Published online Oct 18, 2025. doi: 10.5312/wjo.v16.i10.110233
Revised: June 27, 2025
Accepted: September 19, 2025
Published online: October 18, 2025
Processing time: 136 Days and 22 Hours
Although opioids and non-steroidal anti-inflammatory drugs are commonly used as preemptive analgesics in total knee arthroplasty (TKA), their side effects are a major concern. Tapentadol, a synthetic opioid analgesic, is noted for its higher potency, lower abuse potential, and better gastrointestinal tolerability compared to traditional opioids. However, its efficacy and safety as preemptive analgesia in TKA have not been evaluated.
To hypothesize that preemptive use of tapentadol significantly reduces posto
Ninety patients undergoing unilateral TKA were randomized to receive either tapentadol (single dose of 100 mg sustained-release, n = 45) or a matched placebo 1 hour before surgery. Postoperative pain was assessed using the visual analog scale (VAS), and total pain reduction scores were recorded. Total rescue analgesic consumption and side effects were monitored for 24 hours. Blood samples were collected 6 hours postoperatively to measure plasma levels of cholecystokinin (CCK) (a potential biomarker of pain) and tapentadol using enzyme-linked im
The baseline characteristics of both groups were comparable. The 24-hour VAS scores, the primary outcome, were significantly lower in the tapentadol group median [interquartile range (IQR)] [1.0 (1.0-3.0)] compared to the placebo group [3.5 (2.0-5.0)]. Significant differences in VAS scores were observed at 4 hours, 6 hours, and 12 hours postoperatively (P < 0.05). Requests for rescue analgesia were significantly delayed in the tapentadol group (P = 0.01), and the total dose of analgesics used was significantly lower [median (IQR): 3 (2-4)] compared to the placebo group [4.5 (3-5), P = 0.001]. No major adverse events were observed in either group. Plasma tapentadol concentrations correlated well with pain intensity, whereas no correlation was found between CCK levels and pain intensity.
A preemptive single dose of 100 mg oral tapentadol is safe, effective, and significantly reduces postoperative pain and rescue analgesic requirements in TKA patients. This approach may reduce opioid dependence and support enhanced recovery protocols.
Core Tip: This randomized, double-blind, placebo-controlled trial evaluated the efficacy and safety of a single preemptive 100 mg dose of sustained-release tapentadol in patients undergoing total knee arthroplasty (TKA). Tapentadol significantly reduced postoperative pain scores, delayed the need for rescue analgesia, and lowered total analgesic consumption without increasing opioid-related side effects. Its plasma concentration correlated strongly with pain intensity, while cholecystokinin levels did not. With a favorable safety profile and analgesic efficacy, tapentadol may be a valuable component of multimodal analgesia in TKA, offering an opioid-sparing strategy with reduced gastrointestinal and central nervous system side effects. Further multicenter validation is warranted.
- Citation: Bhattacharjee S, Srinivasan A, Tripathy SK, Doki SK, Hota D. Efficacy and safety of pre-emptive tapentadol on pain control in total knee arthroplasty: A randomized, double-blind, placebo-controlled trial. World J Orthop 2025; 16(10): 110233
- URL: https://www.wjgnet.com/2218-5836/full/v16/i10/110233.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i10.110233
Effective postoperative pain management in total knee arthroplasty (TKA) is crucial for early patient recovery. Proper pain control facilitates early rehabilitation, enhances functional outcomes, reduces morbidity, shortens hospital stays, and increases patient satisfaction[1-3]. Currently, multimodal analgesia is the standard approach for managing pain in total hip and knee arthroplasty. Preemptive analgesia, a key component of this strategy, involves administering analgesics before surgery to reduce the sensitization of peripheral and central pain pathways caused by surgical tissue damage[4-7].
Evidence suggests that preemptive analgesia may be more effective than similar treatments initiated after surgery due to its protective effect on nociceptive pathways. This approach can potentially reduce immediate postoperative pain and prevent the development of chronic pain. Common preemptive analgesics include non-steroidal anti-inflammatory drugs (NSAIDs), gabapentinoids, and opioids[4-7]. However, these medications are associated with various adverse effects. Opioids and gabapentinoids can cause drowsiness, drug dependence, constipation, and respiratory depression, while NSAIDs can damage the gastric mucosa and impair renal function. The efficacy of these drugs in postoperative pain control also varies across studies[5,6].
Tapentadol is a newer synthetic opioid analgesic used for pain management following TKA and total hip arthroplasty[7]. It acts as a central μ-opioid receptor agonist with additional norepinephrine reuptake inhibition (Supplementary Figure 1). Tapentadol has a relatively low affinity for the μ-opioid receptor, resulting in a lower potential for opioid-related adverse effects such as respiratory depression and constipation. Additionally, tapentadol has opioid-sparing effects. Approved by the United States Food and Drug Administration in 2008 for the treatment of moderate to severe acute pain in adults[7,8], tapentadol[8-12] has shown superior or equivalent efficacy compared to oxycodone/naloxone in postoperative pain management, with fewer cases of constipation and shorter hospital stays[13-17].
However, tapentadol has not been studied as a preemptive analgesic in arthroplasty. Therefore, this randomized controlled study aims to evaluate the efficacy and safety of preemptive tapentadol in TKA.
