Published online Jul 18, 2024. doi: 10.5312/wjo.v15.i7.675
Revised: May 7, 2024
Accepted: June 3, 2024
Published online: July 18, 2024
Processing time: 100 Days and 2.4 Hours
Gout is a disease characterized by hyperuricemia, and resultant deposition of uric acid crystals in tissues. While typically manifested as intraarticular crystals or tophi, gout can also cause pathology at entheses. Gouty deposition within ten
A 56-year-old female presented to clinic after feeling her left knee pop and coll
Failed patellar tendon reconstruction due to gouty infiltration is treated with dermal allograft augmented Achilles tendon reconstruction with bone block.
Core Tip: We present a case of a 56-year-old female with severe systemic tophaceous gout who presented with a rupture of her revision patellar tendon reconstruction due to severe gouty infiltration. The major takeaway from this case is that with multiple failed reconstructions of the patellar tendon, both structural and biologic reinforcement were sought to provide this patient the best chance for recovery and a substantial reconstruction. We provided a robust structural construct as well as biologic reinforcement with the use of an Achilles tendon allograft with a wedge-shaped bone block and a dermal allograft incorporated into the reconstruction.
- Citation: Edge CC, Widmeyer J, Protzuk O, Johnson M, O’Connell R. Gouty destruction of a patellar tendon reconstruction and novel revision reconstruction technique: A case report. World J Orthop 2024; 15(7): 675-682
- URL: https://www.wjgnet.com/2218-5836/full/v15/i7/675.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i7.675
Patellar tendon rupture is a relatively rare phenomenon. These injuries are often secondary to trauma, however, chronic inflammatory states, such as gout, have been shown to increase risk of patellar tendon rupture[1-4].
Gout is a disease characterized by hyperuricemia, and resultant deposition of uric acid crystals in tissues. While typically manifested as intraarticular crystals or tophi, gout can also cause pathology at entheses[5,6]. Gouty deposition within tendinous structures put them at risk for traumatic and degenerative rupture. Furthermore, allografts can also be at risk of rupture in the setting of severe gout, as described following Anterior Cruciate Ligament reconstruction[7]. We present the case of a 56-year-old female with severe gouty disease who sustained a re-rupture of a patellar tendon allo
The literature review was performed using PubMed with the search items: “Patellar tendon reconstruction; gout tendinopathy; biologics in tendon reconstruction”. Descriptive literature from the years 1995-2023 were reviewed in
A 56-year-old female who presents with inability to bear weight in her left leg or extend her left knee.
She presented to clinic in December of 2022 after feeling her left knee pop and collapse beneath her while descending stairs. She had immediate pain and inability to bear weight or extend her left knee.
She has a history of severe tophaceous gout and left patellar tendon rupture and primary repair with transosseous suture at age 37 in January, 2004. This repair failed nearly two years later and was revised with a patellar tendon advancement with cerclage wire reinforcement in December, 2005. This revision repair failed, requiring reconstruction in November 2006 with Achilles tendon allograft with bone block fixation (Table 1). Each time, it was felt that gouty depositions led to failure. She was followed closely by rheumatology and treated with multiple medications including allopurinol, feb
| Date | Event |
| January, 2004 | Injury: Initial left patellar tendon rupture |
| Intervention: Primary repair | |
| December, 2005 | Injury: Rupture of patellar tendon repair |
| Intervention: Patellar tendon advancement with cerclage wire reinforcement | |
| November, 2006 | Injury: Re-rupture of patellar tendon |
| Intervention: Reconstruction of patellar tendon with Achilles tendon allograft | |
| December, 2022 | Injury: Re-rupture of patellar tendon reconstruction |
At presentation, she had a palpable defect in her left patellar tendon, ecchymosis into the calf with tenderness and swelling. She had a 40-degree extensor lag. On exam, she was neurovascularly intact without any ligamentous instability of the knee.
