracy but require intraoperative imaging, whereas RA platforms such as TiR eliminate post-registration radiation exposure[1]. A synthesis of these trade-offs is essential to guide clinical adoption and future research.
The primary objective of this systematic review is to compare perioperative outcomes - including screw placement accuracy, operative efficiency, and radiation exposure - between RA and fluoroscopy- or navigation-guided techniques across TLIF, LLIF, and OLIF procedures. Building on this foundation, we further aim to evaluate RA’s impact on radio
This systematic review was developed following the PRISMA 2020 guidelines[16] and methodological recommendations from the Cochrane Handbook for Systematic Reviews of Interventions. The review protocol was prospectively registered in the PROSPERO database (CRD420251064278).
Population: Adult patients undergoing LIF (TLIF, LLIF, OLIF) for degenerative spinal pathologies. Intervention: RA pedicle screw placement. Comparator: FG or navigation-guided techniques. Outcomes: Screw accuracy, perioperative efficiency (operative time, radiation exposure), radiographic outcomes (sagittal alignment), and clinical benefits (PROs, revision rates).
Studies were included if they compared RA vs FG and/or navigation-guided pedicle screw placement in LIF procedures - specifically TLIF, LLIF, or OLIF - for adult patients with degenerative spinal pathologies. Eligible studies had to report at least one outcome related to screw placement accuracy, perioperative efficiency (operative time, radiation exposure, EBL), radiographic outcomes (sagittal alignment correction, proximal FJV), or clinical benefits [PROs such as Visual Analog Scale (VAS) - or Oswestry disability index (ODI), revision rates, or long-term complications like screw loosening]. Randomized controlled trials (RCTs) and observational studies, including both prospective and retrospective cohorts, were considered for inclusion. Case reports, conference abstracts, reviews, cadaveric or animal studies, and studies lacking a comparative group or with fewer than ten participants per arm were excluded. Only full-text articles were eligible, and no language restrictions were applied.
A comprehensive search of the literature was conducted across five major databases: PubMed, EMBASE, Web of Science, Scopus, and the Cochrane Library. The search included records from inception to May 2025.
Search terms included a combination of Medical Subject Headings terms and keywords such as “robot-assisted”, “robotic surgery”, “fluoroscopy-guided”, “navigation-guided”, “TLIF”, “LLIF”, “OLIF”, “lumbar fusion”, and “pedicle screw placement”. Boolean operators “AND” and “OR” were used to refine the search. Reference lists of relevant reviews and included articles were manually screened for additional eligible studies.
Two independent reviewers screened the titles and abstracts of all retrieved articles. Full-text articles were obtained for studies that appeared to meet the inclusion criteria or where eligibility was uncertain. Discrepancies were resolved through discussion or consultation with a third reviewer. The selection process was documented using a PRISMA flow diagram.
Data were extracted independently by two reviewers using a standardized form. Extracted data included the following: Study characteristics (first author, year, country, design, sample size), type of surgical procedure (TLIF, LLIF, OLIF), robotic platform used, comparator technique, and all reported outcomes.
Screw placement accuracy was typically assessed using the Gertzbein-Robbins classification system[6]. Other data extracted included radiation dose and duration, operative time, EBL, FJV, revision surgeries, sagittal alignment correction, and PROs measures (e.g., VAS, ODI). Any disagreements were resolved by consensus.
A meta-analysis was planned to quantitatively synthesize outcomes across studies, provided that the included studies reported sufficient and compatible data, such as standard deviations, confidence intervals, or event counts required for pooled effect estimates. Prior to analysis, the uniformity of outcome definitions and measurement methods (e.g., screw placement accuracy, operative time, EBL) was evaluated to determine the feasibility of quantitative synthesis. In cases where substantial methodological or clinical heterogeneity was identified, or where essential quantitative data were missing or inconsistently reported, a narrative synthesis was conducted following PRISMA 2020 guidelines[16]. This approach ensured that pooling was applied only when methodologically justified and statistically appropriate.
A sensitivity analysis was performed, excluding studies involving hybrid techniques [e.g., RA unilateral biportal en
The risk of bias for RCTs was evaluated using the Cochrane Risk of Bias Tool (RoB 2.0)[17], which examines randomization, deviations from intended interventions, missing data, outcome measurement, and selection of reported results. For observational studies, the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool was employed[18]. This tool assesses confounding, selection bias, intervention classification, and measurement and reporting biases. Assessments were performed independently by two reviewers, with disagreements resolved through discussion or by a third reviewer.
The certainty of the evidence for each outcome was rated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach[19]. Factors assessed included risk of bias, inconsistency, indirectness, imprecision, and publication bias. The quality of evidence was classified as high, moderate, low, or very low.
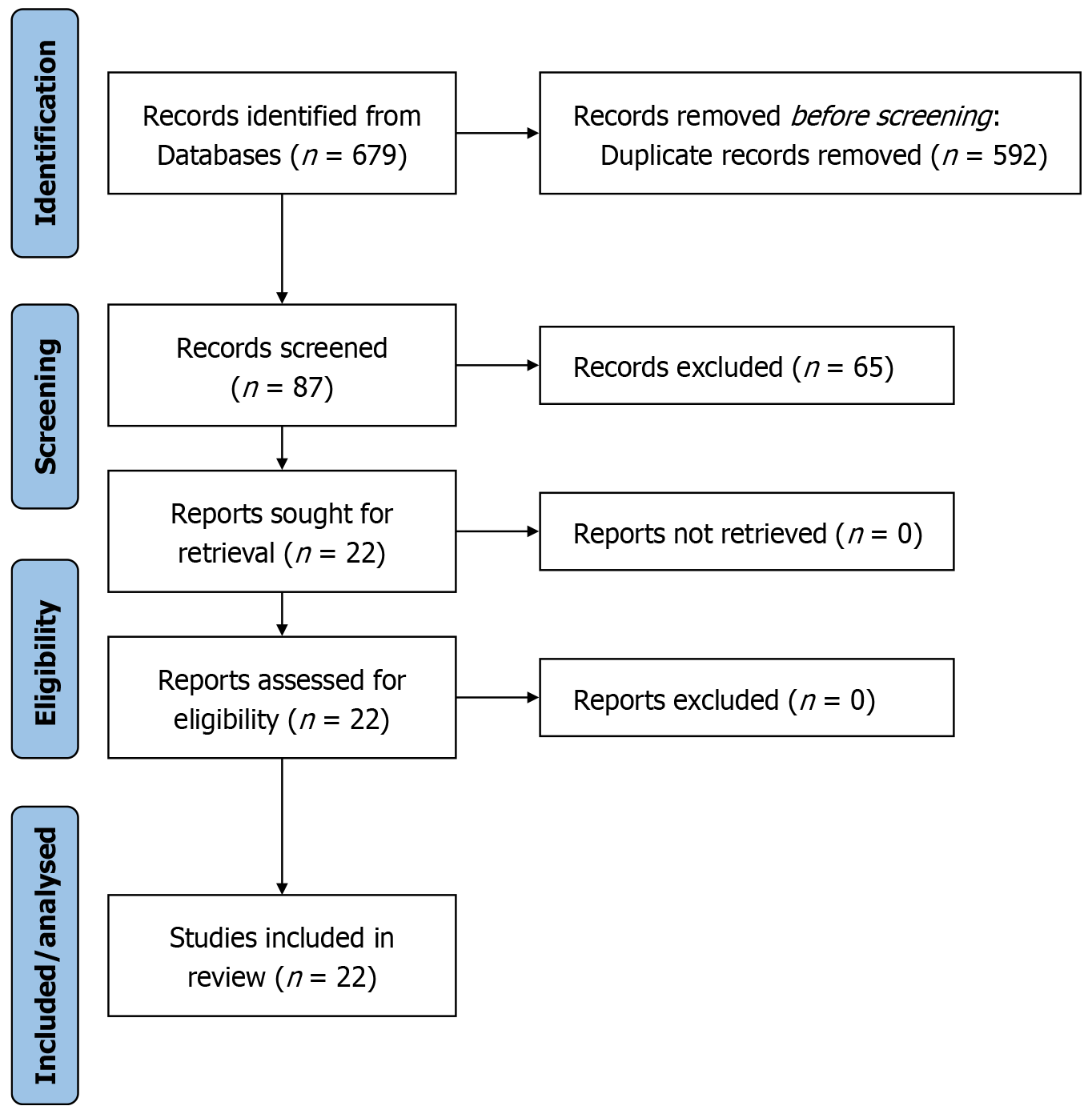
As illustrated in the PRISMA flow diagram (Figure 1), an initial pool of 679 records was subjected to a rigorous selection process. After removing duplicates and applying predefined exclusion criteria - which notably excluded non-RA studies - 87 articles underwent full-text review. Ultimately, 22 studies met the eligibility criteria and were included in this sys
A total of 22 studies met the eligibility criteria and were included in this systematic review (Table 1)[1-7,11,15,20-32]. These comprised 1 RCT[24], 4 prospective cohort studies[1,11,20,21], and 17 retrospective cohort studies[2-7,15,22,23,25-32] conducted across diverse geographic regions. The studies investigated RA vs FG, NG, freehand, or open techniques across primarily TLIF/minimally invasive surgery (MIS)-TLIF, with fewer OLIF and LLIF procedures. In one study[32], a three-arm comparison was performed: RA MIS-TLIF (group A), RA UBE-T/PLIF (group B), and traditional MIS-TLIF (group C).
