Published online Aug 18, 2024. doi: 10.5312/wjo.v15.i8.773
Revised: July 19, 2024
Accepted: July 29, 2024
Published online: August 18, 2024
Processing time: 185 Days and 16.4 Hours
There is concern regarding potential long-term cardiotoxicity with systemic distribution of metals in total joint arthroplasty (TJA) patients.
To determine the association of commonly used implant metals with echocardiographic measures in TJA patients.
The study comprised 110 TJA patients who had a recent history of high chro
Higher cobalt concentrations were associated with increased left ventricular end-diastolic volume (estimate 5.09; 95%CI: 0.02-10.17) as well as left atrial and right ventricular dilation, particularly in men but no changes in cardiac function. Higher titanium concentrations were associated with a reduction in left ventricle global longitudinal strain (estimate 0.38; 95%CI: 0.70 to 0.06) and cardiac index (estimate 0.08; 95%CI, -0.15 to -0.01).
Elevated cobalt and titanium concentrations may be associated with structural and functional cardiac changes in some patients. Longitudinal studies are warranted to better understand the systemic effects of metals in TJA patients.
Core Tip: This study evaluated echocardiographic measures in 110 prospectively recruited total joint arthroplasty patients who had a recent history of elevated metal concentrations. Elevated cobalt and titanium concentrations were positively associated with some structural and functional cardiac changes.
- Citation: Brennan PC, Peterson SM, O'Byrne TJ, Laporta ML, Wyles CC, Jannetto PJ, Kane GC, Vassilaki M, Maradit Kremers H. Blood metal concentrations and cardiac structure and function in total joint arthroplasty patients. World J Orthop 2024; 15(8): 773-782
- URL: https://www.wjgnet.com/2218-5836/full/v15/i8/773.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i8.773
Total joint arthroplasty (TJA) is one of the most common surgical procedures in the United States and is expected to increase further in the coming years with a projected yearly incidence of nearly 5 million total hip arthroplasty (THA) and total knee arthroplasties (TKA) by the year 2040[1]. More than 7 million Americans are currently living with at least one TJA[2].
Although the TJA implant designs and materials can vary, most are made of metals including cobalt-chromium alloys, stainless steel, and titanium combined with ceramics and polymers[3]. Chronic exposure to these aforementioned metals can occur as metal containing implants are subjected to repeated mechanical and oxidative stresses that subsequently release metal ions locally and systemically[4]. Locally, metal ions released from both metal-on-metal (MoM) implants and metal-on-polyethylene are associated with adverse local tissue reactions[5,6]. In particular, cobalt was first identified as cardiotoxic in 1967 when Quebec beer drinkers developed acute cardiomyopathy after drinking beer that used cobalt as a foam-stabilizing agent[7]. Considering the history of cobalt-related cardiotoxicity, several studies over the last decade investigated the potential for cardiotoxicity associated with MoM implants with a focus on cobalt-chromium ion release[8-12]. In a postmortem study, cobalt concentration was significantly higher in the myocardial cells of patients who underwent a THA and this was postulated to arise from alterations in cardiac calcium handling[7]. In particular, three echocardiographic studies in THA patients showed higher metal concentrations in patients with MoM implants but no clinically significant associations between increased metal concentrations and cardiac dysfunction[13-15]. Even titanium, a biocompatible inert material, was attributed to osteolysis through the formation of titanium dioxide[16]. Due to the high prevalence of TJA and the potential of metal ions to be distributed systemically, concerns remain regarding the potential for long-term cardiotoxicity stemming from the systemic effects of metal ions[4,7].
With this background, the aim of the current study was to examine the association between three commonly used implant metals (cobalt, chromium, titanium) with echocardiographic measures and cardiac biomarkers in THA and TKA patients regardless of their implant type.
The study population comprised 110 TJA patients prospectively recruited at a large tertiary care medical center between March 2019 and December 2022. Patients were invited to participate based on having elevated blood metal concentrations of either titanium, cobalt, and/or chromium, as flagged in the electronic medical records (concentrations higher than 1 ng/mL for cobalt and chromium, and higher than 2 ng/mL for titanium) over the previous one-year period and had not undergone a revision procedure since their elevated laboratory measurements.
All patients consented to the use of their medical data for research purposes. The Institutional Review Board approved the study and a listing was maintained on clinicaltrials.gov through the duration of the study. All patients had a previous surgical history of at least one prior THA, TKA, or hip resurfacing procedure. The surgical history was ascertained through self-report and the institutional Total Joint Registry.