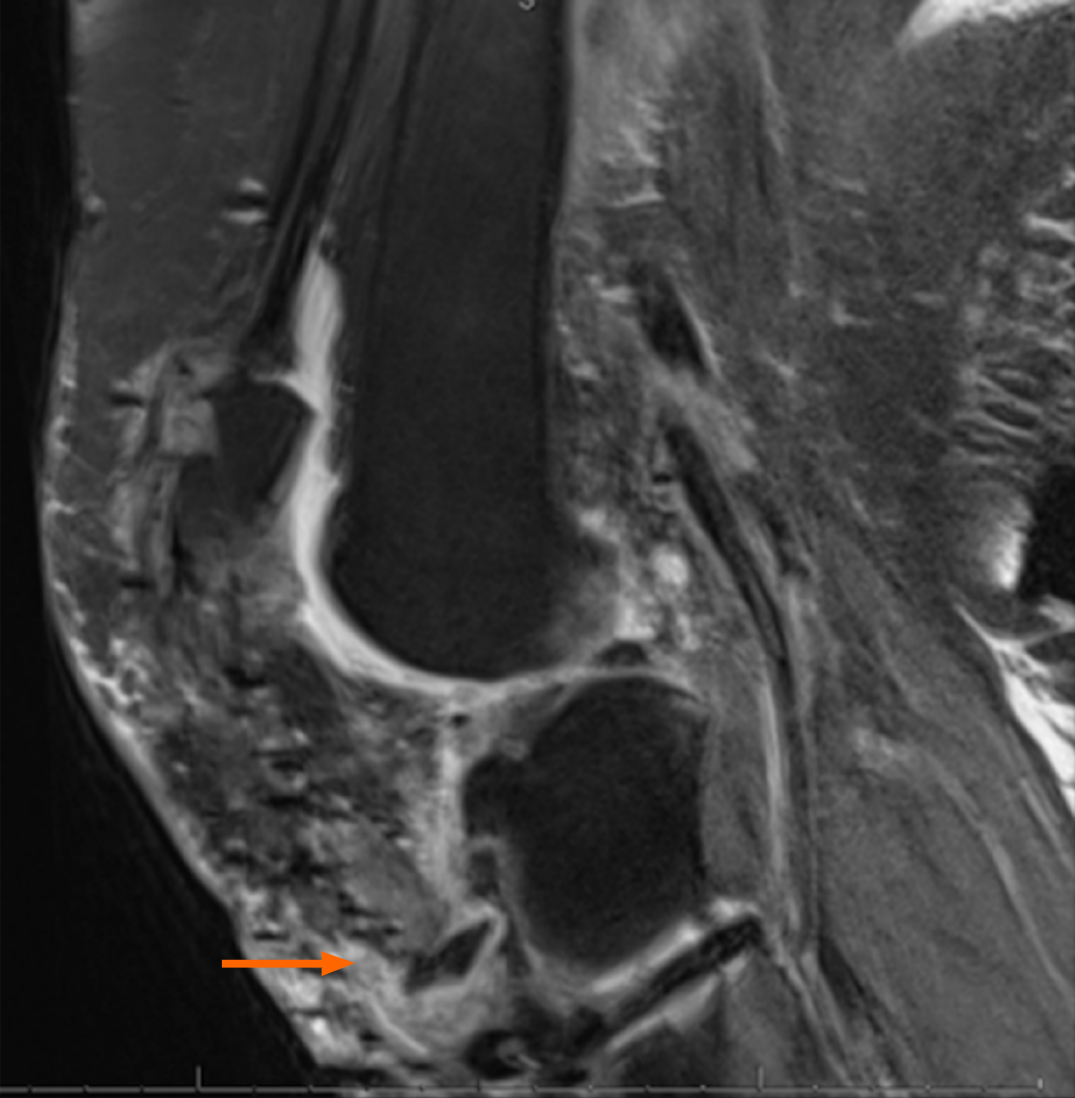
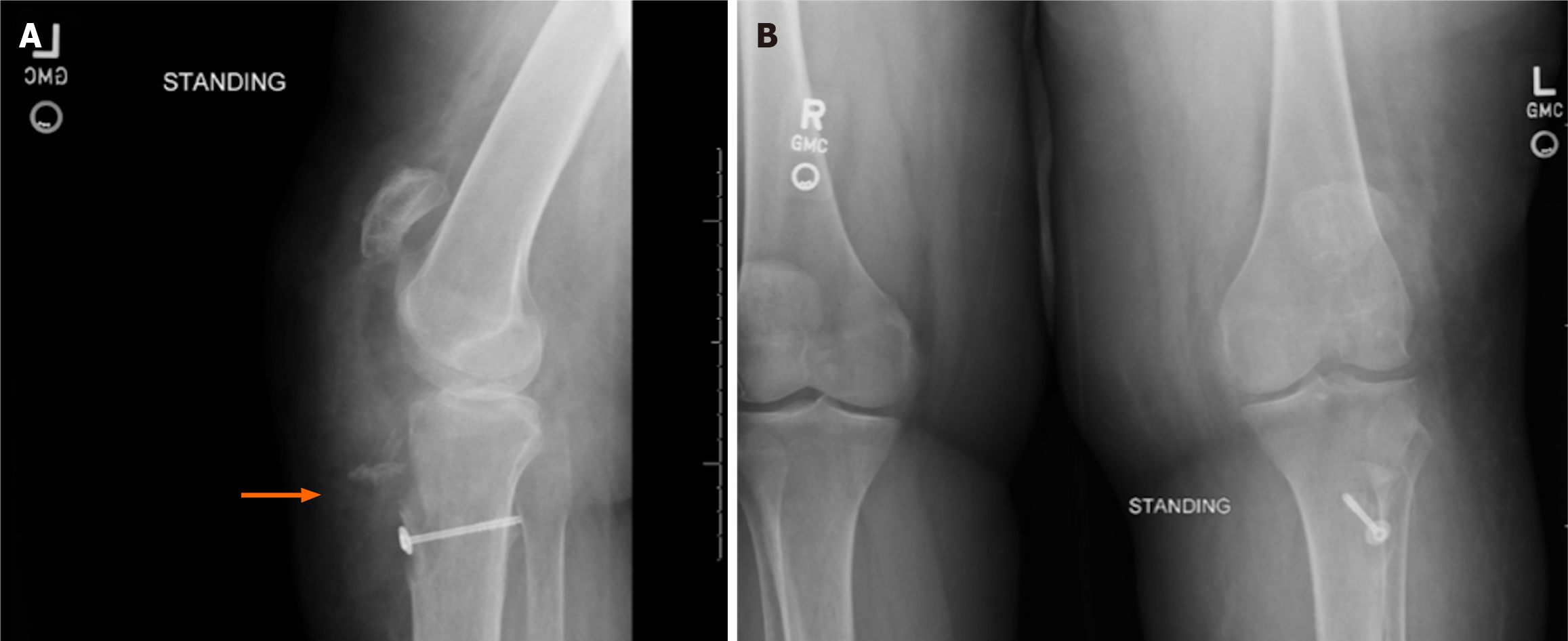
A left knee magnetic resonance imaging (Figure 1) demonstrated ruptured patellar tendon allograft reconstruction and avulsion fracture at the tibial tubercle. Contralateral knee radiographs were obtained to determine patellar height (Figure 2). The patient was placed into a knee immobilizer with plans for revision reconstruction with allograft augm