| Ref. | Country | Study design | Procedure type | Sample size (RA/comparator) | Robotic platform | Comparator type |
| Wang et al[1], 2023 | China | Prospective | MIS-TLIF | 61/62 | TiRobot | Freehand fluoroscopy |
| Tong et al[2], 2024 | China | Retrospective | OLIF | 16/22 | Third-gen Mazor X navigation robot | Fluoroscopy |
| Shafi et al[3], 2022 | United States | Retrospective | MIS-TLIF | 92/130 | ExcelsiusGPS | Intraoperative navigation |
| Griepp et al[4], 2024 | United States | Retrospective | MIS-TLIF | 50/133 | Mazor X Stealth robot | Fluoroscopy-assisted |
| Lin et al[5], 2022 | Taiwan | Retrospective | MIS-TLIF | 75/149 | ROSA | Freehand fluoroscopy |
| Li et al[6], 2025 | China | Retrospective | LLIF | 31/28 | TianJi Robot | Traditional LLIF |
| Heath et al[7], 2024 | Taiwan | Retrospective | MIS-TLIF | 42/58 | ROSA ONE | O-arm navigation |
| Chang et al[11], 2022 | China | Prospective | MIS-TLIF | 26/32 | TiRobot | MIS-TLIF |
| Lai et al[15], 2022 | Taiwan | Retrospective | TLIF | 29/79 | Renaissance | Freehand fluoroscopy |
| Zhang et al[20], 2019 | China | Prospective | TLIF | 43/44 | TiRobot | FG |
| Zhang et al[21], 2019 | China | Prospective | TLIF | 50/50 | Robot assisted | FG |
| Chen et al[22], 2021 | China | Retrospective | MIS-TLIF | 52/52 | TiRobot | Freehand open TLIF |
| Cui et al[23], 2021 | China | Retrospective | MIS-TLIF | 23/25 | TiRobot | Open TLIF surgery |
| Feng et al[24], 2020 | China | RCT | OLIF | 40/40 | TiRobot | Open freehand fluoroscopy |
| Li et al[25], 2024 | China | Retrospective | MIS-TLIF | 58/56 | Tianji Robot | Freehand fluoroscopy |
| Han et al[26], 2021 | China | Retrospective | OLIF vs TLIF | 28/33 | TiRobot | MIS-TLIF |
| De Biase et al[27], 2021 | United States | Retrospective | MIS-TLIF | 52/49 | Mazor X | FG |
| Fayed et al[28], 2020 | United States | Retrospective | MIS-TLIF | 103/90 | ExcelsiusGPS | FG |
| Yang et al[29], 2019 | China | Retrospective | MIS-TLIF | 30/30 | Robot-assisted surgical system | FG |
| Schatlo et al[30], 2014 | Germany | Retrospective | TLIF | 55/40 | Mazor | FG |
| Li et al[31], 2024 | China | Retrospective | RA MIS-TLIF (group A) vs RA UBE-T/PLIF (group B) vs traditional MIS-TLIF (group C) | A: 27, B: 30, C: 26 | Tianji Robot (3rd Gen) | Traditional MIS-TLIF |
| Li et al[32], 2022 | China | Retrospective | RA MIS-TLIF | 33/39 | Tianji Robot | Traditional MIS-TLIF |
The total sample size included 2313 patients, with 1046 undergoing RA-guided procedures and 1267 receiving com
Screw placement accuracy was reported in all studies (Table 2), except Lai et al[15], which did not provide screw placement accuracy data (grade A, A + B) or pedicle breach rates. The majority of studies used the Gertzbein-Robbins classification system. RA techniques consistently demonstrated higher rates of perfect (grade A) placement, ranging from 83.6% to 99.7%, with statistically significant improvements in most studies. In contrast, FG, freehand fluoroscopy, open, and NG techniques showed perfect placement rates typically between 69.5% and 96.7%. Clinically acceptable screw placement (grades A + B) was consistently high (> 90%) across both techniques.
| Ref. | Classification | RA accuracy (grade A) | Comparator accuracy (grade A) | Statistical significance | Clinically acceptable screws (A + B) | Statistical significance | Operative time (RA vs comparator) | EBL (mL) (RA vs comparator) | Radiation exposure (RA vs comparator) | Notes |
| Wang et al[1], 2023 | Gertzbein-Robbins | 85.4% | 69.5% | P < 0.001 | 97.1% vs 95.0% | NS | 160.25 ± 12.13 vs 154.35 ± 15.00 (P = 0.018) | 78.85 ± 33.52 vs 82.90 ± 20.91 (NS) | Surgeon: 13.28 ± 3.09 vs 94.87 ± 6.02 (P < 0.001) patient: NSD (NS) | Less disc height loss at adjacent segments (P < 0.001) |
| Tong et al[2], 2024 | Gertzbein-Robbins | 95.4% | 85.5% | P < 0.05 | 98.4% vs 95.5% | P = 0.04 | 158.8 minutes vs 129.9 minutes (P < 0.05) | 89.8 vs 117.3 (P < 0.05) | 13.3 vs 48.5 fluoroscopy counts (P < 0.05) | Longer operative time in RA but reduced blood loss and radiation exposure. Higher screw accuracy (98.4% vs 95.5%) |
| Shafi et al[3], 2022 | Gertzbein-Robbins | 88.5% | 88.4% | NS | 97.4% vs 95.3% | NS | Not reported | Not reported | Surgeon: Significantly lower with RN (P < 0.001) | Fewer high-grade breaches (0% RN vs 1.2% ION, P = 0.05) |
| Griepp et al[4], 2024 | Gertzbein-Robbins | Not directly reported; lower revision rate (0%) in RA suggests higher accuracy | Not reported; higher revision rate (2.4%) in FA group | P = 0.03 (lower revision rate in RA group) | Not reported | Not reported | 33.3 ± 8.57 minutes/screw vs 30.7 ± 6.87 minutes/screw, P = 0.125) | 161.4 ± 365.7 vs 155.1 ± 194.7 (NS) | 4.9 ± 7.6 vs 20.3 ± 14.0 mGy/screw (P < 0.001) | RA had longer anesthesia time (49.1 minutes/screw vs 43.6 minutes/screw, P = 0.009) |
| Lin et al[5], 2022 | Gertzbein-Robbins | 99.7% | 98.2% | P = 0.04 | 99.7% vs 98.2% | P = 0.04 | 280.7 vs 251.4 minutes (NS) | 313.7 vs 431.6 mL (P = 0.019) | Not reported | RA reduced blood loss significantly. Shorter operative time for 4-level surgeries with RA. No difference in complications or pain outcomes |
| Li et al[6], 2025 | Gertzbein-Robbins | 96.8% | 92.9% | NS | 99.2% vs 98.2% | NS | 147 minutes vs 165 minutes (P = 0.04) | 124.4 vs 138.9 (NS) | 54.6 seconds vs 87.8 seconds (P < 0.01) | Shorter fluoroscopy and operative time in RA, no significant difference in blood loss |
| Heath et al[7], 2024 | Gertzbein-Robbins | 98.0% | 80.0% | P < 0.001 | 100% vs 92.1% | P = 0.003 | 263.5 minutes vs 243.4 minutes (P = 0.28) | 340.6 vs 256.6 (NS) | Not quantified (O-arm used in both groups) | Longer operative time for RA in 2-level fusions (324.7 vs 266.4 min, P = 0.03). No medial breaches or revisions in either group |
| Chang et al[11], 2022 | Gertzbein-Robbins | 99.1% | 93.7% | P < 0.05 | 100% vs 98.4% | P = 0.001 | 208 ± 15.2 minutes vs 161 ± 7.9 minutes (P = 0.02) | 25 ± 10 vs 100 ± 20 (P = 0.01) | Reduced (implied, not quantified) | RA-TLIF had shorter incisions (1.4 cm vs 2.5 cm, P = 0.01). Lower screw misplacement rate (0.9% vs 6.3%, P < 0.05). Steep learning curve noted |
| Lai et al[15], 2022 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 259.0 minutes vs 225.0 minutes (NS) | 400.0 vs 366.7 mL (NS) | Not reported | Lower screw loosening rate with RA (4.3% vs 10.2%, P = 0.049). Similar complication rates (24.1% both groups). RA screws placed closer to upper endplate (P < 0.001) |
| Zhang et al[20], 2019 | Gertzbein-Robbins | 93.2% | 85.8% | P = 0.02 | 98.3% vs 93.6% | P = 0.02 | 165.3 ± 58.9 minutes vs 154.7 ± 46.0 minutes (P = 0.349) | 187.2 ± 95.2 mL vs 373.2 ± 320.3 mL (P = 0.001) | Dose: 25.9 ± 14.2 μSv vs 70.5 ± 27.3 μSv (P < 0.001) time: 93.5 ± 37.9 seconds vs 70.5 ± 28.3 seconds (P = 0.002) | Fewer facet violations: 5 vs 24 screws (P = 0.001). Lower revisions: 0 (RA) vs 2 (FG). Learning curve noted for RA (longer setup time) |
| Zhang et al[21], 2019 | Gertzbein-Robbins | 85.0% | 71.0% | P = 0.017 | 98.0% vs 94.0% | NS | 184.7 minutes vs 117.8 minutes (P < 0.001) | 171.6 vs 362.0 mL (P = 0.001) | 30.3 vs 65.3 μSv (P < 0.001) | Longer op time but less radiation in RA |
| Chen et al[22], 2021 | Gertzbein-Robbins | 92.3% | 77.4% | P < 0.001 | 98.6% vs 96.6% | NS | 169.67 minutes vs 135.48 minutes (P < 0.001) | 92 vs 261 mL (P < 0.001) | 1.26 minutes vs 0.54 minutes (P < 0.001) | Longer op time but less blood loss in RA |
| Cui et al[23], 2021 | Gertzbein-Robbins | 94.6% | 85.0% | P = 0.025 | 100% vs 100% | NS | RA MIS-TLIF: 135.1 ± 11.2 minutes. Open TLIF: 102.2 ± 7.1 minutes (P = 0.002) | RA MIS-TLIF: 173.6 ± 17.9 mL open TLIF: 332.1 ± 23.5 mL (P = 0.005) | Not reported | RA MIS-TLIF showed longer operative time (learning curve) but significantly reduced blood loss and postoperative drainage (97.5 mL vs 261.3 mL, P < 0.001). Faster recovery: Shorter hospitalization (7.3 days vs 10.0 days) and time to ambulation (1.5 days vs 2.9 days, P < 0.05). Less muscle atrophy: Paraspinal muscle cross-sectional area decreased by 3.9% (vs 14.5% in open TLIF, P = 0.016) |
| Feng et al[24], 2020 | Gertzbein-Robbins | 98.2% | 93.1% | P = 0.039 | 100% vs 99.4% | NS | 196.25 minutes vs 230.63 minutes (P < 0.05) | 165 mL vs 237.5 mL (P < 0.05) | Not reported | Efficiency benefit with RA |
| Li et al[25], 2024 | Gertzbein-Robbins | 87.5% | 70.1% | P < 0.001 | 98.3% vs 96.9% | NS | 158.5 minutes vs 146.4 minutes (P < 0.001) | 58.5 vs 52.8 (NS) | Surgeon: 13.8 vs 74.7 fluoroscopy counts (P < 0.001) | Longer operative time in RA but reduced surgeon radiation exposure. Lower adjacent segment degeneration (0.63 mm vs 0.92 mm height loss, P = 0.001) |