Patients who agreed to participate traveled to a single day of in-person appointments where they underwent a single blood draw to measure metal (cobalt, chromium, titanium) concentrations and troponin, and N-terminal pro b-type natriuretic peptide (NT-proBNP) as general indicators of heart health. The cardiac biomarkers were measured using an electrochemiluminescent immunoassay and the metals assayed using inductively coupled plasma mass spectrometry within an International Organization for Standardization Class-7 Laboratory[17,18]. In the present study, cobalt and chromium were analyzed in whole blood while titanium was analyzed in serum. Due to ordering and lab errors, one patient was missing the chromium level, one patient was missing the NT-proBNP level, and two patients were missing the troponin level.
All patients also underwent comprehensive transthoracic echocardiogram (GE E95, General Electric Healthcare) consisting of two-dimensional (2D), three-dimensional (3D), Doppler and speckle-tracking systolic strain echocardiography on the same day as the blood draw. All patients received an ultrasound enhancement agent (Lumason, Bracco Diagnostics) and underwent enhanced measurement of 2D biplane volumes and unenhanced 3D volumes as recom
Patient characteristics, laboratory values and echocardiographic findings were summarized using descriptive statistics [mean ± SD, median, interquartile range (IQR), count, percentage]. Many patients had “undetectable” metal concentrations (< 1 ng/mL), which were converted to numeric by using the highest numeric under the undetectable (e.g. 0.99 ng/mL). Metal measurements were treated as binary (detectable vs undetectable) or continuously on a log transformed scale (levels < 1 ng/mL was converted to 0.99 ng/mL). The association of log transformed metal concentrations (exposure) with echocardiographic measures and cardiac biomarkers (outcomes) were examined using age and sex-adjusted linear and logistic regression models. For significant results, β coefficients and odds ratio (OR) estimates from regression models were back-transformed to facilitate interpretation in original scales. All estimates represent the change corresponding to a doubling of the recorded metal level (e.g. from 1 ng/mL to 2 ng/mL).