Combined with the mechanism of injury, preoperative physical exam and imaging, the intraoperative findings of tophaceous gout within the patellar tendon, and the lab results showing uric acid crystals, the final diagnosis was patellar tendon reconstruction re-rupture secondary to gouty infiltration.
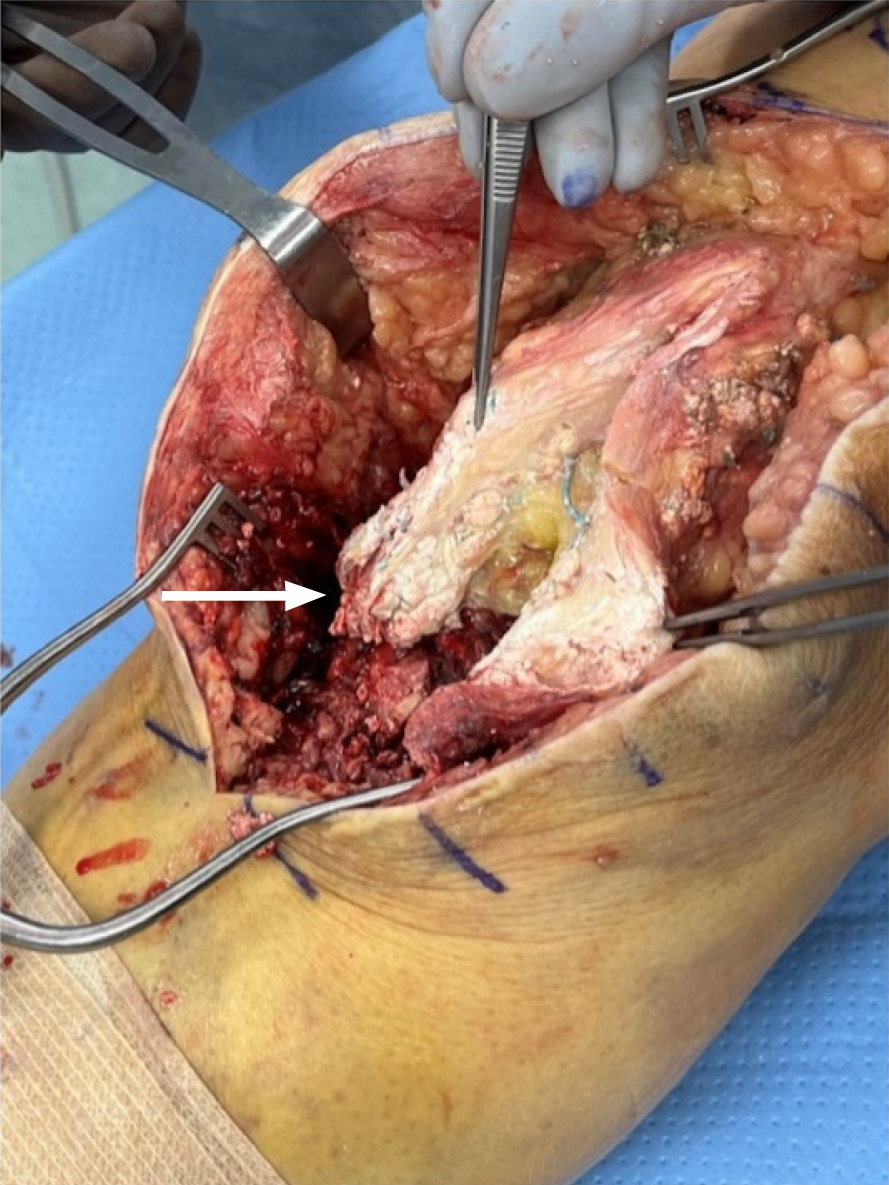
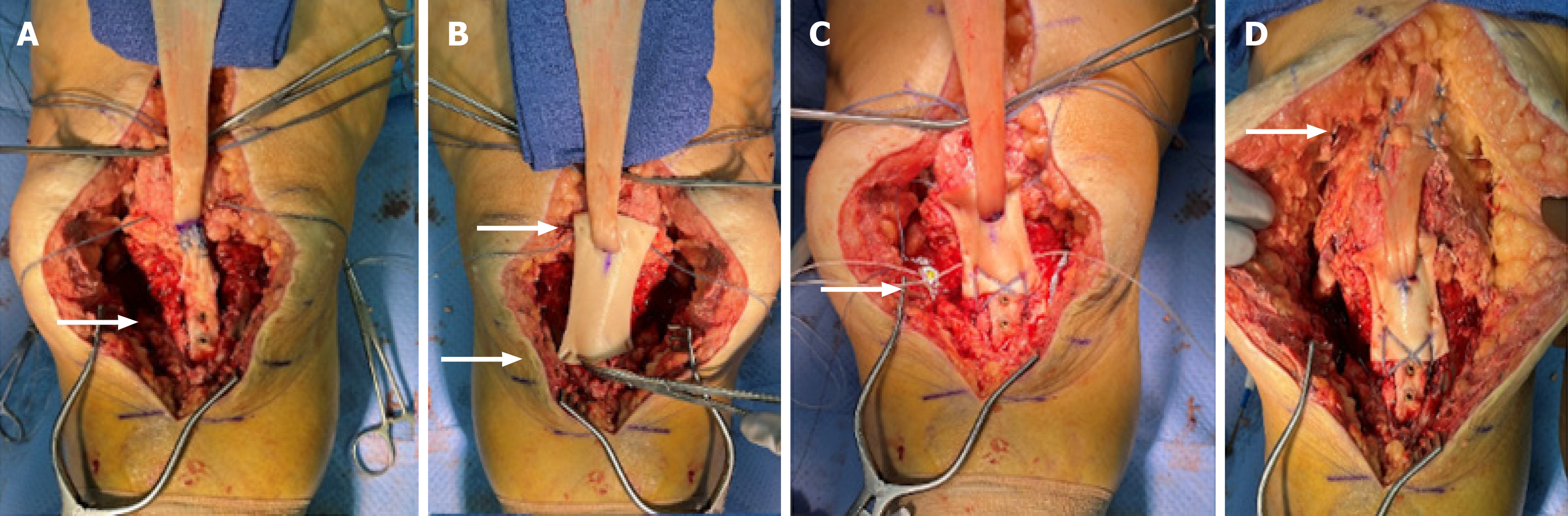
An anterior approach to knee was made using prior incision. Extensive gouty material was found within the allograft tendon and the surrounding tissues. This was debrided and sent to rule out infection and confirm the diagnosis of gout. Consistent with preoperative imaging, the allograft was ruptured at the tibial tubercle with loose tibial hardware. The tibial hardware was removed and gouty material within the previous graft insertion site was debrided. The graft was excised off the distal pole of the patella. Extensive gouty deposition was visualized within the joint (Figure 3). Synovial samples were sent which returned as uric acid crystals.