| Han et al[26], 2021 | Gertzbein-Robbins | 92.9% | 90.9% | NS | 97.3% vs 96.2% | NS | OLIF: 164.9 ± 56.0 minutes MIS-TLIF: 121.5 ± 48.2 minutes (P < 0.01) | OLIF: 142.4 ± 89.4 mL MIS-TLIF: 291.5 ± 72.3 mL (P < 0.01) | Not reported | OLIF had significantly longer operative time but less blood loss. OLIF required position change (lateral to prone), contributing to longer time |
| De Biase et al[27], 2021 | Gertzbein-Robbins | 97.4% | 93.9% | NS | 99.8% vs 99.3% | NS | 241 minutes vs 246 minutes (NS) | 73.8 mL vs 73.9 mL (NS) | 31.5 vs 59.5 mGy (P = 0.035) | RA reduced radiation |
| Fayed et al[28], 2020 | Gertzbein-Robbins | 94.2% | 96.7% | NS | 98.1% vs 100% | NS | Not reported | Not reported | Not reported | RA-PPS: 5.8% breach rate (6/103 screws), with 1.9% significant breaches (> 2 mm). FG-PPS: 3.3% breach rate (3/90 screws), with 1.1% significant breaches. Learning curve: 5 breaches in first 48 screws (10 cases) vs 1 breach in next 55 screws (10 cases). Lateral breaches (4/6) linked to facet hypertrophy/skiving |
| Yang et al[29], 2019 | Gertzbein-Robbins | 93.8% | 73.8% | P = 0.012 | 98.5% vs 96.9% | NS | Not reported | Not reported | Not reported | The study focused on accuracy (pedicle screw placement) and facet joint violation, not perioperative efficiency metrics. RA group had significantly lower pedicle wall penetration (6.2% vs 26.2%) and facet joint violation (5.1% vs 15.6%) compared to FG group. No severe deviations (Neo grade III) in RA group |
| Schatlo et al[30], 2014 | Gertzbein-Robbins | 83.6% | 79.8% | NS | 91.4% vs 87.1% | NS | 205 minutes vs 189 minutes (NS) | 375 mL vs 713 mL (P < 0.01) | Not reported | Lower blood loss in RA |
| Li et al[31], 2024 | Gertzbein-Robbins | 96.3% | 95.0% | P < 0.05 | A: 99%, B: 98%, C: 91% | P < 0.05 | A: 146.9 ± 10.8, B: 172.5 ± 13.2, C: 169.0 ± 13.6 (A < B/C, P < 0.05) | A: 89.3 ± 11.3, B: 74.4 ± 14.6, C: 111.6 ± 20.9 (B < A < C, P < 0.05) | Significantly lower in A/B vs C (P < 0.05) | Group A had shortest operation time. Group B had least blood loss. Group C had highest radiation exposure |
| Li et al[32], 2022 | Gertzbein-Robbins | 99.24% | 91.03% | P = 0.002 | Not specifically reported in A + B form, but grade A alone was 99.24% in RA | Not explicitly reported for A + B, only for grade A | 154.75 ± 7.32 vs 172.22 ± 14.82 (P = 0.001) | 89.49 ± 18.63 vs 121.48 ± 20.55 (P = 0.001) | Fluoroscopy: 59.54 ± 6.56 vs 70.67 ± 9.70 (P = 0.001) | Robot group had shorter hospital stays (3.86 ± 1.17 days vs 5.03 ± 0.73 days) |
In Li et al[31], a three-arm comparison showed that RA MIS-TLIF (group A) and RA UBE-T/PLIF (group B) both achieved high clinically acceptable placement rates (99% and 98%, respectively), significantly superior to the traditional MIS-TLIF group (91%).
Griepp et al[4] did not report specific Gertzbein-Robbins grades but inferred high accuracy from the absence of revision surgeries for misplaced screws in both RA and FA groups. Their reported clinically acceptable placement rates align with literature-reported ranges (RA: 85.4%-99.2%; FA: 70%-93%).
Figure 2A illustrates the comparative accuracy of pedicle screw placement across all included studies. RA consistently achieved higher rates of perfect (grade A) screw placement (ranging from 85.4% to 99.7%) compared to the broad range seen in traditional comparator methods (approximately 60% to 93%). Notable examples include Heath et al[7], where RA demonstrated a significant advantage (98% vs 80%, P < 0.001), and Shafi et al[3], where accuracy was nearly identical between techniques (88.5% vs 88.4%, P = 0.969). The figure highlights these inter-study variations and consistently favorable outcomes for RA systems.
Perioperative outcomes - including operative time, blood loss, and radiation exposure -showed considerable variability across studies, reflecting differences in surgical complexity, learning curves, and robotic platforms (Table 2). RA tech
Operative times were often comparable or slightly longer in RA groups, attributed to the robotic setup and initial learning curve. For example, Heath et al[7] found longer operative times in RA for 2-level fusions (324.7 minutes vs 266.4 minutes, P = 0.03). However, studies like Li et al[6] and Chang et al[11] showed improved efficiency with RA, particularly in multilevel procedures and after the learning curve plateau. In Li et al[31], group A (RA MIS-TLIF) achieved the shortest operative time and group B had the lowest blood loss, while group C (traditional MIS-TLIF) had the highest radiation exposure. Figure 2B illustrates operative time differences between RA and comparator techniques across studies, reflecting the overall trend toward slightly longer but increasingly efficient RA procedures as familiarity with robotic systems grows.
Seventeen studies reported radiographic outcomes, with five providing quantitative data on sagittal alignment [lumbar lordosis (LL), segmental lordosis (SL), and pelvic incidence-LL (PI-LL) mismatch] and most reporting FJV rates (Table 3). RA techniques consistently demonstrated improvements in sagittal alignment in short-term follow-up, particularly in LLIF and OLIF procedures. For example, Li et al[6] found postoperative increases in LL (45.2° to 51.5°), SL (24.0° to 29.3°), and PI-LL (13.0° to 7.8°, P < 0.01), although these improvements were not sustained at final follow-up.
| Ref. | Procedure | Sagittal alignment changes | Facet joint violation (RA vs comparator) | Notes |
| Wang et al[1], 2023 | MIS-TLIF (RA vs FA) | Not reported | FJV grades: RA: 89.8% grade 0 (no violation) FA: 62.1% grade 0 (P < 0.001) - mean FJV grade lower in RA (0.24 vs 0.50; P < 0.001) | Adjacent segment: Less disc height loss at proximal adjacent segment in RA (0.69 mm vs 0.93 mm; P < 0.001). Fusion rates: No difference (BSF-3: 88.5% RA vs 85.5% FA; P = 0.616) |
| Tong et al[2], 2024 | RA-OLIF vs FG OLIF | Sagittal alignment parameters (LL, SL, PI-LL) not explicitly reported | RA-OLIF: 4.7% FJV rate (61 grade 0, 2 grade 1, 1 grade 2) | RA-OLIF had higher screw accuracy (98.4% grade A/B vs 95.5% in fluoroscopy; P = 0.015) |
| Focus on screw accuracy and FJV rates | Fluoroscopy: 19.3% FJV rate (71 grade 0, 11 grade 1, 5 grade 2, 1 grade 3) | Shorter-term benefits: Lower VAS-back at 3 days post-op (P = 0.003) | ||
| (P = 0.009) | ||||
| Shafi et al[3], 2022 | MIS-TLIF (RN vs ION) | Not reported | FJV rates: RN: 5.0% ION: 1.3% (P = 0.0017) | Screw dimensions: RN allowed larger screw diameters (7.25 mm vs 6.72 mm; P < 0.001) and longer screws (48.4 mm vs 45.6 mm; P < 0.001) |
| Accuracy: Similar “ideal” (grade A) screw rates (88.5% RN vs 88.4% ION; P = 0.969), but RN eliminated high-grade breaches (0% grade E vs 1.2% in ION; P = 0.051) | ||||
| Endplate breaches: Higher in RN (6.9% vs 1.3%; P = 0.001), but most were clinically insignificant | ||||
| Griepp et al[4], 2024 | MIS-TLIF (RA vs O-arm navigation) | Not reported | Lateral breaches: RA: 4/210 (1.9%, all grade B)- ON: 24/304 (7.89%, grades C-D) medial breaches: 0 in both groups | No revisions for malposition in either group |
| Lin et al[5], 2022 | MIS-TLIF (robot-guided vs freehand) | Not reported | Not reported | Pedicle screw breach rates: Robot-guided (0.27%) vs freehand (1.75%), P = 0.04 |
| Lateral breaches more common in freehand group (9/12 breaches). No medial breaches with robotics | ||||
| Li et al[6], 2025 | RA-SP-LLIF vs traditional LLIF | Significant postoperative improvements in LL (45.2°-51.5°), SL (24.0°-29.3°), and PI-LL (13.0°-7.8°) (P < 0.01). Gains in LL/SL/PI-LL were not sustained at final follow-up (P > 0.05 vs baseline). No difference in PT/SS changes | Not explicitly reported, but the high screw accuracy (99.2% RA vs 98.2% traditional) suggests low risk | Comparable fusion rates (Bridwell grade) and complications between groups |
| Heath et al[7], 2024 | RA-MIS-TLIF vs ON-MIS-TLIF | Sagittal alignment parameters (LL, SL, PI-LL) not explicitly reported | RA-MIS-TLIF: 0% breach rate (100% grades A/B) | No reoperations for screw malposition in either group |
| Focus on screw accuracy and breach rates | ON-MIS-TLIF: 7.89% breach rate (92.1% grades A/B; P < 0.001) | |||
| No medial breaches in either group | ||||
| Chang et al[11], 2022 | PE RA-TLIF vs MIS-TLIF | Not reported | Not reported | Screw accuracy: Robot 0.9% vs fluoroscopy 6.3% (P < 0.05) |