This prospective, randomized, double-blind, parallel-group, placebo-controlled trial was conducted to evaluate the efficacy and safety of preemptive tapentadol in TKA. All adult patients (> 18 years) of either sex undergoing unilateral TKA at our tertiary care center between July 2017 and December 2019 were screened for eligibility. Patients on tricyclic antidepressants, monoamine oxidase inhibitors, serotonin and norepinephrine reuptake inhibitors, opioids, and those with a history of bronchospastic disease, seizure, and substance abuse or those with American Society of Anesthesiologists score > 3 were excluded. Patients with known allergy to tapentadol, similar group of medication intake, renal disease, liver disease, malignancy, or psychiatric illness were also excluded. A voluntary, written informed consent form was obtained from all eligible participants, and the study was initiated following approval of the Institutional Ethics Committee (No. T/IM-NF/Pharma/02/17). The trial was registered at clinicaltrials.gov (No. NCT03351517).
Patients were randomized using a variable block randomization technique, implemented through the blockrand package in R, to ensure balanced allocation between groups while maintaining unpredictability. The randomization sequence was generated with variable block sizes to assign participants in a 1:1 ratio to receive either a single dose of sustained-release (SR) tapentadol (100 mg capsule) or a placebo (100 mg capsule) prior to undergoing TKA.
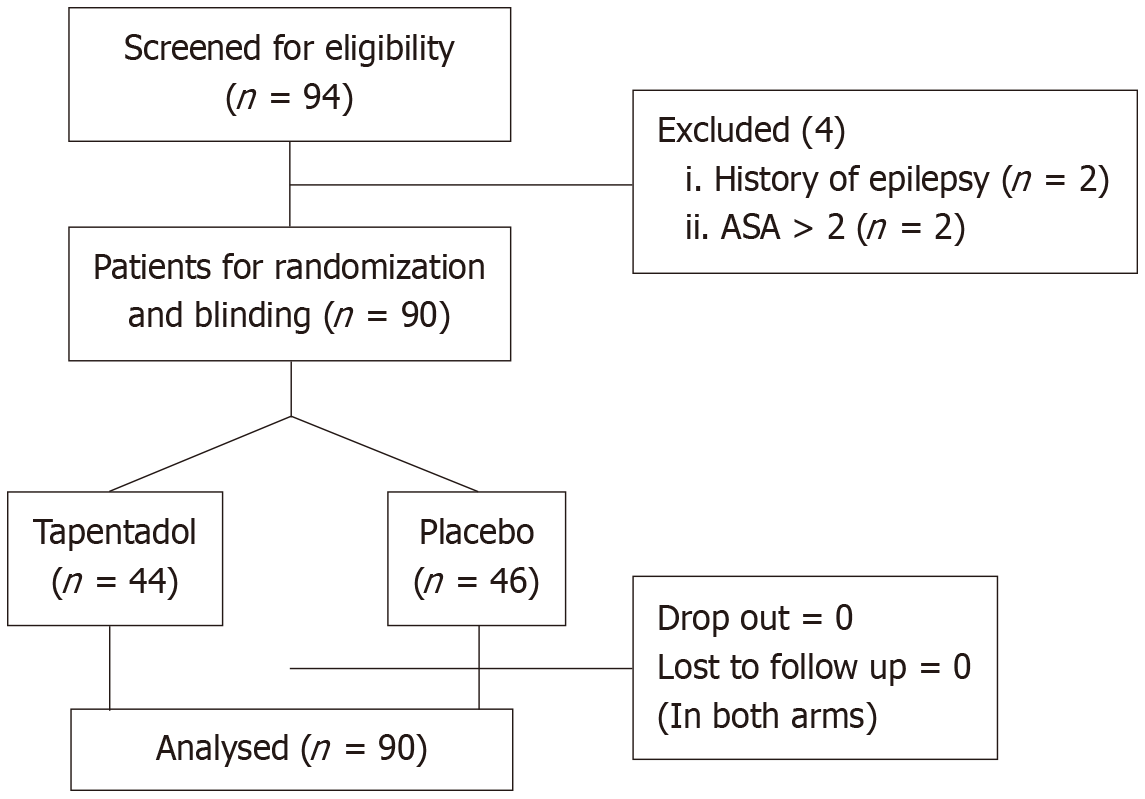
To maintain allocation concealment, the randomization list was stored securely, and the treatment assignments were placed in sequentially numbered, sealed opaque envelopes. Each envelope contained the treatment code corresponding to the randomization sequence and was opened only at the time of participant enrollment. The study medications were identically prepared, labeled, and coded to be indistinguishable, ensuring blinding for both the participants and the research team. This double-blind approach minimized bias in treatment allocation and outcome assessment, enhancing the validity of the study (Figure 1). The baseline clinical parameters and pain score [visual analog scale (VAS)] of the patients were evaluated, and the drug was administered one hour prior to surgery.
All the TKA surgery was performed by a single surgeon (Tripathy SK) uniformly in a standard fashion. The posterior stabilized cemented components (Zimmer Nexgen LPS) were implanted under regional anesthesia and tourniquet control. The patients received chemical and mechanical prophylaxis for venous thromboembolism in the postoperative period, and they were mobilized as early as possible. The pain medications included intravenous infusion of Paracetamol (1 mg four times a day) every six hours and Diclofenac injection (75 mg intramuscularly/intravenous, maximum twice daily) on demand. Codeine phosphate was reserved for patients if the pain was not controlled by the above two medications. Tramadol was used instead of Diclofenac as rescue medication in case of patients with renal impairment
The postoperative pain scores were assessed at 0 hour, 2 hours, 4 hours, 6 hours, 12 hours, and 24 hours of surgery.