The mean age of the 110 TJA patients was 68.8 (SD: 9.2 years) years, 45 (40.9%) were women and 108 (98.2%) were white (Table 1). Only 4 patients were current smokers and 1 had a previous history of heart failure. A total of 79 (71.8%) patients had a history of THA only, 11 (10.0%) TKA only and 20 (18.2%) patients had both surgeries. Of the 99 patients with at least 1 THA, 52 had a history of MoM implants, 5 had ceramic-on-poly implants and 3 had both MoM and ceramic-on-poly implants (remaining had metal-on-poly implants). The average time since the first THA or TKA surgery was 13.4 (SD: 5.7 years) years. A total of 59 patients (53.6%) had at least one revision for their TJA. Detectable metal concentrations were found for chromium (n = 43, 39.5%), cobalt (n = 51, 46.4%) and titanium (n = 89, 80.9%) (Table 2). Median (IQR) metal concentrations among detectable were 1.9 (1.2 ng/mL, 3.8 ng/mL) ng/mL for chromium, 3.7 (1.9 ng/mL, 6.3 ng/mL) ng/mL for cobalt and 3.0 (2.0 ng/mL, 5.0 ng/mL) ng/mL for titanium.
| Characteristic | Total |
| Age (years) | |
| Mean (SD) | 68.8 (9.2) |
| Median (IQR) | 69.0 (63.0, 75.0) |
| Min, Max | 44.0, 93.0 |
| Women | 45 (40.9) |
| Race | |
| Pacific Islander | 2 (1.8) |
| White | 108 (98.2) |
| Smoking history | |
| Current smoker | 4 (3.6) |
| Previous smoker | 36 (32.7) |
| Never smoker | 58 (52.7) |
| History of heart failure | 1 (0.9) |
| Body mass index (kg/m2) | |
| Mean (SD) | 30.3 (6.0) |
| Median (IQR) | 30.0 (62.2, 34.3) |
| Min, Max | 18.8, 47.0 |
| Body surface area (m2) | |
| Mean (SD) | 2.1 (0.3) |
| Median (IQR) | 2.1 (1.9, 2.2) |
| Min, Max | 1.4, 2.7 |
| Systolic blood pressure, mmHg | |
| Mean (SD) | 134.0 (18.9) |
| Median (IQR) | 132.0 (122.0, 144.0) |
| Min, Max | 90.0, 187.0 |
| Diastolic blood pressure, mmHg | |
| Mean (SD) | 77.6 (8.8) |
| Median (IQR) | 78.0 (72.0, 83.0) |
| Min, Max | 60.0, 108.0 |
| TJA history | |
| Joint | |
| Hip (total hip arthroplasty) | 79 (71.8) |
| Knee (total knee arthroplasty) | 11 (10.0) |
| Both | 20 (18.2) |
| Revision surgery | 59 (53.6) |
| Years between first TJA surgery and study visit | |
| Mean (SD) | 13.4 (5.7) |