| Fusion rates: 87.3% (robot) vs 91.8% (fluoroscopy, P = 0.53) | ||||
| Smaller incisions, less blood loss with robotics | ||||
| Lai et al[15], 2022 | MIS-TLIF (robot vs fluoroscopy) | Not reported | Not reported | Screw loosening: Robot 4.3% vs fluoroscopy 10.2% (P = 0.049) |
| Robot screws placed closer to upper endplate (ratio 0.35 vs 0.39, P < 0.001). Loosening linked to age, multilevel fusion, and endplate distance ratio | ||||
| Zhang et al[20], 2019 | TLIF with pedicle screws | Not reported for LL/SL/PI-LL | RA: 5/176 screws (2.8%) violated facets FG: 24/204 screws (11.8%) (P=0.001) | RA achieved higher perfect screw placement (grade A: 93.2% vs 85.8%, P = 0.020) |
| No severe breaches (grade E) in RA vs 2 in FG | ||||
| Zhang et al[21], 2019 | TLIF with percutaneous pedicle screws | Not reported for LL/SL/PI-LL | RA: 4/100 screws (4%) violated facets (grades 1-2) FG: 26/100 screws (26%) (grades 1-3) (P < 0.001) | RA eliminated severe FJV (grade 3 0% vs 3% in FG) |
| Larger screw-to-facet distance (4.16 mm vs 1.92 mm, P < 0.001) | ||||
| Chen et al[22], 2021 | RA MIS-TLIF vs open TLIF | Not reported | Not reported | RA advantages: Higher screw accuracy (92.3% grade A vs 77.4%), faster early pain relief (VAS/ODI at 1 month) |
| Both groups: Similar 1-year fusion rates (94.2% vs 92.3%) | ||||
| Cui et al[23], 2021 | RA-MIS-TLIF vs open TLIF | Alignment restored in both groups (no quantitative LL/SL data) | RA: 0% (no revisions) vs open: 5% (5 screws revised) | RA: Reduced paraspinal muscle atrophy (P = 0.016) at 2-year follow-up |
| Feng et al[24], 2020 | RA-OLIF vs freehand OLIF | Alignment restored via indirect decompression (no quantitative LL/SL data) | RA: 1.8% (3/170 screws breached) vs freehand: 6.9% (12/174 screws breached) | RA: Reduced blood loss (P = 0.022) and eliminated postoperative drainage |
| Li et al[25], 2024 | RA-MIS-TLIF vs fluoroscopy-MIS-TLIF | Sagittal alignment parameters (LL, SL, PI-LL) not explicitly reported | RA-MIS-TLIF: 0.13 ± 0.43 FJV grade (90.1% grade 0) | Reduced adjacent segment disc height loss (0.63 ± 0.38 mm vs 0.92 ± 0.35 mm; P = 0.001) |
| Focus on screw accuracy, FJV, and adjacent segment degeneration | Fluoroscopy: 0.43 ± 0.68 FJV grade (66.1% grade 0) (P < 0.001) | Comparable fusion rates (BSF grades; P = 0.522) | ||
| Han et al[26], 2021 | OLIF vs MIS-TLIF | Not reported | Not reported | OLIF advantages: Higher disc height (12.4 vs 11.2 mm) and fusion rate (96% vs 87%) |
| Both groups: Similar screw accuracy (97.3% vs 96.2%) | ||||
| De Biase et al[27], 2021 | RA vs FG MI-TLIF | Not reported | Not reported | RA advantages: 50% lower radiation dose (31.5 vs 59.5 mGy) |
| Both groups: 0% screw breaches, similar revision rates (1 vs 2 cases) | ||||
| Fayed et al[28], 2020 | RA-PPS (ExcelsiusGPS) vs FG-PPS | Not explicitly measured; alignment inferred from screw accuracy | RA: 5.8% breaches (4 lateral, 2 grade E) vs FG: 3.3% breaches (1 medial) | RA breaches linked to facet hypertrophy; no revisions needed. Short learning curve (1.9% significant breaches after initial cases) |
| Yang et al[29], 2019 | MIS-TLIF with percutaneous pedicle screws | Screw insertion angle: RA: 23.8° ± 6.1° vs fluoroscopy: 18.4° ± 7.2° (P = 0.017) | RA: 5.1% (grades I-II) fluoroscopy: 15.6% (grades I-III), including 2.1% severe (grade III) | RA reduced severe deviations (Neo grade III: 0% vs 3.1%) and improved pedicle screw accuracy (93.8% grade 0 vs 73.8%) |
| Schatlo et al[30], 2014 | Lumbar fusion (open/percutaneous) | Not reported for LL/SL/PI-LL | RA: 8.6% poor trajectory (grades C-E/R) | Lateral misplacement most frequent (RA: 47% of deviations; FG: 39%) |
| FG: 12.9% (grades C-E) (P = 0.09) | No difference in clinically acceptable screws (A/B: 91.4% RA vs 87.1% FG, P = 0.19) | |||
| Li et al[31], 2024 | RA MIS-TLIF/UBE-T/PLIF | Not reported | Not reported | Screw accuracy significantly better in groups A/B vs C (P < 0.05) |
| Li et al[32], 2022 | RA MIS-TLIF | Not reported | Not reported | Higher screw accuracy in robot group (P = 0.002) |
RA also reduced FJV rates compared to FG or NG methods. Tong et al[2] reported a significant reduction in FJV rates (4.7% with RA vs 19.3% with FG, P = 0.009), while Li et al[25] noted lower mean FJV grades in RA procedures (0.13 vs 0.43, P < 0.001). High-grade breaches (grades C-E) were nearly eliminated in RA groups, as shown by Heath et al[7] (0% vs 7.89%, P < 0.001). Adjacent segment degeneration - quantified as reduced disc height loss - was also lower in RA patients (e.g., 0.63 mm vs 0.92 mm, P = 0.001 in Li et al[25]).
Figure 2C shows the comparison of FJV rates across studies. The consistent reduction of FJV rates in RA groups highlights their improved precision and potential for reducing adjacent segment degeneration.
Most included studies reported significant postoperative improvements in PROs such as VAS and ODI scores, with no clinically meaningful differences between RA and comparator groups at final follow-up (Table 4). For example, Li et al[6] and Wang et al[1] found nearly identical improvements in VAS and ODI at two years (P > 0.05). Some studies, including Tong et al[2] and Feng et al[24], noted better early postoperative pain control in RA groups, although these differences were transient.
| Ref. | VAS/ODI improvement | Revision rate (RA vs comparator) | Fusion success | Notes |
| Wang et al[1], 2023 | VAS back: Pre-op 6.92 → 0.90 (RA), 6.78 → 0.71 (FA) at 2 years (P > 0.05) | RA: 1 lateral wall violation (adjusted intraoperatively) | RA: 88.5% (BSF-3) | RA showed fewer facet violations (P < 0.001) |
| VAS leg: Pre-op 7.70 → 0.54 (RA), 7.56 → 0.44 (FA) (P > 0.05) | FA: 1 anterior vertebral perforation (abdominal pain), 1 nerve root irritation (required revision) | FA: 85.5% (BSF-3) (P > 0.05) | Less disc height loss at adjacent segments in RA (P < 0.001) | |
| ODI: Pre-op 70.90 → 15.23 (RA), 71.00 → 14.89 (FA) (P > 0.05) | ||||
| Tong et al[2], 2024 | VAS-back: Significantly lower in robot group at 3 days post-op (2.19 vs 3.18, P < 0.05); no difference at 3/6 months | 1 case vs 1 case | Not reported | No complications like infection or dural tear reported in either group |
| VAS-leg: No significant difference at any time point | ||||
| ODI: No significant difference at any time point | ||||
| Shafi et al[3], 2022 | Not reported | RA: No high-grade breaches (grade E) | Not reported | Higher facet violations in RN (5.0% vs 1.3%, P < 0.001), but no clinically significant breaches |
| ION: 1.2% high-grade breaches (17 screws, P = 0.05) | ||||
| Griepp et al[4], 2024 | ODI: Significant improvement in both groups at 6mo (Δ18.6 robot vs Δ18.2 fluoroscopy) and 12mo (Δ20.7 vs Δ22.4), with similar MCID achievement rates (P > 0.05). NRS back pain: Significant improvement in both groups at 6 months (Δ2.8 vs Δ2.3) and 12 months (Δ2.6 vs Δ2.8), with no inter group differences (P > 0.05) | RA: 1 revision (infection-related hardware removal). Fluoroscopy group: 3 revisions (2 infections, 1 foraminotomy) | High | Low rates in both groups (4.9% overall), with no neurological injuries |
| Screw malposition: 0 revisions in both groups | ||||
| Lin et al[5], 2022 | Similar (P > 0.05) | RA: 1.3% intraop (K-wire malposition), 4.0% postop (CSF leak, wound infection) FG: 1.3% intraop (durotomy), 4.0% postop (screw malposition, wound infection) (P = 0.99 for postop surgery-related complications) | Not reported | RA reduced pedicle breaches (0.27% vs 1.75%, P = 0.04) and blood loss (P = 0.019) |
| Li et al[6], 2025 | VAS-back: 6.3 → 1.8 (RA) vs 6.1 → 1.7 (traditional) | 0% (RA) vs 0.9% (traditional) | 90.3% vs 85.7% grade I | RA: 4 paresthesias; traditional: 2 paresthesias |
| ODI: Comparable at 2 years | ||||
| Heath et al[7], 2024 | VAS/ODI: Not explicitly reported in the study. Clinical safety was confirmed by maintained neurological status postoperatively | RA: 0 revisions for screw malposition. Navigation group: 0 revisions for screw malposition | High (no difference) | No medial breaches or neurological complications in either group |
| Chang et al[11], 2022 | VAS for back pain: Better in PE RA-TLIF (1.3 ± 0.4) vs MIS-TLIF (2.1 ± 0.1), P < 0.05.ODI: No significant difference (17 ± 5 vs 21 ± 8, P = 0.09) | No revisions reported. Misplacement rate: 0.9% (PE RA-TLIF) vs 6.3% (MIS-TLIF), P < 0.05 | Fusion rate: 87.3% (PE RA-TLIF) vs 91.8% (MIS-TLIF), P = 0.53 | PE RA-TLIF showed reduced surgical trauma and faster recovery |