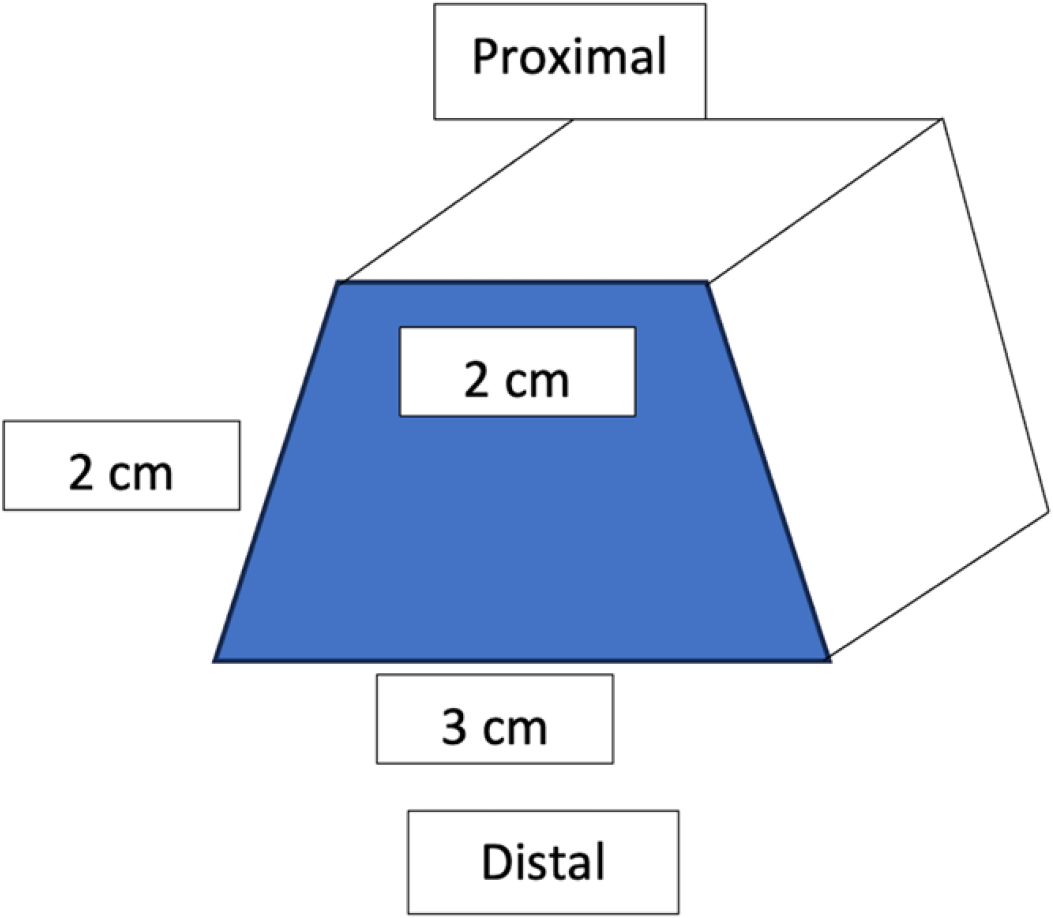
An Achilles tendon allograft (Lifenet, Virginia Beach, VA, United States) with a bone plug was prepared for the revision procedure. The bone plug was shaped into a trapezoid with the wider limb distally to act as a wedge and prevent superior migration [approximately 3 cm (Length) × 2 cm (Depth) × 2 cm (Width)] (Figure 4). A corresponding site was prepared at the tibial tubercle. The bone block was then secured with two 4.0mm partially threaded cannulated screws with washers (DePuy Synthes, West Chester, PA) (Figure 5). Two #5 FiberWire sutures (Arthrex, Naples, FL) were placed as locking Kraków stitch through the tendon portion of the allograft. These were pulled through three longitudinal trans-osseous tunnels drilled through the patella. These were provisionally tensioned and clamped with the knee in full extension.