| Median (IQR) | 12 (10.0, 16.0) |
| Min, Max | 2.0, 31.0 |
| Metal concentrations (ng/mL) | Total | Men | Women |
| Detectable chromium, | 43 (39.5) | 28 (43.8) | 15 (33.3) |
| Mean (SD) | 3.8 (4.7) | 3.0 (3.5) | 5.2 (6.3) |
| Median (IQR) | 1.9 (1.2, 3.8) | 1.9 (1.3, 2.9) | 2.8 (1.2, 5.8) |
| Min, max | 1.1, 21.4 | 1.1,18.5 | 1.2,21.4 |
| Detectable cobalt | 51 (46.4) | 36 (55.4) | 15 (33.3) |
| Mean (SD) | 4.9 (4.3) | 5.2 (4.7) | 4.1 (3.2) |
| Median (IQR) | 3.7 (1.9, 6.3) | 4.1 (2.1, 6.9) | 3.2 (1.9, 5.2) |
| Min, max | 1.1, 24.6 | 1.1, 24.6 | 1.1, 12 |
| Detectable titanium | 89 (80.9) | 49 (75.4) | 40 (88.9) |
| Mean (SD) | 4.8 (5.1) | 4.6 (6.1) | 5.1 (3.5) |
| Median (IQR) | 3.0 (2.0, 5.0) | 3 (1,5) | 4 (3,7) |
| Min, Max | 1.0, 35.0 | 1,35 | 1,15 |
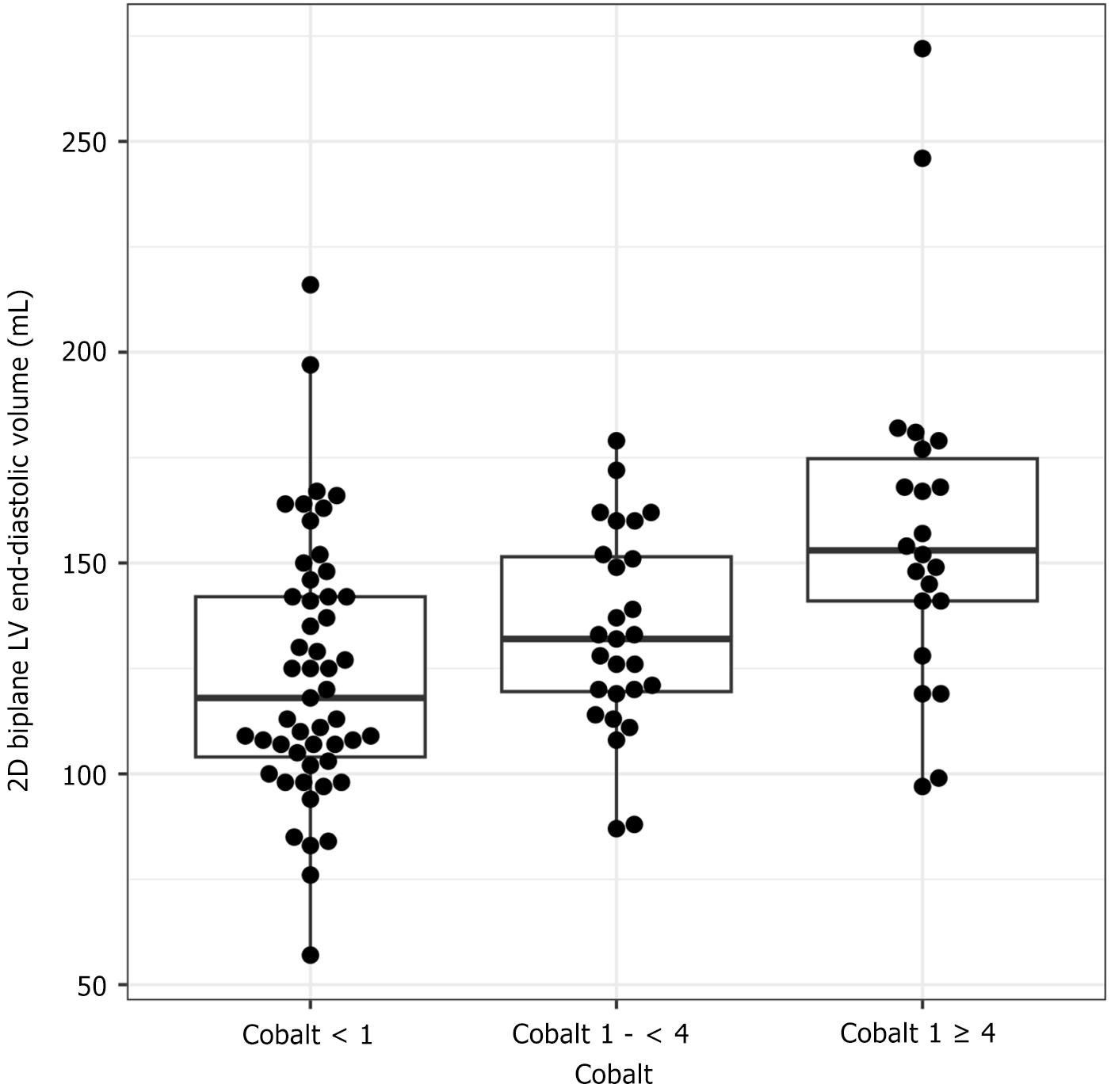
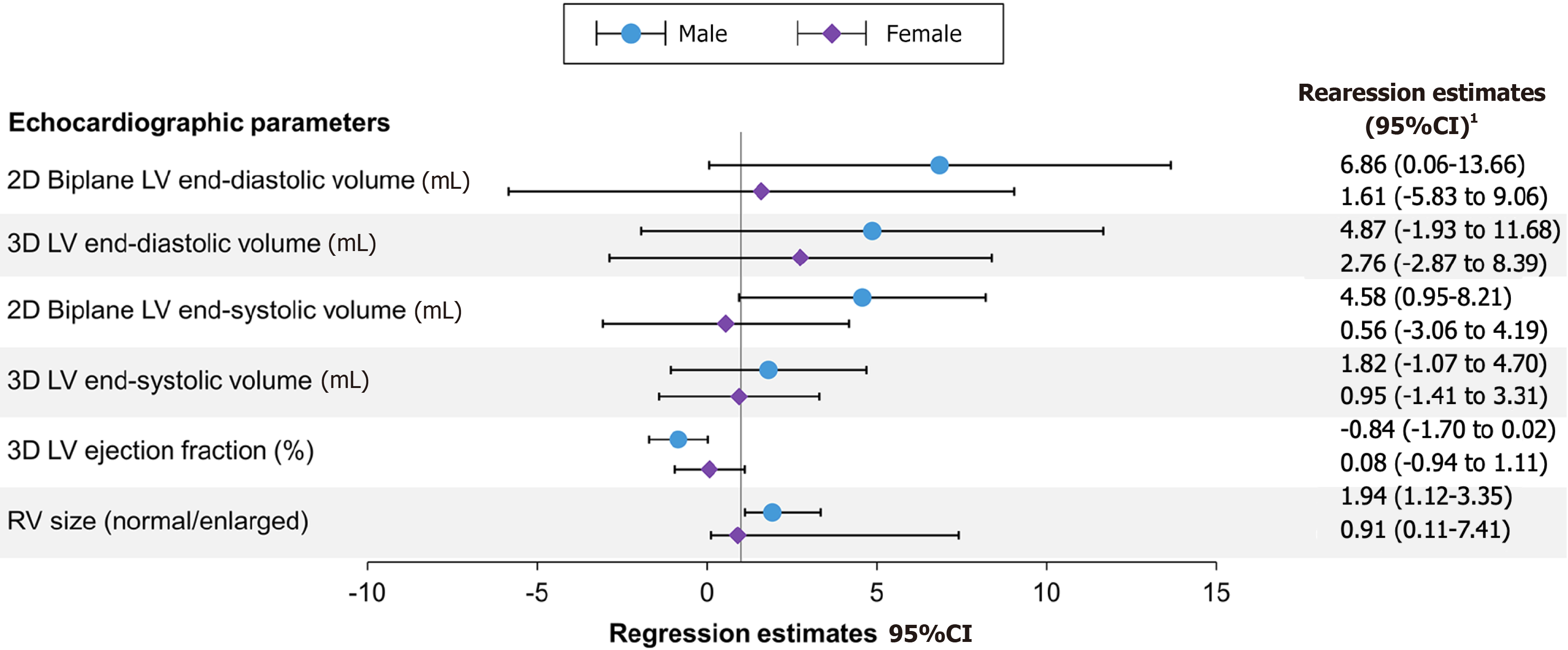
Chromium concentrations were not significantly associated with any echocardiographic findings (Table 3). Higher cobalt concentrations were associated with left and right ventricular dilatation. Higher cobalt concentrations were associated with increased left ventricular (LV) volumes as indicated with larger 2D biplane LV end-diastolic (estimate 5.09; 95%CI: 0.02-10.17), equating to a 5%-10% increase in LV volume per doubling in cobalt concentrations, and end-systolic volumes (estimate 3.13; 95%CI: 0.40-5.87) (Figure 1). For instance, patients with cobalt concentrations ≥ 4 ng/mL had an average 22.8 unit (95%CI: 7.4-38.1 P = 0.004) higher LV volumes than those with cobalt concentrations £1 ng/mL after adjusting for age and sex. This association was seen in men but not in women (Figure 2). Men had higher 2D biplane LV end-diastolic (estimate 6.86; 95%CI: 0.06-13.66; P = 0.05) and end-systolic volumes (estimate 4.58; 95%CI: 0.95-8.21; P = 0.01). Higher cobalt concentration was also associated with right ventricle enlargement in men (OR 1.94; 95%CI: 1.12-3.35; P = 0.02) but not in women (OR 0.91; 95%CI: 0.11-7.41; P = 0.93). Left atria were also larger in those with higher cobalt concentrations (estimate 4.16; 95%CI: 0.79-7.53), similarly in men and women. There was no association between cobalt concentrations and LV systolic function.