| Lai et al[15], 2022 | VAS-leg/back and ODI (preop to 12-month): VAS-leg: Preop 8.0 → 0.0 (Ro) vs 0.0 (FG) VAS-Back: Pre-op 8.0 → 2.0 (Ro) vs 3.0 (FG) ODI: Pre-op 57.78 → 26.67 (Ro) vs 28.89 (FG) (all P < 0.05 for improvement; no intergroup differences) | Complications: RA TLIF: 24.1% (7/29): 3 screw loosening, 3 cage subsidence, 2 infections. FG TLIF: 24.1% (19/79): 14 screw loosening, 2 cage subsidence, 3 infections | Not reported | Less screw loosening in RA (P = 0.049) |
| Revisions: 1 broken rod (FG TLIF) | ||||
| Zhang et al[20], 2019 | Not reported | RA: 0% (0/176 screws) | Not reported | FJV: RA-PPS: 5 screws vs FG-PPS: 24 screws (P = 0.001) |
| Comparator: 1.0% (2/204 screws) | Blood loss: Reduced in RA group (187.2 mL vs 373.2 mL, P = 0.001 | |||
| Zhang et al[21], 2019 | Not reported | RA: 0% (0/100 screws); FG: 1% (1/100 screws) | Not reported | FJV: RA: 4% (4/100) vs FG: 26% (26/100) (P = 0.0001) |
| Severe FJV (grade 3): Only in FG group (3 screws) | ||||
| Intra-pedicle accuracy (grade A): RA: 85% vs FG: 71% (P = 0.017) | ||||
| Blood loss: RA: 171.6 mL vs FG: 362.0 mL (P = 0.001) | ||||
| Chen et al[22], 2021 | Similar (P > 0.05) | None | 94.2% vs 92.3% (NS) | RA showed shorter hospital stay |
| Cui et al[23], 2021 | VAS: 6.9 → 2.1 (RA) vs 6.5 → 3.7 (open) (P = 0.004); ODI: Comparable at 2 years | 0% (RA) vs 5% (open) | Comparable | RA: 1 transient numbness; open: 1 screw loosening |
| Feng et al[24], 2020 | VAS back pain: Immediate post-op: 2.15 (RA) vs 3.35 (comparator) (P < 0.05) ODI: No significant difference between groups at any time point | RA: 0.01% (1/170 screws) comparator: 3.4% (6/174 screws) | Not reported | RA group had shorter operative time, less blood loss, and no postoperative drainage |
| Complications: RA (1 hip flexor weakness); comparator (1 infection, 1 hip flexor weakness, 1 delayed wound healing) | ||||
| Li et al[25], 2024 | VAS-back/Leg: No significant difference between groups pre-op, post-op 3 days, or final follow-up (P > 0.05). ODI: No significant difference at any time point (P > 0.05) | RA: 1 screw revision (penetrated outer pedicle wall, adjusted intraoperatively). Freehand group: 2 screws revised (penetrated anterior cortex, causing transient abdominal pain; 1 screw irritated nerve root, requiring immediate revision) | No significant difference in fusion status (BSF grading) between groups (P > 0.05) | Lower FJV in robot group (0.13 grades vs 0.43 grades, P < 0.001). Less adjacent segment disc height loss in robot group (0.63 mm vs 0.92 mm, P = 0.001) |
| Han et al[26], 2021 | VAS back pain: Lower in OLIF at 1 week (2.8 vs 3.5, P < 0.05) and 3 months (1.6 vs 2.1, P < 0.05). ODI: Lower in OLIF at 3 months (22.3 vs 26.1, P < 0.05) - no differences in leg pain VAS | No revisions reported.Complications: OLIF (7/28) vs MIS-TLIF (5/33), all resolved conservatively | Fusion rate: Higher in OLIF (96% vs 87%, P < 0.01). Disc height: Greater in OLIF (12.4 mm vs 11.2 mm, P < 0.01 | Higher fusion in OLIF with RA OLIF had less blood loss (142.4 vs 291.5 mL, P < 0.01) and shorter hospital stays (3.2 vs 4.2 days, P < 0.01) |
| De Biase et al[27], 2021 | Not reported | Revisions: 1/52 (RA, pseudoarthrosis) vs 2/49 (FG, no surgery required) | Fusion status: No significant differences (pseudoarthrosis rates: 2% RA vs 4% FG, P = 0.523). | Lower radiation in RA group. Operative time, blood loss, hospital stay, and complication rates were similar |
| Fayed et al[28], 2020 | Not reported | RA: 0% (0/103 screws); comparator: 1.1% (1/90 screws) | Not reported | Complications: No revisions required in RA-PPS group; 1 revision in FG-PPS group (medial breach) |
| Yang et al[29], 2019 | Not reported | RA: 0% (0/130 screws); comparator: 3.1% (4/130 screws) | Not reported | FJV: RA-PPS: 5.1% (5/98 screws); FG-PPS: 15.6% (15/96 screws) (P = 0.021) |
| Complications: No severe facet violations (Babu grade III) in RA-PPS group; 2 cases in FG-PPS group | ||||
| Schatlo et al[30], 2014 | Not reported | Robot-assisted: 2.5% (6/244 screws revised intraoperatively); FG: 1 revision surgery (for radiculopathy due to screw malposition) | Not reported | Neurological injury occurred in 1 FG case (resolved after revision) |
| No significant difference in infection rates (robot: 1.8%, fluoroscopy: 2.5%) | ||||
| Blood loss was significantly lower in the robot-assisted group | ||||
| Li et al[31], 2024 | Significant improvement at 6 months (P < 0.05), no difference between groups (P > 0.05) | A: 0, B: 0, C: 0 | Not reported | Macnab excellent/good rates: A: 96%, B: 93%, C: 92% (P > 0.05). No difference in complications (P > 0.05) |
| Li et al[32], 2022 | No significant difference between groups (P > 0.05) | 0/33 vs 2/39 (screw reinsertion) | Not reported | Macnab excellent rate: 91% (RA) vs 87% (conventional group), P = 0.900. Complications: 9% vs 20% |
Fusion success rates were consistently high across studies, with no significant differences in most cases. One exception was Han et al[26], who reported higher fusion rates with RA-assisted OLIF (96% vs 87%, P < 0.01).
Complication rates were generally low and comparable between groups, with some studies reporting slight advantages of RA in terms of reduced pedicle breach rates, FJVs, and revision rates for screw malposition (e.g., Lin et al[5]: 0.27% vs 1.75%, P = 0.04; Zhang et al[20]: 0% vs 4.5%). RA was also associated with reduced blood loss, shorter hospital stays, and fewer screw loosening cases (e.g., Lai et al[15]: 3 cases vs 14 cases, P = 0.049). Griepp et al[4] found similar revision rates but more infection-related revisions in the fluoroscopy group. These findings highlight the technical advantages of RA while suggesting its long-term clinical impact may vary by context. Figure 2D illustrates the revision rates across all included studies, highlighting the consistent trend of reduced revision rates in the RA groups compared to comparators. These data emphasize the improved surgical precision offered by RA techniques.
Only the study by Li et al[25] mentioned the economic implications of robotic technology in spine surgery. While no formal cost-effectiveness analysis was provided, the authors acknowledged the potential economic challenges posed by these advanced technologies and highlighted the need for collaborative efforts among healthcare stakeholders to develop appropriate funding models. No other included studies reported data or discussions specifically addressing the cost-effectiveness of robotic-assisted spine surgery.
A meta-analysis was performed for the outcome of pedicle screw placement accuracy using a random-effects model (DerSimonian-Laird method) due to observed heterogeneity. Odds ratios with 95% confidence intervals (CIs) were calculated. The meta-analysis was performed using Python 3.11 with the Matplotlib and Numerical Python libraries, which also generated the forest plot, following Cochrane and PRISMA 2020 guidelines[16]. Statistical heterogeneity was assessed using the I2 statistic, with values > 50% indicating moderate to high heterogeneity (Figure 3A).
A meta-analysis was conducted to evaluate EBL across three studies comparing RA and FG techniques. The pooled standardized mean difference was -0.28 (95%CI: -0.47 to -0.08), indicating a modest but statistically significant reduction in intraoperative blood loss in favor of RA procedures. No significant heterogeneity was detected (I2 = 0%), suggesting consistency among the included studies (Figure 3B).
A random-effects meta-analysis was performed to compare operative time between RA and conventional techniques. The pooled standardized mean difference was 0.01 (95%CI: -0.30 to 0.31), indicating no significant difference in operative time between the groups. Moderate heterogeneity was observed (I2 = 66%), suggesting some variability across studies (Figure 3C).
No meta-analysis was conducted for radiation dose due to substantial heterogeneity in how this outcome was reported. Specifically, some studies presented fluoroscopy time in seconds or minutes, others reported absorbed radiation dose in mGy or μSv, while several measured radiation indirectly using the number of fluoroscopic images taken. Given the lack of standardized units and definitions, and the absence of at least three studies using comparable metrics, pooling was not methodologically justifiable. Therefore, findings related to radiation exposure were synthesized qualitatively.