Two knotless fiber tack anchors (Arthrex, Naples FL) were then placed on the medial and lateral borders of the inferior pole of the patella. A 3 mm thick dermal allograft (Arthrex, Naples, FL) was then secured to the inferior pole of the patella with the previously placed knotless fiber tack anchors. A transverse slit in the graft was created and the excess proximal Achilles tendon allograft was passed from posterior to anterior through this opening. The distal portion of the dermal allograft was secured to the tibial tubercle in the speed bridge fashion with four swivel lock anchors (Arthrex, Naples, FL) and fiber tape overlying the bone block. The graft was tensioned and the transosseous sutures through the patella were tied, completing the patellar tendon reconstruction. Appropriate patellar height was confirmed with fluoroscopy of the contralateral knee. Accessory sutures from the anchors in the tibial tubercle were utilized to reinforce the graft medially and laterally. FiberWire suture was used to repair the medial and lateral retinaculum to incorporate the graft into the repair. The remaining allograft tendon was brought superiorly and reinforced into the quadriceps tendon with #2 FiberWire in a figure-of-eight pattern. Prior to closure, the knee was brought to 60 degrees of flexion without excessive tension on the construct or gapping.
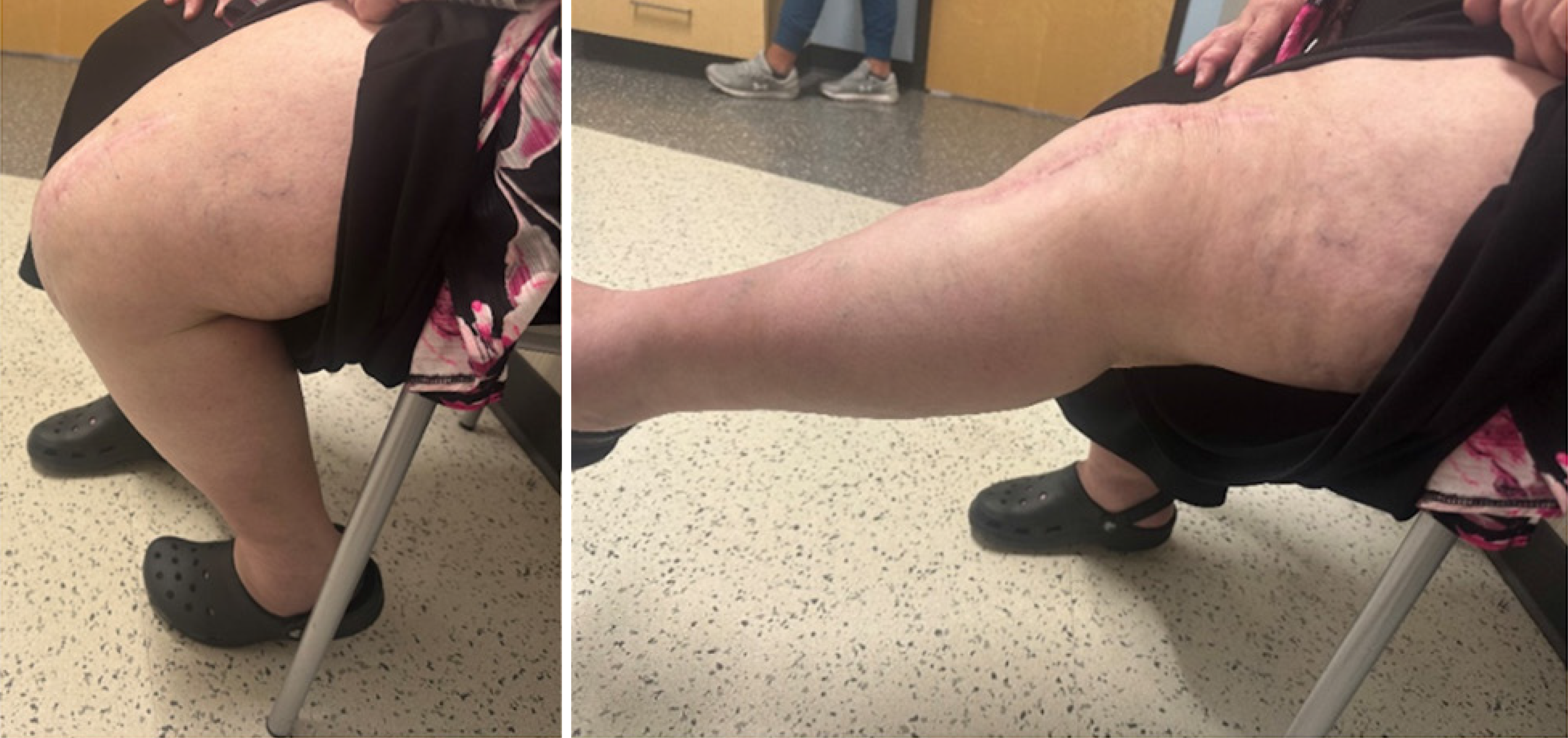
Postoperatively, the patient was made non-weightbearing in the left lower extremity. Due to habitus and recurrent ruptures, we did not feel a knee immobilizer would provide adequate initial immobilization. Thus, she was placed into a long leg cast for two weeks. She was then transitioned to a custom fitted Total Range of Motion brace locked in extension and was allowed to bear weight. Isometric quad exercises were started. At one month, she began passive flexion exercises with a progression of 15 degrees per week. At nine weeks, she had full passive extension and seventy degrees of flexion in the left knee. She was still limited to weightbearing with the brace in extension until her quad strength improved. At three months, she had full active extension to resistance without extensor lag, as well as 90 degrees of active flexion. At five months, patient was riding a stationary bicycle and was ascending and descending stairs. Her active range of motion was from 10-115 degrees with full passive extension. At twelve months, she had no pain and felt she had improved function. Her extensor lag improved from 10 to 5 degrees (Figure 6). She returned to work and her usual daily and recreational activities.