| Measure available | Mean (SD); Median (interquartile range) | Regression estimates (95%CI)1 | |||
| Measure (unit) | Chromium | Cobalt | Titanium | ||
| LV size and systolic function | |||||
| 2D Biplane LV end-diastolic volume (mL) | 100 | 134.04 (34.14); 129.5 (109, 153) | 1.20 (-5.23 to 7.64) | 5.09 (0.02-10.17)a | -3.67 (-8.55 to 1.22) |
| 3D LV end-diastolic volume (mL) | 95 | 130.38 (32.14); 126 (110, 147) | 0.95 (-4.59 to 6.50) | 4.09 (-0.57 to 8.74)a | -1.69 (-6.35 to 2.96) |
| 2D Biplane LV end-systolic volume (mL) | 99 | 50.76 (18.22); 48 (38, 60) | 0.44 (-3.05 to 3.92) | 3.13 (0.40-5.87) | -1.53 (-4.20 to 1.14) |
| 3D LV end-systolic volume (mL) | 94 | 49.24 (14.2); 48 (38, 57) | 0.44 (-1.86 to 2.75) | 1.46 (-0.50 to 3.42) | -1.16 (-3.09 to 0.76) |
| 3D LV ejection fraction | 94 | 62.12 (4.34); 62 (60, 65) | -0.10 (-0.88 to 0.68) | -0.51 (-1.17 to 0.15) | 0.07 (-0.59 to 0.73) |
| LV global longitudinal strain | 108 | -19.74 (2.12); -20 (-21, -19) | -0.15 (-0.55 to 0.26) | -0.27 (-0.60 to 0.07) | -0.38 (-0.70 to 0.06)b |
| LV stroke volume (mL) | 108 | 94.5 (17.22)/91 (82.5, 105) | -0.28 (-3.43 to 2.87) | 1.59 (-0.99 to 4.16) | -2.69 (-5.24 to 0.15)b |
| LV cardiac output (l/minute) | 109 | 6.12 (1.3); 5.84 (5.22, 6.8) | -0.01 (-0.35 to 0.16) | 0.01 (-0.20 to 0.22) | -0.20 (-0.40 to 0.004)a |
| LV cardiac index (l/minute/m2) | 108 | 2.92 (0.4)/2.82 (2.68,3.16) | 0.01 (-0.08 to 0.09) | 0.01 (-0.06 to 0.07) | -0.08 (-0.15 to -0.01)b |
| LV diastolic function | |||||
| LV diastolic function (normal/abnormal) | 110 | Normal 42 (38.2) | Reference | Reference | Reference |
| Abnormal 68 (61.8) | 1.21 (0.78-1.87) | 1.14 (0.80-1.62) | 0.67 (0.47-0.97)b | ||
| Medial E/e' ratio | 106 | 10.76 (3.7)/10 (8.3,12.5) | -0.38 (-1.02 to 0.27) | -0.05 (-0.60 to 0.49) | 0.18 (-0.34 to 0.70) |
| LA biplane vol index (mL/m2) | 107 | 31.32 (9.02); 30 (25, 37) | 1.26 (-1.36 to 3.86) | 2.72 (0.60-4.84)b | -0.24 ( -2.3 to 1.82) |
| LA reservoir strain | 105 | 29.9 (6.9); 29.5 (26.2, 33.9) | 0.002 (-1.38 to 1.39) | 0.083 (-1.06 to 1.22) | 1.602 (0.55-2.66)c |
| Right heart parameters | |||||
| RV systolic pressure (mmHg) | 80 | 28.34 (5.34); 28 (23.5, 32) | -0.41 (-1.54 to 0.73) | -0.22 (-1.19 to 0.75) | -0.28 (-1.15 to 0.58) |
| RV size (normal/enlarged) | 110 | Normal 99 (90) | Reference | Reference | Reference |
| Abnormal 11 (10) | 1.66 (0.91-3.05) | 1.80 (1.08-2.99)b | 1.22 (0.74-2.01) | ||
| RV basal diameter (mm) | 53 | 40.48 (6.3); 41 (36, 46) | 0.37 (-1.18 to 1.92) | 1.17 (-0.18 to 2.51)a | -0.17 (-1.42 to 1.08) |
| Tricuspid annular plane systolic excursion (mm) | 91 | 23.58 (3.7); 23 (21, 26) | -0.34 (-1.21 to 0.53) | 0.11 (-0.61 to 0.83) | 0.17 (-0.50 to 0.84) |
| RV Basal Lateral Systolic Strain | 34 | -25.06 (4.88); -26 (-29, -24) | 1.19 (-0.64 to 3.01) | 0.40 (-1.50 to 2.29) | -0.59 (-2.12 to 0.94) |
| Other parameters | |||||
| Mid ascending thoracic aortic diameter (mm) | 82 | 35.9 (4.08); 36 (33, 38) | 0.16 (-0.65 to 0.98) | 0.43 (-0.27 to 1.13) | -0.71 (-1.38 to -0.05)b |
| N terminal pro b-type natriuretic peptide (pg/mL) | 109 | 153.4 (269.3); 85 (42, 138) | -20.47 (-72.32 to 31.38) | 10.01 (-32.78 to 52.80) | 13.41 (-27.74 to 54.55) |
| Troponin (ng/mL) | 108 | 13.4 (11.3); 11 (7, 15) | -0.97 (-2.94 to 0.99) | -0.85 (-2.47 to 0.77) | 0.22 (-1.34 to 1.78) |