To isolate the effect of pure robotic guidance, a sensitivity analysis was conducted, excluding studies that utilized hybrid techniques (e.g., RA unilateral biportal endoscopy or navigation-combined PLIF). After removing the hybrid group (Li et al[31], group B), pooled estimates for screw placement accuracy and EBL remained consistent with the primary analysis, suggesting that the observed benefits were due to robotic guidance rather than confounding by endoscopic or NG factors. This reinforces the independent value of robotic assistance in enhancing procedural precision and safety.
The methodological quality and risk of bias of the included studies were assessed using the RoB 2.0 tool for RCTs (Table 5) and the ROBINS-I tool for observational studies (Table 6). For the single RCT by Feng et al[24], the overall risk of bias was judged as low across all domains, including randomization, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of reported results (Table 5).
| Ref. | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of reported result | Overall risk of bias |
| Feng et al[24], 2020 | Low | Low | Low | Low | Low | Low |
| Ref. | Confounding | Selection bias | Classification of interventions | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of reported result | Overall risk of bias |
| Wang et al[1], 2023 | Low | Low | Low | Low | Low | Low | Low | Low |
| Tong et al[2], 2024 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Shafi et al[3], 2022 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Griepp et al[4], 2024 | Moderate | High | Low | Low | Low | Low | Low | Moderate |
| Lin et al[5], 2022 | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Li et al[6], 2025 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Heath et al[7], 2024 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Chang et al[11], 2022 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Lai et al[15], 2022 | High | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Zhang et al[20], 2019 | High | High | Low | Low | Low | Moderate | Moderate | High |
| Zhang et al[21], 2019 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Chen et al[22], 2021 | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Cui et al[23], 2021 | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Li et al[25], 2024 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Han et al[26], 2021 | High | Moderate | Low | Low | Low | Moderate | Moderate | High |
| De Biase et al[27], 2021 | High | Moderate | Low | Low | Moderate | Moderate | Moderate | High |
| Fayed et al[28], 2020 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Yang et al[29], 2019 | Moderate | Moderate | Low | Low | Low | Low | Low | Moderate |
| Schatlo et al[30], 2014 | High | Moderate | Low | Low | Low | Low | Low | Moderate |
| Li et al[31], 2024 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Li et al[32], 2022 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
Among the 22 observational studies assessed using ROBINS-I (Table 6), one study[1] was rated as low risk of bias across all domains. The majority of studies (n = 16) were deemed to have a moderate overall risk of bias, primarily due to concerns regarding confounding and selection bias. Four studies (Table 6) were assessed as having a high overall risk of bias, largely due to serious confounding, selection bias, or issues in measurement and reporting of outcomes.
These findings indicate that while the RCT exhibited robust methodology, the observational studies varied in quality, with some demonstrating notable methodological limitations that should be considered when interpreting the results.
The overall certainty of evidence for key outcomes was assessed using the GRADE framework (Table 7). Outcomes included screw placement accuracy, operative time, radiation exposure, EBL, FJV, sagittal alignment, and PROs.
| Outcome | Number of studies | Study design(s) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality (GRADE) | Comments/explanation |
| Screw accuracy (grade A) | 1 RCT, 21 observationals | RCT, observational | Moderate | Not serious | Not serious | Not serious | Undetected | Moderate | Consistent large effect across multiple studies despite observational nature |
| Operative time | 1 RCT, 17 observationals | RCT, observational | Moderate | Serious | Not serious | Serious | Undetected | Low | Risk of confounding and moderate heterogeneity across studies (I2 = 66%) |
| Blood loss | 1 RCT, 17 observationals | RCT, observational | Low | Not serious | Not serious | Not serious | Undetected | Low | Multiple studies had critical ROBINS-I domains; small-to-moderate effect size |
| FJV | 1 RCT, 12 observationals | RCT, observational | Moderate | Not serious | Not serious | Not serious | Undetected | Moderate | RA reduced FJV rates by 50%-75% |
| Sagittal alignment | 0 RCT, 7 observationals | Observational | Moderate | Serious | Not serious | Serious | Undetected | Low | Limited data; heterogeneous measurements |
| Patient-reported outcomes | 1 RCT, 14 observationals | RCT, observational | Moderate | Not serious | Not serious | Not serious | Undetected | Moderate | No long-term differences between RA and comparators |
For screw placement accuracy (grade A), the certainty of evidence was rated as moderate, based on consistent and substantial effects observed across 1 RCT and 21 observational studies. In contrast, operative time was downgraded to low certainty due to the risk of confounding and moderate heterogeneity (I2 = 66%) across 1 RCT and 17 observational studies.
EBL, assessed in 1 RCT and 17 observational studies, was also rated as low certainty, primarily due to critical risks of bias in ROBINS-I (selection and confounding), despite consistent directionality of effect.
A meta-analysis was not feasible for radiation exposure due to heterogeneity in outcome definitions and measurement units (e.g., mGy, μSv, fluoroscopy time, image count). Consequently, this outcome was not graded, although consistent narrative evidence supported a reduction in surgeon exposure with RA. For FJV, moderate certainty was retained based on consistent reductions (50%-75%) observed across 1 RCT and 12 observational studies. Sagittal alignment, supported by 7 observational studies, was rated as low certainty due to limited data and heterogeneous radiographic measures. Finally, PROs were downgraded to low certainty due to variation in outcome measurement tools, lack of long-term follow-up, and potential for confounding, despite data from 1 RCT and 14 observational studies.
In summary, while screw placement accuracy and facet preservation show moderate-certainty evidence in favor of RA, outcomes such as operative time, EBL, PROs, and sagittal alignment are supported by low-certainty evidence due to limitations in study design, inconsistency, or imprecision. Radiation exposure was not graded due to incompatible data formats. No publication bias was detected for any outcomes.
This systematic review critically evaluated the comparative outcomes of RA vs freehand, FG, open, and NG techniques in lumbar fusion procedures, focusing on key domains including screw placement accuracy, perioperative efficiency, radiographic outcomes, clinical outcomes, complications, risk of bias, and evidence quality. Across 22 studies involving 2313 patients, our findings offer comprehensive insights into the evolving role of robotic systems in spine surgery.
Our analysis of screw placement accuracy consistently demonstrated superior outcomes with RA techniques (grade A accuracy up to 99.7%) compared to traditional methods, corroborating the findings of Hiyama et al[33] who reported significant improvements in RA-LLIF. Pham et al[34] similarly noted higher accuracy of pedicle screw placement with RA, emphasizing that these improvements stem from precise robotic guidance during screw trajectory planning and placement. Fan et al[35] further supported this, reporting fewer grade C-E breaches in RA-assisted cases, reducing the need for revision surgeries.
The perioperative data in our review showed that while RA often led to longer operative times (attributed to robotic setup and the learning curve), these techniques significantly reduced intraoperative blood loss and radiation exposure to the surgical team. This observation aligns with Passias et al[36], who reported lower radiation exposure per screw in the RA group. Similarly, Li et al[37] and Vardiman et al[38] noted lower intraoperative blood loss and reduced need for repeated fluoroscopy in RA cases. Interestingly, Singhatanadgige et al[39] highlighted that RA MIS-TLIF achieved the shortest operative time among three comparator techniques, underscoring the potential for enhanced efficiency once the learning curve is overcome.
Several cohort studies have quantitatively characterized the learning curve associated with RA pedicle screw pla
These findings suggest that the neutral pooled effect on operative time in our meta-analysis likely reflects a mixture of early and experienced RA implementation phases. Surgeon proficiency appears to be a key determinant of operative efficiency, and future studies should control for this factor when comparing robotic and conventional workflows.
Economic evaluation is a critical factor influencing the adoption of robotic spine surgery. A single-center cost-effectiveness model estimated annual savings of approximately 608546 dollars - driven by fewer revisions (about 9.5 avoided cases), reduced infections, and shorter hospital stays - despite high up-front capital costs[44]. By contrast, a matched-cohort study by Ezeokoli et al[45] reported 11% higher day-of-surgery costs and 16% higher total encounter costs for RA lumbar fusion compared to freehand instrumentation, primarily due to increased supply use and operating room time.
More recent analyses have found RA systems to be cost-effective when revision rates are reduced and time savings per procedure are modest (< 4000 dollars per case)[46]. For example, a cost-utility model comparing RA MIS-TLIF with non-robotic MIS-TLIF showed the robotic approach to be cost-effective in 63% of simulations, based on a willingness-to-pay threshold of 50000 dollars per quality-adjusted life year[47]. Data from a Singaporean center also indicate that for two-level OLIF procedures, robotic guidance saved about stochastic gradient descent 1500 per patient due to reduced operative time alone[43]. Additionally, Menger et al’s cost-effectiveness study[44] in an academic center demonstrated estimated annual savings of approximately 608546 dollars following the implementation of RA spine surgery, despite the system’s high initial acquisition cost. These savings were primarily attributed to reductions in revision surgeries (about 9.5 cases), surgical site infections, and hospital length of stay. These findings align with our meta-analytical results and further support the economic feasibility of robotic systems, particularly in institutions with sufficient case throughput.
Taken together, these data suggest that while the initial acquisition and per-case costs of robotic navigation are high, benefits - such as reduced revision surgery, shorter hospital stays, lower infection rates, and cumulative operating room efficiencies - may offset expenses, especially in high-volume centers. We highlight the need for further robust, multi-institutional cost-effectiveness and cost-utility studies that incorporate long-term outcomes, quality-adjusted life years, and real-world revision data to inform implementation strategies and health policy decisions.
Our findings were further supported by a sensitivity analysis excluding hybrid techniques, such as RA UBE-T/PLIF. Even after removing these procedures, the benefits of RA - particularly in screw placement accuracy and reduced blood loss - remained significant, indicating that the observed outcomes were not confounded by additional techniques such as endoscopy or intraoperative navigation. This analysis supports the conclusion that robotic guidance independently improves perioperative outcomes, a finding consistent across multiple platforms and LIF approaches. However, the predominance of TLIF procedures and the underrepresentation of OLIF and LLIF techniques in the meta-analyzed data may still limit generalizability, highlighting the need for further research focused on platform- and approach-specific outcomes.