International Knee Documentation Committee (IKDC) Subjective Knee Evaluation[8], Lower Extremity Functional Scale[9], and Lysholm Knee Scoring Scale[10] outcome measures were collected at follow ups (Table 2). All scores improved at all time points between 2 and 12 months.
| Months post-op | Lower extremity functional scale | IKDC subjective knee form | Lysholm knee scale |
| 2 months post-op | 26 | - | - |
| 3 months post-opp | 42.5 | 55% | 52 |
| 5 months post-op | 53 | - | - |
| 12 months post-op | 59 | 69% | 82 |
Although gouty destruction of tendons has been described, there are no current reports of complete destruction of a patellar tendon allograft leading to failure; as in this patient[7,11]. Due to extensive destruction of surrounding tissues including tibial tubercle bone, augmentation of both soft tissue and bone were necessary for adequate healing.
Dermal allografts provide biologic and noninflammatory properties as well as time-zero structural integrity[12]. Tendon allografts must undergo multiple stages of healing and maturation in order to achieve long term structural integrity. In this process, the graft must undergo revascularization to support subsequent cellular repopulation and matrix remodeling[13,14]. The decellularized human dermal matrix functions as a collagen scaffold that facilitates host tissue integration and thus can assist in tendon allograft maturation.
Other forms of bioaugmentation, such as platelet rich plasma, bone marrow aspirate concentrate, and bio-inductive patches, have been gaining popularity as adjuncts in the treatment of tendon injuries. Like dermal allograft, bio-inductive patches provide porous animal collagen scaffold that promotes tissue formation. While they do not enhance strength of the repair directly, once incorporated, increased tendon thickness and tissue healing has been implicated in improved pain and functional outcomes[15,16]. One must consider that these adjuncts can be costly, do not provide any structural enhancement to the repair, and have only been used in patellar tendon reconstruction in limited cases[17-20]. While few reports exist regarding the application of bio-inductive patches to patellar tendon repair/reconstruction, there are promising short and mid-term results[19-21]. Due to the low incidence of patellar tendon reconstruction and varied reconstructive options, there have been no large scale comparative studies to determine optimal technique, however, gracilis and semitendinosus are often used[22,23]. The addition of bioaugmentation to the well-established outcomes of tendinous allografts yields a promising solution for the problem of patellar tendon tears necessitating reconstruction in poor healing environments.
Our patient’s self-reported outcome scores correspond to fair functionality and some difficultly performing moderate to high-strain activities[8-10]. Furthermore, this patient progressed to 115 degrees of flexion, with regression to 5 degrees of extensor lag. It is possible that as she began ambulating, the micro-elasticity of the allograft allowed for a small amount of lengthening, which resulted in a slight lag. Most importantly, the patient has progressed to independence with the ability to exercise and work without pain.