Higher titanium concentrations were associated with measures of reduced LV systolic function as indicated by a reduction in LV global longitudinal systolic strain (estimate 0.38; 95%CI: -0.70 to -0.06) which is a sensitive measure of left ventricle contraction. For instance, patients with titanium concentrations ≥ 3 ng/mL had an average 1.3 (95%CI: 2.2-0.4, P = 0.005) reduction in LV global longitudinal strain than those with titanium concentrations £1 ng/mL after adjusting for age and sex. LV stroke volume, cardiac output, and cardiac index were similarly lower in patients with high titanium concentrations (Table 3). For instance, the normal range for cardiac index is 2.5-4.2 L/min/m2. A 0.08 unit decrease in cardiac index equates from 1.9% to 3.2% decrease from normal cardiac function when titanium concentration was doubled (e.g. from 1 ng/mL to 2 ng/mL). On the contrary, higher titanium concentrations were associated with better left ventricle diastolic function (OR 0.67; 95%CI: 0.47-0.97) and higher left atrial reservoir strain (OR 1.60; 95%CI: 0.55-2.66). No significant associations were observed between metal concentrations and NT-proBNP and troponin levels.
There is concern regarding the systemic effects of increased metal concentrations in TJA patients. However, evidence has been limited to anecdotal case reports or large registry-based studies with limited data on clinical findings or echocardiographic abnormalities. In this study, we investigated the association of systemic chromium, cobalt, and titanium concentrations with echocardiographic measures of cardiac structure and function and cardiac labs troponin and NT-proBNP. Overall, we found that cobalt was associated with both left and right ventricular dilation in men and titanium was associated with significant changes in LV systolic function (i.e., cardiac index in Table 3). No significant associations were observed for chromium.
Besides case reports and registry-based studies, three previous studies are similar to ours and examined the correlation between metal concentrations and echocardiographic findings. Prentice et al[15] compared 35 MoM hip resurfacing patients with 35 matched asymptomatic THA patients with conventional implants. Patients with MoM implants had higher systemic cobalt and chromium concentrations (1.75 µg/L vs 0.38 µg/L and 1.27 µg/L vs < 0.30 µg/L for chromium), but relatively minor differences in cardiac ejection fraction and LV end-diastolic diameter.
In another study, 90 THA patients were split into three groups based on bearing type and blood metal concentrations, and cardiac function was evaluated using both cardiac magnetic resonance imaging and echocardiography[13]. There was no evidence for an association between metal concentrations and cardiac structure or function. Despite the large sample size similar to ours and 3 groups of THA patients with a wide spectrum of chromium and cobalt concentrations, linear associations between metal concentrations and LV structural measures were not reported. Splitting patients into 3 separate groups may have resulted in loss of power. Finally, 75 THA patients with the same modular implants (49 MoM bearings) were evaluated at two visits for cobalt, chromium, and titanium concentrations and an echocardiogram at the second visit[14]. Cobalt and chromium concentrations were higher in MoM patients, but titanium concentrations and cobalt-chromium ratios were not different between groups. Cobalt and chromium concentrations increased between time-points in the MoM patients with significantly lower global longitudinal strain than compared with the non-MoM group. Cobalt concentrations were associated with tricuspid annular plane systolic excursion, an indicator of right ventricular function. We similarly observed several associations between cobalt and titanium concentrations and cardiac function measures. Consistent with the previous study, we found an association between cobalt and right ventricular size as well as an association with LV volume, not described in earlier studies.