Radiographic parameters, such as LL, SL, and PI-LL mismatch, were improved following RA-assisted procedures in our review, with reductions in FJV rates compared to traditional methods. These trends are in agreement with Hiyama et al[33], who showed that larger interbody cages in RA-LLIF improved spinal alignment metrics, and Li et al[37], who observed significantly lower FJV rates with robotic assistance. Such radiographic improvements contribute to long-term spinal stability and may reduce the risk of adjacent segment degeneration.
Interpretation of FJV outcomes in our review was limited by variability in reporting metrics. While several studies used binary outcomes (violation present/absent), others employed quantitative or ordinal grading systems to assess the severity or proximity of screw breach relative to the facet joint (e.g., ≤ 1 mm breach as a minor violation, or multigrade scales)[25]. Due to this inconsistency, we conducted a narrative synthesis that preserved each study’s reporting format and focused on comparative directionality rather than absolute rates. A reduction in FJV with RA was consistently found across formats. However, the lack of standardized definitions precluded meta-analysis and introduced some uncertainty in estimating the clinical relevance of observed differences. Standardizing FJV classification systems in future robotic spine surgery studies will be essential to improve outcome comparability and more precisely quantify the protective effect of robotic screw placement on adjacent segment integrity.
Despite the clear technical advantages of RA, our review noted that PROs (VAS, ODI) at final follow-up were generally similar between RA and comparator groups. This mirrors the findings of Tian et al[48] and Wang et al[1], who found no significant differences in long-term pain and functional outcomes. Early postoperative pain relief, however, was more favorable in RA-assisted cases[24,37]. Complication rates were low across studies, with RA associated with fewer reoperations for malpositioned screws, supporting findings by Griepp et al[4] and Li et al[6].
In our systematic review, only one study briefly addressed the potential economic impact of robotic-assisted spine surgery[25], suggesting that its higher precision and safety must be weighed against possible financial challenges. This underscores the importance of future research focusing on the cost-effectiveness of these technologies to inform decision-making and ensure equitable access. Robust economic evaluations are crucial for assessing the sustainability and real-world applicability of robotic systems in spinal surgery.
The majority of studies included in our review were retrospective cohorts, raising concerns about selection bias and confounding. These challenges were similarly acknowledged[8-10], highlighting the need for more robust prospective designs. The one included RCT[24] demonstrated low risk of bias, underscoring the value of high-quality randomized trials in clarifying the comparative benefits of RA.
Our GRADE evaluation indicated moderate-quality evidence supporting the benefit of RA techniques in improving pedicle screw placement accuracy and reducing FJVs, based on consistent findings across multiple studies[15,20,21]. Conversely, operative time, EBL, PROs, and sagittal alignment were supported by low-quality evidence, primarily due to high risk of bias, inconsistency, and imprecision. Although RA was consistently favored for reducing radiation exposure, this outcome was not formally graded due to incompatible reporting formats (e.g., dose, time, or fluoroscopy image count). These findings align with prior literature highlighting both the precision benefits of RA and the current limitations in evidence certainty[35,49,50].
Our systematic review has several limitations. While we were able to conduct meta-analyses for pedicle screw placement accuracy, EBL, and operative time, the quality of evidence was constrained by clinical and methodological heterogeneity. Notably, definitions and measurement methods varied across studies, particularly for outcomes such as radiation exposure, which could not be synthesized quantitatively due to inconsistent reporting units (e.g., mGy, μSv, fluoroscopy time, image counts). Additionally, the diversity of comparator techniques, variability in surgeon experience, and the use of different robotic platforms introduce potential confounding. Most included studies were single-center and observational, limiting generalizability. Future research should prioritize multicenter RCTs with standardized protocols, consistent outcome definitions, and extended follow-up to evaluate not only safety and efficacy but also cost-effectiveness and patient-centered outcomes across different robotic systems.
In summary, our systematic review supports the conclusion that RA-guided lumbar fusion offers consistent technical advantages in screw placement accuracy, radiation reduction, and surgical precision while maintaining comparable long-term clinical outcomes to traditional techniques. The translation of these technical gains into improved patient outcomes and healthcare efficiency will rely on continued research, informed by rigorous multicenter trials and standardized data reporting practices.
RA LIF demonstrates superior pedicle screw placement accuracy and reduced intraoperative blood loss compared to conventional fluoroscopy- or navigation-guided techniques. While no significant difference was observed in operative time, RA systems consistently reduced surgeon radiation exposure and adjacent segment degeneration. Despite these perioperative advantages, long-term clinical outcomes - including pain relief, fusion success, and complication rates - remain comparable across techniques. These findings highlight the clinical precision and safety profile of robotic systems.
Future research should prioritize high-quality, multicenter RCTs that stratify patients by relevant clinical variables - such as obesity status and bone mineral density - to better understand subgroup effects on outcomes. In addition, long-term prospective registries that capture revision rates, hardware failure, and PROs beyond 5 years will be essential for assessing the sustained value of robotic assistance. Finally, adopting standardized outcome definitions - particularly for screw accuracy grading, FJV, and perioperative complications - will enhance cross-study comparability and support more robust future meta-analyses.
| 1. | Wang L, Li C, Wang Z, Li D, Tian Y, Yuan S, Liu X. Comparison of robot-assisted versus fluoroscopy-assisted minimally invasive transforaminal lumbar interbody fusion for degenerative lumbar spinal diseases: 2-year follow-up. J Robot Surg. 2023;17:473-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 2. | Tong M, Zhang S, Zhang W, Mou L, Dong Z, Wang R, Li S, Huang Y. Efficacy and safety of navigation robot-assisted versus conventional oblique lateral lumbar interbody fusion with internal fixation in the treatment of lumbar degenerative diseases: A retrospective study. Medicine (Baltimore). 2024;103:e39261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Shafi KA, Pompeu YA, Vaishnav AS, Mai E, Sivaganesan A, Shahi P, Qureshi SA. Does robot-assisted navigation influence pedicle screw selection and accuracy in minimally invasive spine surgery? Neurosurg Focus. 2022;52:E4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Griepp DW, Caskey J, Bunjaj A, Turnbull J, Alsalahi A, Alexander H, Dragonette J, Sarcar B, Desai S, Tong D, Soo TM, Bono P, Kelkar P, Houseman C, Claus CF, Richards BF, Carr DA. Irradiation safety, anesthesia time, surgical complications, and patient-reported outcomes in the robotic Mazor X versus fluoroscopy guided minimally invasive transforaminal lumbar interbody fusion surgery: a comparative cohort study. Neurosurg Focus. 2024;57:E11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Lin MC, Liu HW, Su YK, Lo WL, Lin CM. Robot-guided versus freehand fluoroscopy-guided minimally invasive transforaminal lumbar interbody fusion: a single-institution, observational, case-control study. Neurosurg Focus. 2022;52:E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Li T, Liao W, Hu J, Zhang W, Yu Y, Wang F, Liu X. Clinical and radiographic comparison of robot-assisted single-position versus traditional dual-position lateral lumbar interbody fusion. J Neurosurg Spine. 2025;42:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Heath DC, Chang HK, Chang CC, Yang HC, Tu TH, Hsu BH, Lin MC, Wu JC, Lin CM, Huang WC, Liu HW. Comparison between robot-assisted and navigation-guided minimally invasive transforaminal lumbar interbody fusion: a multicenter study. Neurosurg Focus. 2024;57:E12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 8. | McKenzie DM, Westrup AM, O'Neal CM, Lee BJ, Shi HH, Dunn IF, Snyder LA, Smith ZA. Robotics in spine surgery: A systematic review. J Clin Neurosci. 2021;89:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Perdomo-Pantoja A, Ishida W, Zygourakis C, Holmes C, Iyer RR, Cottrill E, Theodore N, Witham TF, Lo SL. Accuracy of Current Techniques for Placement of Pedicle Screws in the Spine: A Comprehensive Systematic Review and Meta-Analysis of 51,161 Screws. World Neurosurg. 2019;126:664-678.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 10. | Guan J, Feng N, Yu X, Yang K. Comparison of robot-assisted versus fluoroscopy-guided transforaminal lumbar interbody fusion (TLIF) for lumbar degenerative diseases: a systematic review and meta-analysis of randomized controlled trails and cohort studies. Syst Rev. 2024;13:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Chang M, Wang L, Yuan S, Tian Y, Zhao Y, Liu X. Percutaneous Endoscopic Robot-Assisted Transforaminal Lumbar Interbody Fusion (PE RA-TLIF) for Lumbar Spondylolisthesis: A Technical Note and Two Years Clinical Results. Pain Physician. 2022;25:E73-E86. [PubMed] |