Blood samples were collected from all patients at 6 hours of surgery to assess plasma cholecystokinin (CCK) (a possible biomarker of pain) and tapentadol levels using enzyme-linked immunosorbent assay (ELISA) and high-performance liquid chromatography (HPLC). CCK was chosen due to its proposed role in visceral pain signaling; however, its utility in orthopedic postoperative pain remains exploratory.
The blood samples were centrifuged and the plasma was stored at -80 °C until analysis. Plasma tapentadol levels were quantified using HPLC with ultraviolet detection. Sample preparation included protein precipitation with acetonitrile, followed by centrifugation and injection of the supernatant into the HPLC system. Calibration standards were prepared in pooled plasma with known tapentadol concentrations to construct a standard curve (r² > 0.99). Internal standards were used to correct for recovery variability. Inter-assay and intra-assay coefficient of variation (CV) were below 10%.
CCK levels were measured using a commercially available ELISA kit (Caltech Life Science, Bhubaneshwar, India). Samples and standards were processed in duplicate according to the manufacturer’s instructions. The assay sensitivity was 0.1 pg/mL, with inter-assay and intra-assay CVs below 8%.
Any adverse reactions to the drugs were noted.
The primary objective was to evaluate pain scores (VAS) at 24 hours postoperatively. The secondary objectives were: (1) Evaluation of pain score at 0 hour, 2 hours, 4 hours, 6 hours, and 12 hours; (2) Evaluation of total pain reduction (TOTPAR) (TOTPAR scale: None-Mild-Moderate-Severe) at 0 hour, 2 hours, 4 hours, 6 hours, 12 hours, 24 hours postoperatively[18]; (3) Amount of total rescue analgesic consumption within 24 hours of surgery; and (4) Assessment of plasma concentrations of CCK and tapentadol at 6 hours of surgery.
The TOTPAR scale was used to assess patients' subjective perception of overall pain relief at predefined postoperative intervals. This categorical scale classifies pain relief into four levels: (1) None; (2) Mild; (3) Moderate; and (4) Complete. “None” indicates no noticeable pain relief, while “mild” reflects only slight relief with ongoing discomfort. “Moderate” suggests a clear reduction in pain, though some discomfort remains, and “complete” denotes near-total or total relief from pain. Patients were asked to report their perceived level of pain relief at 0 hour, 2 hours, 4 hours, 6 hours, 12 hours, and 24 hours following surgery. This scale complements the VAS by capturing the qualitative dimension of pain control and analgesic effectiveness. The time of the first request for supplemental analgesia was also recorded. The safety outcomes were evaluated in terms of treatment-emergent adverse events. The common adverse events such as nausea, vomiting, drowsiness, and pruritus were rated on a four-point verbal scale (none, mild, moderate, severe).
The sample size was calculated using the open-source software R (version 3.4.1). A number of 45 participants were needed in each group to detect a difference in the VAS score (scale of 10) of 1.5 (15%) between the two groups with a mean variance of 4 (SD = 2). The power of detection was 90%, allowing a false positive rate of 0.05. A post-hoc analysis confirmed > 90% power with an effect size of 1.05, affirming robustness.
Baseline characteristics between the two groups were compared using the independent t-test or Mann-Whitney U test for continuous variables and the χ2 or Fisher's exact test for categorical variables, as appropriate. Data were reported as mean ± SD or median with interquartile range (IQR) for continuous variables and as counts and percentages for categorical variables.
For the primary efficacy outcome, differences in median 24-hour VAS scores between groups were assessed using the Mann-Whitney U test. Secondary efficacy outcomes, including VAS scores at multiple time points, were analyzed using generalized linear mixed modeling (GLM) to evaluate group effects over time. The TOTPAR scale and time to first supplementary analgesia were compared between groups using Mann-Whitney U tests.
The total consumption of rescue analgesics was analyzed using Mann-Whitney U tests, and the results were presented as median and IQR. Receiver operating characteristic (ROC) curves were generated to assess the predictive relationship between CCK levels and VAS scores, as well as between plasma tapentadol concentrations and pain intensity. The area under the curve (AUC) was calculated, along with sensitivity, specificity, and thresholds for pain relief.
Safety outcomes were analyzed using χ2 or Fisher’s exact tests to compare the incidence of adverse events such as nausea, vomiting, dizziness, and pruritus between groups. P value of less than 0.05 was considered significant.
Ninety patients were randomized to receive either tapentadol (n = 45) or a matched placebo (n = 45) (Figure 1). Baseline characteristics in terms of age, gender, weight, VAS score, duration of surgery and anesthetics used between both the groups were similar (Table 1).