Cobalt and chromium concentrations were undetectable in about half of our patients and were not associated with echocardiographic differences in women. Yet, a relationship between LV dilation was found with cobalt concentrations in men. Furthermore, a doubling of cobalt levels in men was associated with a 1.9-fold increase in the prevalence if right ventricular enlargement. Prior studies have suggested a link between cobalt deposition and cardiac toxicity potentially related to intracellular calcium mishandling[7]. Here a tendency for abnormal ventricular dilation is a well-recognized finding that precedes the development of heart failure[20].
Titanium was the most commonly elevated metal in our study and was associated with several measures of decreased LV systolic function but not diastolic function. Better measurement of small concentrations in cobalt and chromium may allow detection of associations between these metals and heart function measures. Compared with cobalt and chromium, relatively little is known about the potential systemic effects of titanium. Evidence to date comes only from laboratory studies. For example, in an experimental laboratory study in hypertensive rats, intra-tracheal administration of titanium resulted in irreversible hemodynamic impairment and cardiac structural damage[21].
Our findings should be interpreted considering potential limitations. Due to the small sample size, we were unable to perform subgroup analyses by number of surgeries, surgery/implant types, potential reasons for high metal ions (e.g. trunnionosis) or among subgroups of patients such as those with smoking history, other lifestyle factors or selected comorbidities like heart failure. Adverse cardiac effects of high metal concentrations may vary in different ethnic and racial groups. With mostly Caucasian white patients, the generalizability our findings is limited. Our power calculations prior to the study indicated that with a sample size of 100 patients, the linear regression test of correlation = 0 (P = 0.05 two-sided) between the cardiac outcomes and metal concentrations would have 80% power to detect a correlation of 0.27 (with a corresponding r-square of 0.073). The main exposure variable in this study was the metal concentrations which were examined as a log-transformed continues exposure variable. Dichotomizing or categorization of continuous exposure variables for analysis is inefficient and misleading[22,23]. Importantly, we examined cross-sectional associations and observed some significant correlations with cobalt and titanium concentrations, but the magnitude was small. Therefore, these significant findings should be interpreted with caution and bearing in mind the evidence from other lines of research. Ideally, information on longitudinal changes in systemic metal concentrations and cardiac measures would be needed to further interpret causal implications of these cross-sectional findings. Furthermore, in addition to TJA implants, other environmental exposures, such as dental implants and food additives, nutritional status or alcohol intake can contribute to higher systemic levels[24,25]. Strengths include the use of 3D and speckle-tracking echocardiography, which provide much more sensitive markers of cardiac dysfunction than the traditional 2D echocardiography.
In conclusion, elevated cobalt and titanium concentrations may be associated with structural and functional cardiac changes in some patients. Given the high prevalence of TJA and limited evidence on the potential long-term cardiotoxicity of metal implants, more research is warranted to better understand how the systemic distribution of metal ions may affect cardiac function and the temporal relationship and quantitative thresholds for metal concentrations and cardiac pathology.
| 1. | Singh JA, Yu S, Chen L, Cleveland JD. Rates of Total Joint Replacement in the United States: Future Projections to 2020-2040 Using the National Inpatient Sample. J Rheumatol. 2019;46:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 829] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 2. | Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, Jiranek WA, Berry DJ. Prevalence of Total Hip and Knee Replacement in the United States. J Bone Joint Surg Am. 2015;97:1386-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 1270] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 3. | Merola M, Affatato S. Materials for Hip Prostheses: A Review of Wear and Loading Considerations. Materials (Basel). 2019;12:495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 4. | Cheung AC, Banerjee S, Cherian JJ, Wong F, Butany J, Gilbert C, Overgaard C, Syed K, Zywiel MG, Jacobs JJ, Mont MA. Systemic cobalt toxicity from total hip arthroplasties: review of a rare condition Part 1 - history, mechanism, measurements, and pathophysiology. Bone Joint J. 2016;98 -B:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Sutphen SA, MacLaughlin LH, Madsen AA, Russell JH, McShane MA. Prevalence of Pseudotumor in Patients After Metal-On-Metal Hip Arthroplasty Evaluated with Metal Ion Analysis and MARS-MRI. J Arthroplasty. 