Our report suggests that while multi-revision patellar tendon reconstructions are debilitating with a difficult recovery, that a dermal allograft augmented Achilles allograft reconstruction with bone block is affective for treatment of recurrent patellar tendon rupture in the setting of gouty destruction with return to a functional level. This multi-graft technique could be considered for cases of graft revision or other complex patellar tendon reconstruction.
| 1. | Rosso F, Bonasia DE, Cottino U, Dettoni F, Bruzzone M, Rossi R. Patellar tendon: From tendinopathy to rupture. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2015;2:99-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12:300-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Lu M, Johar S, Veenema K, Goldblatt J. Patellar tendon rupture with underlying systemic lupus erythematosus: a case report. J Emerg Med. 2012;43:e35-e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 340] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Pineda C, Amezcua-Guerra LM, Solano C, Rodriguez-Henríquez P, Hernández-Díaz C, Vargas A, Hofmann F, Gutiérrez M. Joint and tendon subclinical involvement suggestive of gouty arthritis in asymptomatic hyperuricemia: an ultrasound controlled study. Arthritis Res Ther. 2011;13:R4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Xu G, Lin J, Liang J, Yang Y, Ye Z, Zhu G, Cao H. Entheseal involvement of the lower extremities in gout: an ultrasonographic descriptive observational study. Clin Rheumatol. 2021;40:4649-4657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Rodas G, Pedret C, Català J, Soler R, Orozco L, Cusi M. Intratendinous gouty tophus mimics patellar tendonitis in an athlete. J Clin Ultrasound. 2013;41:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, Richmond JC, Shelborne KD. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29:600-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1375] [Cited by in RCA: 1667] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 9. | Binkley JM, North American Orthopaedic Rehabilitation Research Network, Stratford PW, Lott SA, Riddle DL. Lower Extremity Functional Scale. PsycTESTS Dataset. 2018;. [DOI] [Full Text] |
| 10. | E Albuquerque RP, Giordano V, Calixto A, Malzac F, Aguiar C, do Amaral NP, Carvalho AC. Analysis on the modified lysholm functional protocol among patients with normal knees. Rev Bras Ortop. 2011;46:668-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Lubis AMT, Reksoprodjo AY, Kuncoro MW, Ifran NN. Post-ACL Reconstruction Graft Failure in Severe Gout Arthritis Patient. Int Med Case Rep J. 2021;14:725-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Acevedo DC, Shore B, Mirzayan R. Orthopedic applications of acellular human dermal allograft for shoulder and elbow surgery. Orthop Clin North Am. 2015;46:377-388, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Yao S, Fu BS, Yung PS. Graft healing after anterior cruciate ligament reconstruction (ACLR). Asia Pac J Sports Med Arthrosc Rehabil Technol. 2021;25:8-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Yang C, Teng Y, Geng B, Xiao H, Chen C, Chen R, Yang F, Xia Y. Strategies for promoting tendon-bone healing: Current status and prospects. Front Bioeng Biotechnol. 2023;11:1118468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 15. | Bragg JT, Shields MV, Salzler MJ. Intrasubstance Patellar Tendon Repair with the Addition of a Bio-inductive Implant. Arthrosc Tech. 2023;12:e11-e15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Mc Millan S, Faoao DO, Ford E. Treatment of an Insertional High Grade Partial Patellar Tendon Tear Utilizing a Bio-Inductive Implant. Arch Bone Jt Surg. 2019;7:203-208. [PubMed] |
| 17. | Hung CY, Lin SJ, Yeh CY, Yeh WL. Effect of Platelet-Rich Plasma Augmentation on Endoscopy-Assisted Percutaneous Achilles Tendon Repair. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Aridici R, Yetisgin A, Boyaci A, Tutoglu A, Bozdogan E, Sen Dokumaci D, Kilicaslan N, Boyaci N. Comparison of the Efficacy of Dry Needling and High-Power Pain Threshold Ultrasound Therapy with Clinical Status and Sonoelastography in Myofascial Pain Syndrome. Am J Phys Med Rehabil. 2016;95:e149-e158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Imam MA, Holton J, Horriat S, Negida AS, Grubhofer F, Gupta R, Narvani A, Snow M. A systematic review of the concept and clinical applications of bone marrow aspirate concentrate in tendon pathology. SICOT J. 2017;3:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Geers BA, Bishai SK. Chronic Midsubstance Patellar Tendon and Retinacular Rupture: Primary Repair Enhancement Using Bioinductive Implant Augmentation. Arthrosc Tech. 2023;12:e1595-e1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Gilmore JH, Clayton-Smith ZJ, Aguilar M, Pneumaticos SG, Giannoudis PV. Reconstruction techniques and clinical results of patellar tendon ruptures: Evidence today. Knee. 2015;22:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Kim WT, Kao D, O'Connell R, Patel NK, Vap A. Clinical Outcomes are Similar Between Graft Types Used in Chronic Patellar Tendon Reconstruction: A Systematic Review. Arthrosc Sports Med Rehabil. 2022;4:e1861-e1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/