| Parameters | Tapentadol (n = 44) | Placebo (n = 46) | P value |
| Mean age (mean ± SD) | 61.07 ± 12.47 | 60.18 ± 11.18 | 0.75 |
| Gender (male) | 36% | 22% | 0.90 |
| Mean body weight (mean ± SD) | 63.50 ± 12.45 | 65.44 ± 11.40 | 0.46 |
| Baseline visual analog scale score for osteoarthritis, median (IQR) | 4 (4-5) | 4 (4-5) | 0.22 |
| The duration between drug administration and surgery (minutes), median (IQR) | 75.28 (62.70-78.03) | 75.87 (60.25-78.88) | 0.30 |
| Duration of surgery(minutes), median (IQR) | 68.20 (65.43-75.15) | 69.04 (66.20-72.33) | 0.16 |
| Baseline cholecystokinin levels, median (IQR) | 160.6 (138.39-206.58) | 158.35 (120.45-197.48) | 0.53 |
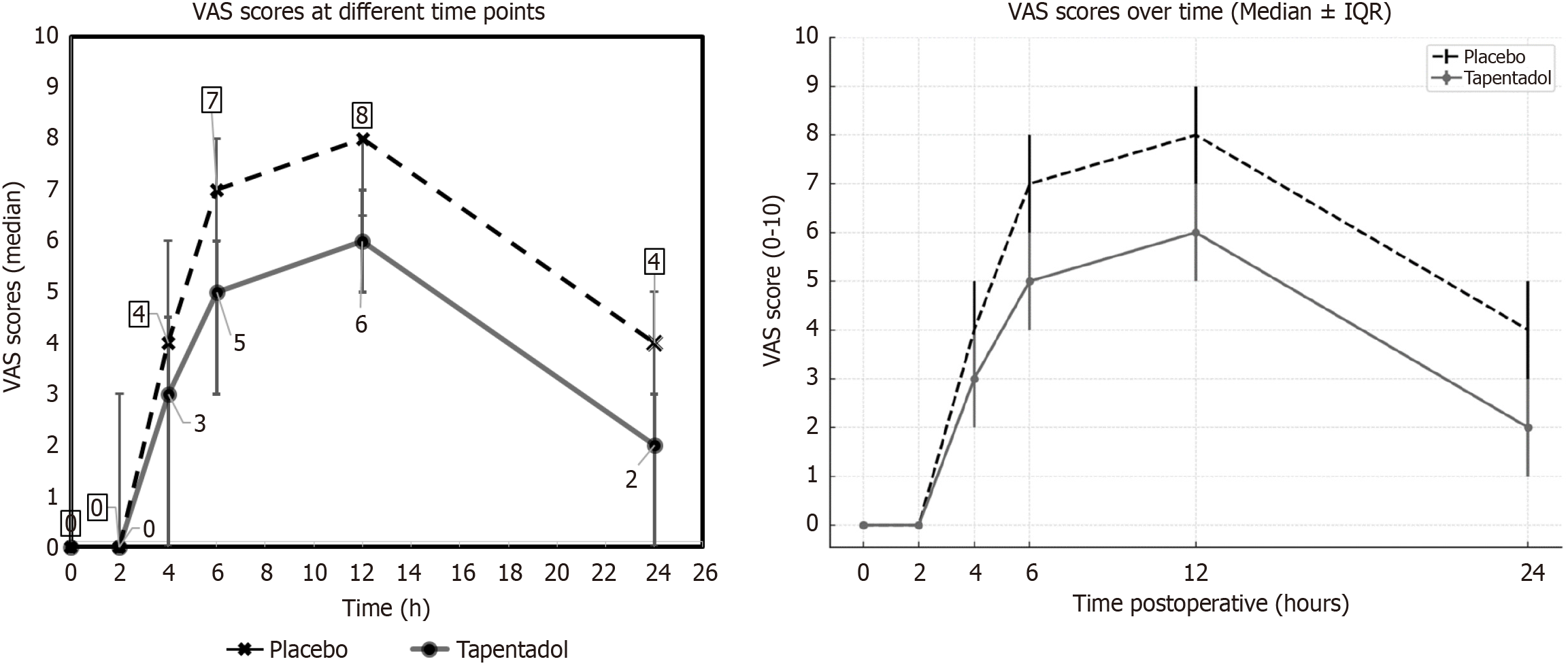
Primary efficacy outcome: The median 24 hours VAS scores for tapentadol and placebo groups were 1.0 (1.0-3.0) and 3.50 (2.0-5.0), respectively. The difference in the VAS score between the groups was 2 and it was statistically significant (P = 0.001; Figure 2). The observed median difference of 2.0 points on the VAS exceeds the established minimum clinically important difference of approximately 1.5 points for postoperative pain in TKA, supporting the clinical as well as statistical significance of these findings.
Secondary efficacy outcomes-clinical: (1) VAS scores at 0 hour, 2 hours, 4 hours, 6 hours, and 12 hours postoperatively: The median VAS scores at 0 hour, 2 hours, 4 hours, 6 hours, and 12 hours were 0, 0, 4, 7 and 8 in the placebo group, whereas these scores were 0, 0, 3, 5 and 6 in the tapentadol group. The difference in the VAS scores was statistically significant (P < 0.0503) at 4 hours, 6 hours, and 12 hours (Figure 2). The GLM showed a significant difference in VAS scores between the two groups independent of the time points (P = 0.001; Figure 2); (2) TOTPAR scale at 0 hour, 2 hours, 4 hours, 6 hours, 12 hours, and 24 hours postoperatively: There was a significant reduction in the severity of pain in the tapentadol group at 0 hour, 2 hours, 4 hours, 6 hours, 12 hours, and 24 hours postoperatively (Table 2). The request for first supplementary analgesia was significantly delayed in the tapentadol group (P = 0.01); and (3) Total doses of rescue analgesic consumption: The total dose (median and IQR) of rescue analgesics used in the tapentadol group [3 (2-4)] was significantly less (P = 0.001) compared to placebo [4.50 (3-5)] (Table 3).