2016;31:260-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Daniel J, Holland J, Quigley L, Sprague S, Bhandari M. Pseudotumors associated with total hip arthroplasty. J Bone Joint Surg Am. 2012;94:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Wyles CC, Wright TC, Bois MC, Amin MS, Fayyaz A, Jenkins SM, Wyles SP, Day PL, Murray DL, Trousdale RT, Anavekar NS, Edwards WD, Maleszewski JJ. Myocardial Cobalt Levels Are Elevated in the Setting of Total Hip Arthroplasty. J Bone Joint Surg Am. 2017;99:e118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Lassalle M, Colas S, Rudnichi A, Zureik M, Dray-Spira R. Is There a Cardiotoxicity Associated With Metallic Head Hip Prostheses? A Cohort Study in the French National Health Insurance Databases. Clin Orthop Relat Res. 2018;476:1441-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Sabah SA, Moon JC, Jenkins-Jones S, Morgan CL, Currie CJ, Wilkinson JM, Porter M, Captur G, Henckel J, Chaturvedi N, Kay P, Skinner JA, Hart AJ, Manisty C. The risk of cardiac failure following metal-on-metal hip arthroplasty. Bone Joint J. 2018;100 -B:20-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Gillam MH, Pratt NL, Inacio MC, Roughead EE, Shakib S, Nicholls SJ, Graves SE. Heart failure after conventional metal-on-metal hip replacements. Acta Orthop. 2017;88:2-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Goodnough LH, Bala A, Huddleston J III, Goodman SB, Maloney WJ, Amanatullah DF. Metal-on-metal total hip arthroplasty is not associated with cardiac disease. Bone Joint J. 2018;100-B:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Deere K, Matharu GS, Ben-Shlomo Y, Wilkinson JM, Blom AW, Sayers A, Whitehouse MR. The risk of all-cause mortality, heart outcomes, cancer, and neurodegenerative disorders with cobalt-chrome-containing total hip arthroplasty implants : an analysis of the National Joint Registry. Bone Joint J. 2022;104 -B:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Berber R, Abdel-Gadir A, Rosmini S, Captur G, Nordin S, Culotta V, Palla L, Kellman P, Lloyd GW, Skinner JA, Moon JC, Manisty C, Hart AJ. Assessing for Cardiotoxicity from Metal-on-Metal Hip Implants with Advanced Multimodality Imaging Techniques. J Bone Joint Surg Am. 2017;99:1827-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Darrith B, Rahman TM, Ananthasubramaniam K, Culvern C, Jacobs JJ, Silverton CD. Echocardiographic Changes in the Context of Metal-on-Metal Versus Nonmetal-on-Metal Total Hip Arthroplasty. J Arthroplasty. 2020;35:3230-3236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Prentice JR, Clark MJ, Hoggard N, Morton AC, Tooth C, Paley MN, Stockley I, Hadjivassiliou M, Wilkinson JM. Metal-on-metal hip prostheses and systemic health: a cross-sectional association study 8 years after implantation. PLoS One. 2013;8:e66186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Yao JJ, Lewallen EA, Trousdale WH, Xu W, Thaler R, Salib CG, Reina N, Abdel MP, Lewallen DG, van Wijnen AJ. Local Cellular Responses to Titanium Dioxide from Orthopedic Implants. Biores Open Access. 2017;6:94-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Day PL, Eckdahl SJ, Maleszewski JJ, Wright TC, Murray DL. Establishing human heart chromium, cobalt and vanadium concentrations by inductively coupled plasma mass spectrometry. J Trace Elem Med Biol. 2017;41:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Yao JJ, Lewallen EA, Thaler R, Dudakovic A, Wermers M, Day P, Eckdahl S, Jannetto P, Bornhorst JA, Larson AN, Abdel MP, Lewallen DG, van Wijnen AJ. Challenges in the Measurement and Interpretation of Serum Titanium Concentrations. Biol Trace Elem Res. 2020;196:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6446] [Cited by in RCA: 9968] [Article Influence: 906.2] [Reference Citation Analysis (0)] |
| 20. | Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113:2851-2860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Rossi S, Savi M, Mazzola M, Pinelli S, Alinovi R, Gennaccaro L, Pagliaro A, Meraviglia V, Galetti M, Lozano-Garcia O, Rossini A, Frati C, Falco A, Quaini F, Bocchi L, Stilli D, Lucas S, Goldoni M, Macchi E, Mutti A, Miragoli M. Subchronic exposure to titanium dioxide nanoparticles modifies cardiac structure and performance in spontaneously hypertensive rats. Part Fibre Toxicol. 2019;16:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Senn S. Dichotomania: an obsessive compulsive disorder that is badly affecting the quality of analysis of pharmaceutical trials. 2005; 13. Available from: https://www.semanticscholar.org/paper/Dichotomania%3A-An-Obsessive-Compulsive-Disorder-that-Senn/8c390cb90465ad1f08b86cee52474859e4dffd77. |
| 23. | van Smeden M. A Very Short List of Common Pitfalls in Research Design, Data Analysis, and Reporting. PRiMER. 2022;6:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Kim KT, Eo MY, Nguyen TTH, Kim SM. General review of titanium toxicity. Int J Implant Dent. 2019;5:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 196] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 25. | Zhou Z, Shi Q, Wang J, Chen X, Hao Y, Zhang Y, Wang X. The unfavorable role of titanium particles released from dental implants. Nanotheranostics. 2021;5:321-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/