| 12. | Pimenta L, Amaral R, Taylor W, Tohmeh A, Pokorny G, Rodrigues R, Arnoni D, Guirelli T, Batista M. The prone transpsoas technique: preliminary radiographic results of a multicenter experience. Eur Spine J. 2021;30:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Soliman MAR, Aguirre AO, Ruggiero N, Kuo CC, Mariotti BL, Khan A, Mullin JP, Pollina J. Comparison of prone transpsoas lateral lumbar interbody fusion and transforaminal lumbar interbody fusion for degenerative lumbar spine disease: A retrospective radiographic propensity score-matched analysis. Clin Neurol Neurosurg. 2022;213:107105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Smith TG, Pollina J, Joseph SA Jr, Howell KM. Effects of Surgical Positioning on L4-L5 Accessibility and Lumbar Lordosis in Lateral Transpsoas Lumbar Interbody Fusion: A Comparison of Prone and Lateral Decubitus in Asymptomatic Adults. World Neurosurg. 2021;149:e705-e713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Lai YP, Lin YH, Wu YC, Shih CM, Chen KH, Lee CH, Pan CC. Robot-Assisted Pedicle Screw Placement Led to Lower Screw Loosening Rate than Fluoroscopy-Guided Technique in Transforaminal Lumbar Interbody Fusion for Lumbar Degenerative Disease: A Single-Center Retrospective Study. J Clin Med. 2022;11:4989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 16. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 5185] [Article Influence: 1037.0] [Reference Citation Analysis (1)] |
| 17. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 18624] [Article Influence: 2660.6] [Reference Citation Analysis (0)] |
| 18. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 12488] [Article Influence: 1248.8] [Reference Citation Analysis (2)] |
| 19. | Aguayo-Albasini JL, Flores-Pastor B, Soria-Aledo V. [GRADE system: classification of quality of evidence and strength of recommendation]. Cir Esp. 2014;92:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Zhang Q, Han XG, Xu YF, Liu YJ, Liu B, He D, Sun YQ, Tian W. Robot-Assisted Versus Fluoroscopy-Guided Pedicle Screw Placement in Transforaminal Lumbar Interbody Fusion for Lumbar Degenerative Disease. World Neurosurg. 2019;125:e429-e434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Zhang Q, Xu YF, Tian W, Le XF, Liu B, Liu YJ, He D, Sun YQ, Yuan Q, Lang Z, Han XG. Comparison of Superior-Level Facet Joint Violations Between Robot-Assisted Percutaneous Pedicle Screw Placement and Conventional Open Fluoroscopic-Guided Pedicle Screw Placement. Orthop Surg. 2019;11:850-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Chen X, Song Q, Wang K, Chen Z, Han Y, Shen H, Li Q. Robot-assisted minimally invasive transforaminal lumbar interbody fusion versus open transforaminal lumbar interbody fusion: a retrospective matched-control analysis for clinical and quality-of-life outcomes. J Comp Eff Res. 2021;10:845-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Cui GY, Han XG, Wei Y, Liu YJ, He D, Sun YQ, Liu B, Tian W. Robot-Assisted Minimally Invasive Transforaminal Lumbar Interbody Fusion in the Treatment of Lumbar Spondylolisthesis. Orthop Surg. 2021;13:1960-1968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Feng S, Tian W, Wei Y. Clinical Effects of Oblique Lateral Interbody Fusion by Conventional Open versus Percutaneous Robot-Assisted Minimally Invasive Pedicle Screw Placement in Elderly Patients. Orthop Surg. 2020;12:86-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Li C, Liu D, Tian YH, Yuan SM, Wang LL, Liu XY. [Efficacy comparison of robot-assisted versus freehand fluoroscopy-assisted minimally invasive transforaminal lumbar interbody fusion for degenerative lumbar spinal diseases]. Zhonghua Yi Xue Za Zhi. 2024;104:3498-3505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Han XG, Tang GQ, Han X, Xing YG, Zhang Q, He D, Tian W. Comparison of Outcomes between Robot-Assisted Minimally Invasive Transforaminal Lumbar Interbody Fusion and Oblique Lumbar Interbody Fusion in Single-Level Lumbar Spondylolisthesis. Orthop Surg. 2021;13:2093-2101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | De Biase G, Gassie K, Garcia D, Abode-Iyamah K, Deen G, Nottmeier E, Chen S. Perioperative Comparison of Robotic-Assisted Versus Fluoroscopically Guided Minimally Invasive Transforaminal Lumbar Interbody Fusion. World Neurosurg. 2021;149:e570-e575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Fayed I, Tai A, Triano M, Sayah A, Makariou E, Voyadzis JM, Sandhu FA. Robot-Assisted Percutaneous Pedicle Screw Placement: Evaluation of Accuracy of the First 100 Screws and Comparison with Cohort of Fluoroscopy-guided Screws. World Neurosurg. 2020;143:e492-e502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Yang JS, He B, Tian F, Liu TJ, Liu P, Zhang JN, Liu SC, Tuo Y, Chu L, Hao DJ. Accuracy of Robot-Assisted Percutaneous Pedicle Screw Placement for Treatment of Lumbar Spondylolisthesis: A Comparative Cohort Study. Med Sci Monit. 2019;25:2479-2487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Schatlo B, Molliqaj G, Cuvinciuc V, Kotowski M, Schaller K, Tessitore E. Safety and accuracy of robot-assisted versus fluoroscopy-guided pedicle screw insertion for degenerative diseases of the lumbar spine: a matched cohort comparison. J Neurosurg Spine. 2014;20:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 31. | Li T, Liao WB, Zhong WJ, Liu XL, Wang F, Hu J. [Robot-assisted minimally invasive transforaminal lumbar interbody fusion in treatment of lumbar degenerative diseases]. Zhongguo Zuzhi Gongcheng Yanjiu. 2024;28:1855-1862. [DOI] [Full Text] |
| 32. | Li T, Liu XL, Wang F, Hu J. [Robot-assisted minimally invasive transforaminal lumbar interbody fusion in the treatment of lumbar degenerative diseases: accuracy and safety of screw placement]. Zhongguo Zuzhi Gongcheng Yanjiu. 2022;26:5812-5818. [DOI] [Full Text] |
| 33. | Hiyama A, Katoh H, Nomura S, Sakai D, Sato M, Watanabe M. Radiographs assessment of changes in the psoas muscle at L4-L5 level after single-level lateral lumbar interbody fusion in patients with postoperative motor weakness. J Clin Neurosci. 2021;90:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Pham MH, Diaz-Aguilar LD, Shah V, Brandel M, Loya J, Lehman RA. Simultaneous Robotic Single Position Oblique Lumbar Interbody Fusion With Bilateral Sacropelvic Fixation in Lateral Decubitus. Neurospine. 2021;18:406-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Fan Y, Peng Du J, Liu JJ, Zhang JN, Liu SC, Hao DJ. Radiological and clinical differences among three assisted technologies in pedicle screw fixation of adult degenerative scoliosis. Sci Rep. 2018;8:890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Passias PG, Brown AE, Alas H, Bortz CA, Pierce KE, Hassanzadeh H, Labaran LA, Puvanesarajah V, Vasquez-Montes D, Wang E, Ihejirika RC, Diebo BG, Lafage V, Lafage R, Sciubba DM, Janjua MB, Protopsaltis TS, Buckland AJ, Gerling MC. A cost benefit analysis of increasing surgical technology in lumbar spine fusion. Spine J. 2021;21:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Li Y, Chen L, Liu Y, Ding H, Lu H, Pan A, Zhang X, Hai Y, Guan L. Accuracy and safety of robot-assisted cortical bone trajectory screw placement: a comparison of robot-assisted technique with fluoroscopy-assisted approach. BMC Musculoskelet Disord. 2022;23:328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Vardiman AB, Wallace DJ, Crawford NR, Riggleman JR, Ahrendtsen LA, Ledonio CG. Pedicle screw accuracy in clinical utilization of minimally invasive navigated robot-assisted spine surgery. J Robot Surg. 2020;14:409-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Singhatanadgige W, Sukthuayat A, Tanaviriyachai T, Kongtharvonskul J, Tanasansomboon T, Kerr SJ, Limthongkul W. Risk factors for polyetheretherketone cage subsidence following minimally invasive transforaminal lumbar interbody fusion. Acta Neurochir (Wien). 2021;163:2557-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Kam JKT, Gan C, Dimou S, Awad M, Kavar B, Nair G, Morokoff A. Learning Curve for Robot-Assisted Percutaneous Pedicle Screw Placement in Thoracolumbar Surgery. Asian Spine J. 2019;13:920-927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Yu J, Zhang Q, Fan MX, Han XG, Liu B, Tian W. Learning curves of robot-assisted pedicle screw fixations based on the cumulative sum test. World J Clin Cases. 2021;9:10134-10142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Torii Y, Ueno J, Iinuma M, Yoshida A, Niki H, Akazawa T. The Learning Curve of Robotic-Assisted Pedicle Screw Placements Using the Cumulative Sum Analysis: A Study of the First 50 Cases at a Single Center. Spine Surg Relat Res. 2022;6:589-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Paramasivam Meenakshi Sundaram P, Lai MC, Kaliya-Perumal A, Oh JY. Overcoming the Learning Curve in Robot-Assisted Spinal Surgery—How Does It Compare to O-Arm Navigation? Surgeries. 2024;5:896-907. [DOI] [Full Text] |
| 44. | Menger RP, Savardekar AR, Farokhi F, Sin A. A Cost-Effectiveness Analysis of the Integration of Robotic Spine Technology in Spine Surgery. Neurospine. 2018;15:216-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 45. | Ezeokoli EU, Pfennig M, John J, Gupta R, Khalil JG, Park DK. Index Surgery Cost of Fluoroscopic Freehand Versus Robotic-Assisted Pedicle Screw Placement in Lumbar Instrumentation: An Age, Sex, and Approach-Matched Cohort Comparison. J Am Acad Orthop Surg Glob Res Rev. 2022;6:e22.00137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 46. | Chumnanvej S, Ariyaprakai K, Pillai BM, Suthakorn J, Gurusamy S, Chumnanvej S. Cost-effectiveness of robotic-assisted spinal surgery: A single-center retrospective study. Laparoendosc Robotic Surg. 2023;6:147-153. [DOI] [Full Text] |
| 47. | Malone H. Commentary: Robotic-Assisted vs Nonrobotic-Assisted Minimally Invasive Transforaminal Lumbar Interbody Fusion: A Cost-Utility Analysis. Neurosurgery. 2022;90:e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Tian W, Xu Y, Liu B, Liu Y, He D, Yuan Q, Lang Z, Lyu Y, Han X, Jin P. Lumbar spine superior-level facet joint violations: percutaneous versus open pedicle screw insertion using intraoperative 3-dimensional computer-assisted navigation. Chin Med J (Engl). 2014;127:3852-3856. [PubMed] |
| 49. | Gouzoulis MJ, Seddio AE, Winter AD, Jabbouri SS, Zhu JR, Rubio DR, Varthi AG, Grauer JN. Robotic-Assisted Versus Navigation-Assisted Posterior Lumbar Fusion : A National Database Study. Spine (Phila Pa 1976). 2024;49:1483-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Kim HJ, Kang KT, Chun HJ, Hwang JS, Chang BS, Lee CK, Yeom JS. Comparative study of 1-year clinical and radiological outcomes using robot-assisted pedicle screw fixation and freehand technique in posterior lumbar interbody fusion: A prospective, randomized controlled trial. Int J Med Robot. 2018;14:e1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/