| Postoperative TOTPAR at different time points | TOTPAR (pain reduction) | Tapentadol (n = 44) | Placebo (n = 46) | Fisher’s exact (P value) |
| TOTPAR at 2 hours | Complete | 40 (91) | 25 (55) | 0.020 |
| Mild | 3 (5) | 10 (26) | ||
| Moderate | 1 (2) | 7 (18) | ||
| None | 0 (0) | 4 (1) | ||
| TOTPAR at 4 hours | Complete | 18 (43) | 4 (4.5) | 0.002 |
| Mild | 6 (16) | 16 (44) | ||
| Moderate | 16 (32) | 23 (50) | ||
| None | 4 (8) | 3 (1.5) | ||
| TOTPAR at 6 hours | Complete | 3 (5) | 3 (5) | 0.006 |
| Mild | 9 (24) | 0 (0) | ||
| Moderate | 22 (51) | 23 (52) | ||
| None | 7 (18) | 16 (42) | ||
| TOTPAR at 12 hours | Complete | 7 (15) | 0 (0) | 0.001 |
| Mild | 17 (38) | 20 (45) | ||
| Moderate | 15 (34) | 2 (4) | ||
| None | 7 (15) | 24 (52) | ||
| TOTPAR at 24 hours | Complete | 14 (31) | 0 (0) | 0.001 |
| Mild | 9 (20) | 4 (9) | ||
| Moderate | 17 (38) | 20 (45) | ||
| None | 5 (1) | 22 (46) |
| Parameters (mean ± SD) | Placebo (n = 38) | Tapentadol (n = 37) | P value | |
| Total analgesic consumption | 4.00 ± 0.86 | 3.10 ± 0.86 | < 0.005 | |
| Individual analgesic consumption in 24 hours (mg) | Paracetamol | 2000 ± 305.00 | 1850 ± 309.00 | 0.004 |
| Diclofenac | 125 ± 35.96 | 72.24 ± 41.78 | 0.008 | |
| Tramadol | 76.67 ± 54.83 | 40.38 ± 51.77 | 0.001 | |
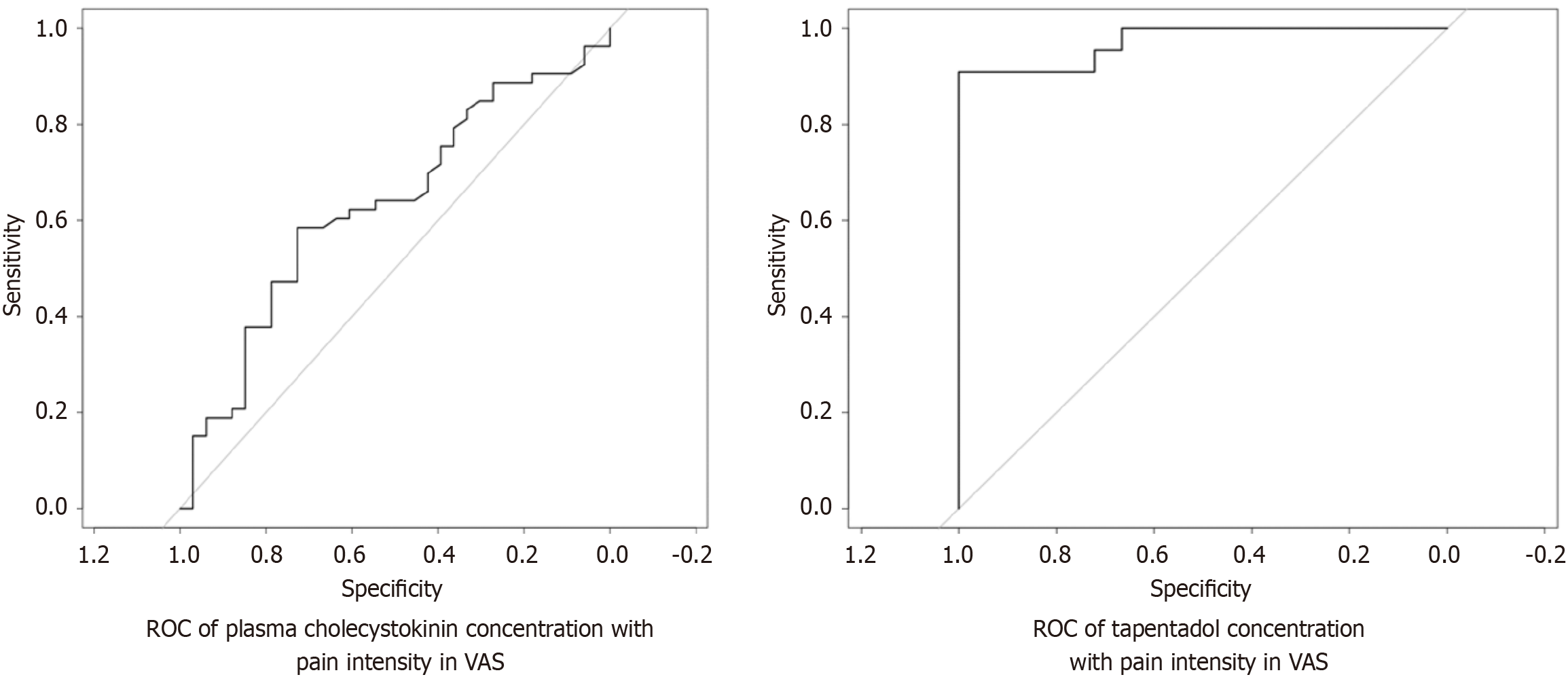
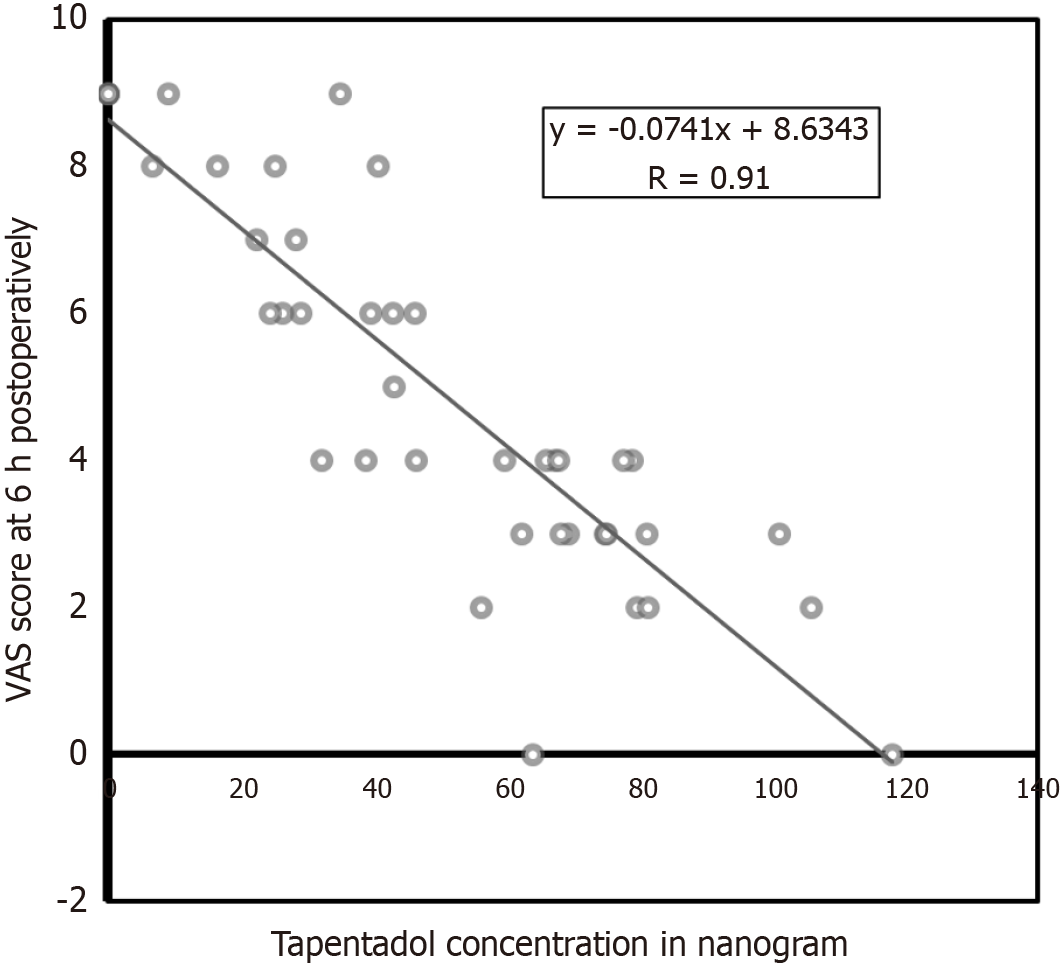
Secondary efficacy outcomes-biochemical: (1) CCK vs pain intensity: The ROC curve of CCK levels was plotted against VAS scores at 6 hours. It revealed AUC of 0.630 and coefficient of determination (R2) of 0.09 (P = 0.35). There was no correlation between CCK levels and pain scores. The sensitivity and specificity of CCK as a biological marker of pain (threshold 0.91) was found to be 0.58 and 0.72, respectively (Figure 3); and (2) Tapentadol vs pain intensity: The tapentadol concentrations were plotted against pain scores at 6 hours postoperatively; it showed a linear relationship with a coefficient of determination of 80% (Figure 4). The ROC curve showed the tapentadol concentration and pain score 4 as responders and above 4 as non-responders. The AUC was 0.97 and coefficient of determination (R2) was 0.8292. This model predicts 83% of data (pain intensity) correctly (P < 0.001, threshold = 46.19, specificity = 1.00, sensitivity = 0.90; Figure 3). The average actual plasma concentration of tapentadol was found to be 56.48 ng/mL whereas its derived threshold value for pain control was 46 ng/mL (P = 0.001).
Secondary safety outcome: There was no significant difference in percentage of patients developing nausea (13% vs 17%, P = 0.78), vomiting (6% vs 6%, P = 0.86), dizziness (6% vs 4%, P = 0.90) and pruritus (0% vs 2%, P = 0.65) between the tapentadol and placebo groups. However, the symptoms were mild and tolerable.
This study found that oral administration of 100 mg extended-release tapentadol as a pre-medication before TKA surgery decreased the postoperative pain and total analgesic consumption without increasing the incidence of opioid-related adverse effects.
Tapentadol has been found to be effective in moderate to severe pain of both acute and chronic onset[19]. In a meta-analysis, Wang et al[20] concluded that 75-100 mg of immediate-release tapentadol had similar pain control to morphine and tramadol. It was associated with a lesser incidence of gastrointestinal adverse effects such as nausea and constipation than other opioids. They suggested that tapentadol can be considered as a first-line opioid for acute pain[20]. There are limited studies on tapentadol in orthopedic surgery, and all these studies have focused only on postoperative pain management[10,13,14,16,18]. Based on the findings of limited randomized control trials, the Orthoevidence team suggested that using tapentadol instead of oxycodone in the multimodal analgesia strategy of hip and knee arthroplasty might be beneficial in terms of better safety and tolerability[21]. Another meta-analysis by Xiao et al[19] had similar recommendations.
Literature to date has only three published studies on tapentadol in hip and knee arthroplasties[21]. These original studies, one non-randomised controlled trial (RCT), and 2 RCTs, suggested the use of tapentadol for postoperative pain management in knee/hip replacement patients[10,14,17]. The non-RCT showed that patients treated with tapentadol after hip arthroplasty had significantly lower postoperative pain than those who received oxycodone/naloxone[10]. However, Rian et al[14] did not find significant differences among tapentadol, oxycodone, and placebo in the multimodal analgesia protocol. Nevertheless, this RCT had a small sample size, and it was underpowered. Although there was no significant difference in pain reduction, both RCTs approved that multimodal analgesia with tapentadol had fewer side effects (i.e., constipation) than with oxycodone. Rian et al[14] also suggested that tapentadol might be beneficial in reducing the length of hospital stay.
There are very few studies on the preemptive analgesic effect of tapentadol. In a randomized controlled trial, Yadav et al[12] used a single preemptive oral dose of tapentadol (75 mg) in laparoscopic cholecystectomy (Supplementary Table 1). They reported that it effectively reduced perioperative analgesic requirements and acute postoperative pain without added side effects. Sethi and co-workers used single-dose pretreatment tapentadol of 100 mg in another trial and compared it with etodolac and ketorolac. They observed a significant reduction in post-treatment endodontic pain compared to etodolac[22].
After an extensive search of the literature, we could find only one study from orthopedics that compared the extended-release tapentadol and oxycodone/naloxone as a preemptive medication[9]. In this RCT, Haeseler et al[9] reported that tapentadol and oxycodone/naloxone are reliable and effective perioperative multimodal oral analgesics. In terms of efficacy, tapentadol was found to be non-inferior but not superior to oxycodone/naloxone. They advocated uniform prophylactic medications for nausea, vomiting, and constipation irrespective of the type of preemptive opioid (Supplementary Table 1)[9, 12]. However, this study had several limitations and biases. There was substantial heterogeneity in patient selection as the author included different types of surgeries such as open reduction internal fixation of fractures, joint replacement, and arthroscopic ligament reconstruction procedures. All the surgeries were performed under general anesthesia, and the intraoperative prophylactic medications for nausea/vomiting were as per the discretion of the anesthetist. The patients were also not blinded to the treatment. We believe the low dose of tapentadol (50 mg) was also not adequate for optimal pain relief.
Contrary to it, our study results showed that 100 mg SR preemptive tapentadol was effective in postoperative pain control up to 24 hours of TKA. There was also a lesser need for rescue analgesics, and the time for the additional analgesia was longer in the tapentadol group compared to placebo. We believe the dual nature of the mechanism of tapentadol might have contributed to its efficacy[8].
The current evidence on tapentadol in hip and knee arthroplasty affirms that this drug is equally effective or superior to oxycodone/naloxone or other commonly used opioids. The significant advantage is its safety profile, and that should be one of the major determinants of use in the geriatric age group. Biondi et al[16] reported that tapentadol is not only effective but also safe and tolerable in patients above 75 years of age.
The present study observed no significant difference in the postoperative incidence of adverse events between preemptive tapentadol and placebo groups. As the analgesic property of tapentadol is derived from its affinity towards the non-opioid receptors mainly with minimal affinity for the opioid receptor, the typical opioid-related adverse effects are less[8].
Tapentadol is absorbed rapidly after oral administration and achieves peak plasma concentration within 1.25-1.5 hours. The elimination half-life is about 4.5 hours[23,24]. Consequently, administration of the drug an hour before the TKA surgery effectively covers the time point of maximal pain stimulus for the period of the surgical procedure. As the effect of spinal anesthesia wears off after 2-4 hours of surgery, both the groups in this study had no pain at 0 hour and 2 hours of surgery. Subsequently, the pain score was significantly different between the groups at all-time points, indicating the better efficacy of tapentadol compared to placebo. The blood levels of tapentadol and pain score at 6 hours were having a significant correlation (odds ratio = -0.97). This suggests that the threshold concentration of tapentadol is 40 ng/dL to achieve the maximal analgesic effect. This finding can be used to assess the pain control in special cases where patients are unable to express their pain intensity through traditional subjective pain scales.
There are certain limitations to this study. The study evaluated only the short-term effects of preemptive tapentadol, and the long-term effects could not be addressed. The study finding cannot be generalized to all categories of orthopedic surgeries. However, the strength of the study is homogeneity in the patient selection. As TKA is a standardized pro
To conclude, the present study established that tapentadol sustained release 100 mg is an effective and safe preemptive analgesic in patients undergoing TKA and incorporation of this drug into daily postoperative pain care system as a preemptive analgesic might be beneficial. Further, more extensive multicentric studies are warranted, focusing on di
The investigators acknowledge M/s. MSN Laboratories (Mumbai, India) for providing study drugs.
| 1. | Kahlenberg CA, Nwachukwu BU, McLawhorn AS, Cross MB, Cornell CN, Padgett DE. Patient Satisfaction After Total Knee Replacement: A Systematic Review. HSS J. 2018;14:192-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 2. | Maheshwari AV, Blum YC, Shekhar L, Ranawat AS, Ranawat CS. Multimodal pain management after total hip and knee arthroplasty at the Ranawat Orthopaedic Center. Clin Orthop Relat Res. 2009;467:1418-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Nakano N, Shoman H, Olavarria F, Matsumoto T, Kuroda R, Khanduja V. Why are patients dissatisfied following a total knee replacement? A systematic review. Int Orthop. 2020;44:1971-2007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Li JW, Ma YS, Xiao LK. Postoperative Pain Management in Total Knee Arthroplasty. Orthop Surg. 2019;11:755-761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 242] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 5. | Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. 2005;100:757-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 417] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 6. | Golladay GJ, Balch KR, Dalury DF, Satpathy J, Jiranek WA. Oral Multimodal Analgesia for Total Joint Arthroplasty. J Arthroplasty. 2017;32:S69-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Grape S, Tramèr MR. Do we need preemptive analgesia for the treatment of postoperative pain? Best Pract Res Clin Anaesthesiol. 2007;21:51-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Roulet L, Rollason V, Desmeules J, Piguet V. Tapentadol Versus Tramadol: A Narrative and Comparative Review of Their Pharmacological, Efficacy and Safety Profiles in Adult Patients. Drugs. 2021;81:1257-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Haeseler G, Schaefers D, Prison N, Ahrens J, Liu X, Karch A. Combatting pain after orthopedic/trauma surgery- perioperative oral extended-release tapentadol vs. extended-release oxycodone/naloxone. BMC Anesthesiol. 2017;17:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | D'Amato T, Martorelli F, Fenocchio G, Simili V, Kon E, Di Matteo B, Scardino M. Tapentadol vs oxycodone/naloxone in the management of pain after total hip arthroplasty in the fast track setting: an observational study. J Exp Orthop. 2019;6:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Wang X, Tay HP, Narayan SW, Penm J, Patanwala AE. Comparison of opioid prescribing upon hospital discharge in patients receiving tapentadol versus oxycodone following orthopaedic surgery. Int J Clin Pharm. 2021;43:1602-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Yadav G, Jain G, Samprathi A, Baghel A, Singh DK. Role of preemptive tapentadol in reduction of postoperative analgesic requirements after laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol. 2016;32:492-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Abstracts and Highlight Papers of the 36th Annual European Society of Regional Anaesthesia & Pain Therapy (ESRA) Congress 2017. Reg Anesth Pain Med. 2017;42:e1-e200. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Rian T, Skogvoll E, Hofstad J, Høvik L, Winther SB, Husby VS, Klaksvik J, Egeberg T, Sand K, Klepstad P, Wik TS. Tapentadol vs oxycodone for postoperative pain treatment the first 7 days after total knee arthroplasty: a randomized clinical trial. Pain. 2021;162:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Afilalo M, Morlion B. Efficacy of tapentadol ER for managing moderate to severe chronic pain. Pain Physician. 2013;16:27-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Biondi DM, Xiang J, Etropolski M, Moskovitz B. Tolerability and efficacy of tapentadol extended release in elderly patients ≥ 75 years of age with chronic osteoarthritis knee or low back pain. J Opioid Manag. 2015;11:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Viscusi ER, Allard R, Sohns M, Eerdekens M. Tapentadol immediate release for moderate to severe acute post-surgery pain. J Opioid Manag. 2019;15:51-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Chen YJ, Chiang CC, Huang PJ, Huang J, Karcher K, Li H. Tapentadol immediate-release for acute postbunionectomy pain: a phase 3, randomized, double-blind, placebo-controlled, parallel-group study in Taiwan. Curr Med Res Opin. 2015;31:2001-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Xiao JP, Li AL, Feng BM, Ye Y, Wang GJ. Efficacy and Safety of Tapentadol Immediate Release Assessment in Treatment of Moderate to Severe Pain: A Systematic Review and Meta-Analysis. Pain Med. 2017;18:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Wang X, Narayan SW, Penm J, Patanwala AE. Efficacy and Safety of Tapentadol Immediate Release for Acute Pain: A Systematic Review and Meta-Analysis. Clin J Pain. 2020;36:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Zhu M, Chang YP, Phillips S, Siddiqua A, Bhandari M. Use of Tapentadol for Postoperative Pain Management in Patients after Knee/Hip Arthroplasty: A Scoping Review. OrthoEvidence. 2021;4:3. |
| 22. | Sethi P, Agarwal M, Chourasia HR, Singh MP. Effect of single dose pretreatment analgesia with three different analgesics on postoperative endodontic pain: A randomized clinical trial. J Conserv Dent. 2014;17:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Terlinden R, Ossig J, Fliegert F, Lange C, Göhler K. Absorption, metabolism, and excretion of 14C-labeled tapentadol HCl in healthy male subjects. Eur J Drug Metab Pharmacokinet. 2007;32:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Tzschentke TM, Christoph T, Kögel B, Schiene K, Hennies HH, Englberger W, Haurand M, Jahnel U, Cremers TI, Friderichs E, De Vry J. (-)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel mu-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther. 2007;323:265-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 301] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/